Summary
Signaling through Toll-like receptor 2 (TLR2) promotes inflammation and modulates CD4+CD25+ regulatory T cells (Tregs). We assessed mechanistically how this molecule would alter immunoregulation in type 1 diabetes (T1D). We also asked whether TLR2 may be involved in our recent discovery that viral infection can protect from autoimmune diabetes by expanding and invigorating regulatory T cells (Tregs). Treatment of prediabetic mice with a synthetic TLR2 agonist diminished T1D and increased the number and function of CD4+CD25+ Tregs, also conferring dendritic cells (DCs) with tolerogenic properties. TLR2 ligation also promoted the expansion of Tregs upon culture with DCs and ameliorated their capacity to prevent the disease. Protection from T1D by lymphocytic choriomeningitis virus (LCMV) infection depended on TLR2. LCMV increased the frequency of CD4+CD25+ Tregs and their production of TGF-β more significantly in wild type than TLR2-deficient mice. Furthermore, LCMV infection in vivo or LCMV-infected DCs in vitro rendered, via TLR2, CD4+CD25+ Tregs capable of diminishing T1D. We identify novel mechanisms by which TLR2 promotes immunoregulation and controls autoimmune diabetes in naïve or infected hosts. This work should help understand T1D etiology and develop novel immune-based therapeutic interventions.
Keywords: Type 1 diabetes, Toll-like receptor 2, immunoregulation, regulatory T cells, dendritic cells, virus
Introduction
T1D is a genetic disease resulting in the destruction of insulin-producing β cells by autoreactive T cells in the pancreatic islets of Langerhans [1]. The importance of additional environmental factors such as infections in the development of this disease has long been reported, but to date whether and how these might trigger or prevent T1D is not understood [2]. It has been proposed that the inflammatory events induced upon anti-infectious immunity enable enhanced presentation of β cell antigens to autoreactive T cells. Pro-inflammatory cytokines cause the up-regulation of class I major histocompatibility molecules (MHC) on β cells, and may thereby “unmask” them for recognition by CD8+ T cells [3]. In addition, concomitant damage to β cells and activation of antigen-presenting cells (APCs) by the infection may promote the presentation of β cell antigens to CD8+ T cells. This has notably been demonstrated in NOD mice using Coxsackievirus B4 (CVB4) [4], or in RIP-LCMV mice which transgenically express LCMV antigens on their β cells and develop autoimmune diabetes following LCMV infection [5–7]. Inflammatory signals not only promote DC and T cell activation but might also directly cause β cell destruction [8–10], therefore strongly contributing to T1D development.
On the other hand, studies in humans and mice suggest that infections and inflammation might play a protective role in T1D; notably, disease can be prevented in NOD mice by infection with a number of viruses [2]. Antiviral immunity may increase resistance to diabetogenic infections, or “distract” the immune system from their detrimental effect [11]. In addition, as we reported recently [12], viral infections may shape the immune system such that diabetogenic T cells are impaired or kept under control by immunoregulatory mechanisms. We found that viral infection triggered the expansion of invigorated CD4+CD25+ Tregs that produced TGF-β and protected from autoimmune diabetes by synergizing with PD-L1. These findings indicated a beneficial role of virally induced inflammation in T1D. A number of studies in mice have underscored the capacity of pro-inflammatory agents to prevent rather than induce T1D when intervening in the absence of β cell damage and autoantigen [13].
Toll-like receptors (TLRs) are usually referred to as “danger-sensing” molecules that play a central part in triggering inflammation and immunity in response to infection [14]. Different TLRs each recognizing specific molecular patterns have been identified in humans and mice. Binding of cognate ligands to TLRs on professional APCs such as dendritic cells (DCs) triggers signaling pathways that lead notably to the production of inflammatory cytokines [15]. In this way, TLR signaling might promote the development of autoimmunity. For instance, both TLR3 [16] and TLR9 [17] signaling can cause T1D when triggered in the presence of β cell antigens. Similarly, TLR2 has been shown to cause APC activation upon binding to byproducts of late apoptotic β cells, and thereby contribute to the initiation of autoimmune responses in T1D [18]. TLR2 binds to molecular motifs present in lipopolysaccharide (LPS), peptidoglycan, lipoteichoic acid, and lipoproteins/lipopeptides expressed by bacterial or parasitic micro-organisms [19–21]. TLR2 also binds to endogenous ligands, such as heat shock protein 60 (HSP60) [22] and possibly other self antigens present within secondary necrotic cells [18] or released during antiviral immunity [23]. Importantly, activation of TLR signaling is not systematically causative for T1D, as treatment with compounds that trigger TLR2 [24], TLR3 [25], TLR4 [26], or TLR9 [27] signaling, when given in the absence of β cell antigen, has a preventive effect in autoimmune diabetes. Interestingly, previous work has shown that CD4+CD25+ Tregs, which play a crucial role in the prevention of autoimmunity, not only express different TLRs, including TLR2 [28–30], but are also functionally regulated directly and indirectly through TLR signaling [31]. Exposure of Tregs to LPS induces their activation and enables them to control T cell-mediated wasting disease [28]. In addition, while binding to TLR2 by endogenous antigens causes APC activation and promotes T1D [18], it was also reported to enhance the function of CD4+CD25+ Tregs [22]. In fact, while activation of TLR2 signaling in CD4+CD25+ Tregs causes a transient loss of their function, it efficiently triggers their expansion [29, 30]. A recent study also suggested that TLR2 (and MyD88) was dispensable for development of T1D in NOD mice [32], thereby contrasting with previous work involving this molecule in the initiation of autoimmune responses directed against β cells [18].
Using the NOD and RIP-LCMV mouse models for T1D, we thus assessed the capacity of TLR2 signaling to modulate immune regulation and alter autoimmunity in this disease. Our results indicate a role for TLR2 in enhancing CD4+CD25+ Tregs and DCs, both in a naïve context or during viral infection, to enable protection from autoimmune diabetes. Therefore, while innate pathways such as TLR2 signaling may contribute to the development of autoimmunity when β cells are damaged, they may also promote immunoregulatory mechanisms that counter autoimmune processes and prevent T1D when β cells are spared. The opposing roles of inflammation in T1D may thus be accounted for by the capacity of innate pathways to trigger both immunity (via β cell damage) and immunoregulation.
Results
Activation of TLR2 signaling during the prediabetic phase diminishes T1D
TLR2 recognizes motifs present in LPS, peptidoglycan, lipoteichoic acid, and lipoproteins/lipopeptides [19–21]. We evaluated the effect of a TLR2 agonist, the lipopetide Pam3CSK4 (P3C), on T1D development in NOD mice. P3C was administered intraperitoneally to NOD mice, twice during the prediabetic phase. We observed that P3C treatment could delay diabetes onset and diminish the incidence of the disease in NOD mice (Figure 1A). In accordance with this observation, infiltration of the pancreatic islets by immune cells was significantly decreased at 11 and 16 weeks of age in P3C-treated compared to naïve NOD mice (Figure 1B). Of note, additional injections of P3C during the prediabetic phase did not ameliorate the protective effect of P3C on T1D (data not shown). Although the observed effect of P3C was modest, these results suggest that activation of the TLR2 signaling pathway systemically before the onset of T1D could confer some extent of protection from this disease.
Figure 1. Activation of TLR2 signaling by P3C in prediabetic NOD mice diminishes type 1 diabetes.
(A) Cumulative diabetes incidence over time in NOD mice injected at 9 weeks of age with either saline (None) or 100 μg P3C twice at 5 days interval (P3C). (B) Insulitis scoring of pancreatic islets from Naïve (None) and P3C-treated (P3C) at 11 and 16 weeks of age.
Activation of TLR2 signaling in vivo increases the number of CD4+CD25+ Tregs and ameliorates their tolerogenic property in T1D
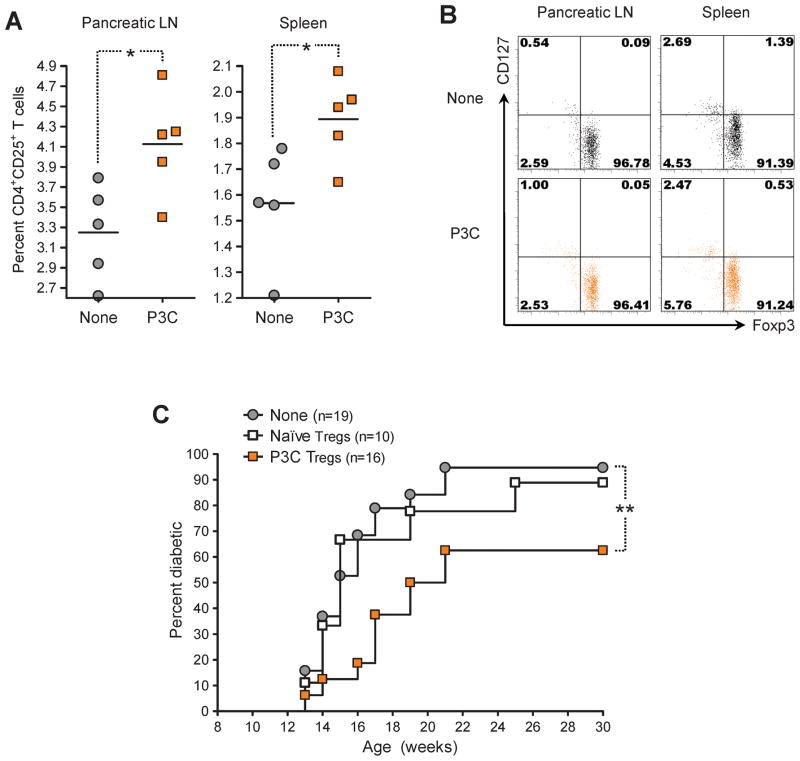
We evaluated whether the capacity of P3C treatment to diminish autoimmune diabetes might be conferred by enhanced CD4+CD25+ Tregs. We treated prediabetic NOD mice with P3C and measured 21 days later the frequency and function of these cells in the pancreatic LN and spleen. We found that injection of P3C induced an increase in the percentage of CD4+CD25+ T cells in these lymphoid organs (Figure 2A). The vast majority of CD4+CD25+ T cells expressed Foxp3 along with low levels of CD127 (Figure 2B), indicating that they were indeed Tregs [33, 34]. Consequently, the frequency of CD4+Foxp3+ T cells was also increased in the pancreatic LN and spleen of P3C-treated mice (data not shown). In order to determine whether the protective effect of P3C treatment also involved an enhancement of Treg function, we monitored diabetes development in NOD mice adoptively transferred with CD4+CD25+ T cells purified from P3C-treated donors. We found that P3C treatment conferred Tregs with the capacity to diminish the incidence of diabetes in these mice (Figure 2C). These observations suggested that stimulation of the TLR2 signaling pathway in vivo could increase the frequency and ameliorate the tolerogenic function of CD4+CD25+ Tregs in spontaneous T1D, in a fashion comparable to viral infection [12].
Figure 2. Activation of TLR2 signaling by P3C in prediabetic NOD mice increases the frequency of CD4+CD25+ Tregs and confers them with protective capacity in type 1 diabetes.
(A) Percentage of CD4+CD25+ T cells in the pancreatic LN and spleen of individual 12-week-old NOD mice injected 21 days prior with either saline (None) or 100 μg P3C (P3C) twice at 5 days interval, measured by flow cytometry. (B) Representative flow cytometry dot plots of Foxp3 and CD127 expression by CD4+CD25+ T cells in the pancreatic LN and spleen of individual 12-week-old NOD mice left untreated (None) or injected 21 days prior with 100 μg P3C twice at 5 days interval. Quadrants were defined based on isotype control stainings showing under 0.2% positive cells for each parameter analyzed. Numbers indicate the percentage of cells in the corresponding quadrants. (C) Cumulative diabetes incidence over time in NOD mice left untreated (None, gray circles) or injected at 12 weeks of age with 106 CD4+CD25+ Tregs purified from age-matched NOD donors injected at 9 weeks of age with either saline (Naïve Tregs, white squares) or 100 μg P3C twice at 5 days interval (P3C Tregs, orange squares).
Activation of TLR2 signaling in vivo confers DCs with the capacity to protect from T1D
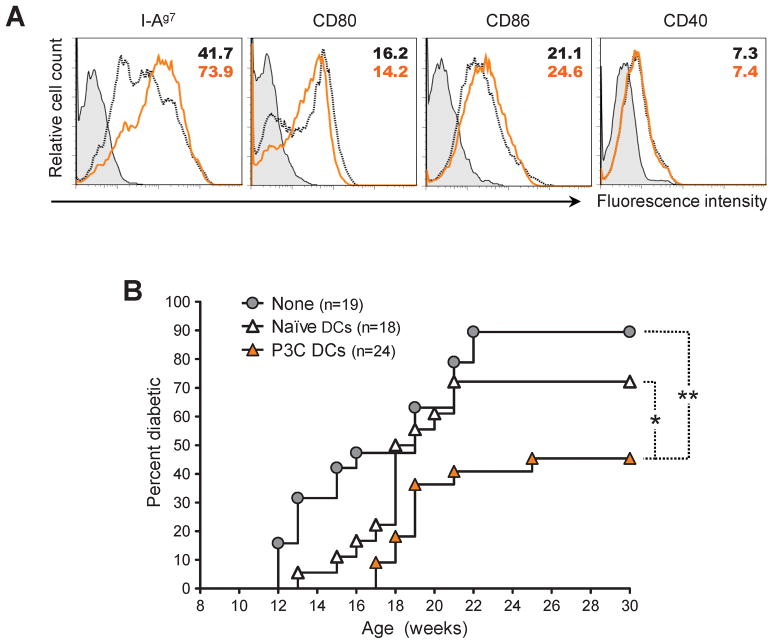
Since DCs constitute the principal immune cell type expressing TLRs, we assessed whether stimulation of these cells through the TLR2 pathway might be able to confer them with protective function in autoimmune diabetes in vivo. We treated 9-week-old NOD mice with P3C, and purified their DCs from the LNs 15 h later. Treatment of prediabetic mice with P3C caused the up-regulation of MHC class II but not CD80, CD86 or CD40 on the surface of DCs (Figure 3A). While we were unable to detect cytokine production by DCs from P3C-treated mice ex vivo, we found that their exposure to P3C in vitro led to increased production of the pro-inflammatory cytokines IL-6, but also of the immunoregulatory cytokine IL-10 (Supplementary Figure 1A). We then adoptively transferred DCs from P3C-treated mice into age-matched, prediabetic NOD mice. We found that DCs from P3C-treated donors significantly reduced diabetes by both delaying the onset of overt disease and diminishing disease incidence, while DCs from naïve mice only had a limited effect on the disease (Figure 3B). These observations suggested that activation of the TLR2 signaling pathway conferred DCs the ability to diminish T1D in vivo.
Figure 3. Activation of TLR2 signaling by P3C in prediabetic NOD mice confers DCs with protective capacity in type 1 diabetes.
(A) Flow cytometry histograms plots of I-Ag7, CD80, CD86, and CD40 expression by CD11c+ cells purified from 9-week-old NOD mice left untreated (black dotted lines) or injected 15 h prior with 100 μg P3C (orange lines). Filled histograms represent isotype control staining. Numbers indicate the MFI of the parameter analyzed. (B) Cumulative diabetes incidence over time in NOD mice left untreated (None, gray circles) or injected at 9 weeks of age with 106 CD11c+ cells purified from age-matched NOD injected 15 h prior with either saline (Naïve DCs, white triangles) or 100 μg P3C (P3C DCs, orange triangles).
CD4+CD25+ Tregs cultured with DCs and a TLR2 agonist efficiently protect from T1D
DCs play a crucial part in activating not only effector T cells, but also Tregs, and previous work has shown that CD4+CD25+ Tregs can be expanded with DCs in vitro and used to treat autoimmune diabetes in vivo [35, 36]. Based on our results thus far, we assessed whether TLR2-mediated stimulation in vitro might expand Tregs capable of diminishing T1D in vivo. DCs and CD4+CD25+ T cells were purified from 9-week-old NOD mice and cultured in the presence or absence of P3C. After 6 days, the DCs were depleted from the culture, and the Tregs were counted and their phenotype assessed. CD4+CD25+ Tregs cultured with DCs and stimulated through TLR2 in vitro had expanded fivefold, whereas cells cultured in the absence of P3C showed no expansion in culture (Figure 4A). Consistent with previous observations by others [29, 30], Foxp3 expression was reduced in TLR2-stimulated Tregs, although not completely lost, and surface expression of CD25 was increased (Figure 4B), although modestly. Expression of CD127 and most notably PD-L1 was also increased on the surface of P3C-stimulated Tregs. Addition of an anti-CD3 antibody to the culture media further promoted the expansion of the Tregs but did not affect them in terms of expression of Foxp3 or CD127 (data not shown). Interestingly, P3C-mediated expansion of Tregs was associated with IL-10 production and depended on the presence of DCs stimulated through TLR2 (either before or during culture with the Tregs) (Supplementary Figure 1). We then assessed the capacity of CD4+CD25+ T cells cultured with DCs and P3C to modulate T1D in vivo. While CD4+CD25+ Tregs cultured with DCs in the absence of P3C could diminish diabetes upon injection into 9-week-old NOD mice, stimulation through TLR2 significantly ameliorated the tolerogenic function of these cells which conferred efficient reduction of the disease (Figure 4C). In sum, exposure of CD4+CD25+ Tregs to DCs stimulated through TLR2 promoted their expansion and markedly increased their tolerogenic function in T1D in vivo.
Figure 4. CD4+CD25+ Tregs cultured with DCs and P3C efficiently prevent type 1 diabetes in NOD mice.
CD4+CD25+ T cells were purified from 9-week-old NOD mice and cultured in the presence of DCs (CD11c+ cells purified from age-matched, syngeneic mice) and 10 U/ml rhIL-2 for 6 days with media alone (Cult.None Tregs) or 2 μg/ml P3C (Cult.P3C Tregs). At the end of the culture, the Tregs were separated from the DCs by negative selection of MHC class-II-expressing cells. (A) Fold expansion of CD4+CD25+ T cells over the 6-day culture period (from duplicate samples). (B) Flow cytometry histograms plots of Foxp3, CD25, CD127, and PD-L1 expression by Tregs purified after culture with DCs in the absence (black dotted lines) or presence (orange lines) of P3C. Filled histograms represent isotype control staining. Numbers indicate the MFI of the parameter analyzed. (C) Cumulative diabetes incidence over time in NOD mice left untreated (None, gray circles) or injected at 10 weeks of age with 106 Tregs cultured with DCs in the absence (Cult.Naïve Tregs, white squares) or presence (Cult.P3C Tregs, orange squares) of P3C.
Virally induced activation of TLR2 signaling during the prediabetic phase diminishes T1D
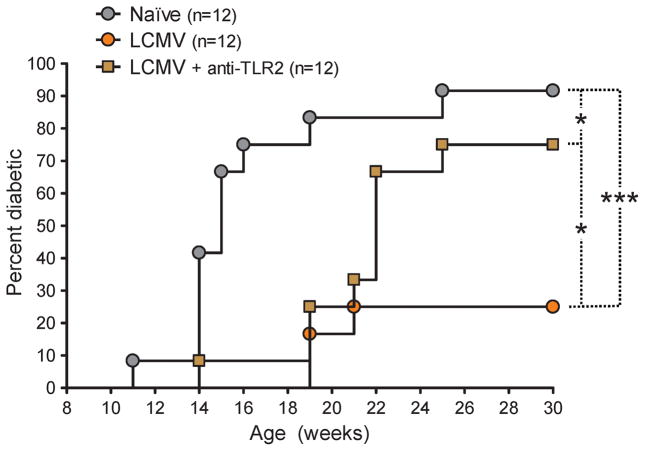
Based on our results thus far and our previous observations in virally mediated prevention of T1D [12], we addressed whether TLR2 neutralization in vivo concomitant to LCMV infection of NOD mice might affect the capacity of the virus to prevent autoimmunity. Anti-TLR2 blocking mAbs were administered to prediabetic, 9-week-old NOD mice along with LCMV and again 5 days later, and development of diabetes was monitored. We observed that LCMV delayed the onset of diabetes but failed to significantly reduce disease incidence when administered to NOD mice in the context of TLR2 blockade (Figure 5). In a different set of experiments, treatment of LCMV-infected NOD mice with isotype control mAbs had no effect on T1D development (data not shown), indicating the selective effect of anti-TLR2 mAbs. These observations suggested that activation of TLR2 signaling during LCMV infection contributed to the capacity of this virus to diminish T1D.
Figure 5. Activation of TLR2 signaling by LCMV infection in prediabetic NOD mice diminishes type 1 diabetes.
Cumulative diabetes incidence over time in NOD mice left untreated (None) or infected at 9 weeks of age with LCMV and left untreated (LCMV) or injected simultaneously and 5 days later with 50 μg of TLR2 blocking mAb (LCMV + anti-TLR2).
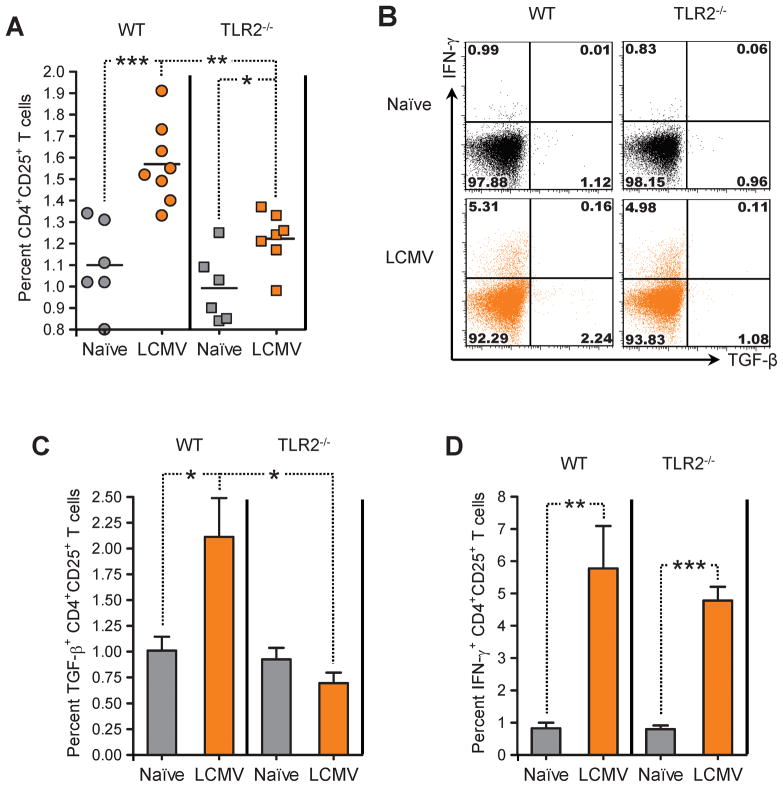
Viral infection of TLR2-deficient mice less markedly enhances the frequency of CD4+CD25+ Tregs and does not increase their production of TGF-β
Our previous work showed that reduced incidence of autoimmune diabetes following LCMV infection was caused by increased numbers of invigorated CD4+CD25+ Tregs producing TGF-β [12]. We thus assessed whether LCMV infection would still enhance Tregs in vivo when TLR2 signaling was impaired. In order to fully disrupt TLR2 signaling, we used mice rendered deficient in TLR2 protein expression by selective mutation of the TLR2 gene (TLR2−/−), on the C57BL/6 (B6) background. We found that LCMV infection increased the percentage of CD4+CD25+ T cells in the spleen of WT B6 mice (Figure 6A), similar to our earlier observation in NOD mice [12]. However, this effect of LCMV appeared hindered in TLR2−/− B6 mice, which showed a mildly but significantly lower increase in CD4+CD25+ T cell frequency after infection. In both WT and TLR2−/− mice infected with LCMV, the majority of CD4+CD25+ T cells expressed Foxp3 and low levels of CD127 (data not shown), indicating that these cells were Tregs indeed. In B6 mice infected 21 days prior with LCMV, a fraction of CD4+CD25+ T cells were capable of TGF-β production upon polyclonal stimulation (Figure 6B and C), similar to our previous observation in NOD mice [12] but to a lesser extent (possibly reflecting intrinsic differences in TGF-β production in these two different genetic backgrounds). Although production of TGF-β by CD4+CD25+ T cells from WT mice challenged with LCMV was low, it was virtually absent in LCMV-immune TLR2−/− mice (Figure 6C). Interestingly, CD4+CD25+ T cells from both WT and TLR2−/− mice infected with LCMV were capable of producing IFN-γ (Figure 6D). These results suggested that the ability of LCMV infection to increase CD4+CD25+ Treg frequency and TGF-β (but not IFN-γ) production in vivo was dependent on TLR2.
Figure 6. LCMV infection of TLR2-deficient B6 mice fails to enhance the frequency of CD4+CD25+ Tregs and their production of TGF-β in the spleen.
(A) Percentage of CD4+CD25+ T cells in the spleen of individual age-matched WT (circles) or TLR2−/− (squares) B6 mice left untreated (gray symbols) or infected 21 days prior with LCMV (orange symbols), measured by flow cytometry. (B) Representative flow cytometry dot plots of TGF-β and IFN-γ production by CD4+CD25+ T cells in the spleen of individual age-matched WT or TLR2−/− B6 mice left untreated (Naïve) or infected 21 days prior with LCMV, measured by flow cytometry after polyclonal stimulation. Quadrants were defined based on isotype control stainings showing under 0.2% positive cells for each parameter analyzed. Numbers indicate the percentage of cells in the corresponding quadrants. (C–D) Percentage of TGF-β-(C) and IFN-γ-producing CD4+CD25+ T cells in the spleen of WT or TLR2−/− B6 mice left untreated (Naïve) or infected 21 days prior with LCMV, measured by flow cytometry after polyclonal stimulation (4 to 7 mice per group).
Viral infection confers CD4+CD25+ Tregs protective capacity in T1D via TLR2 and DCs
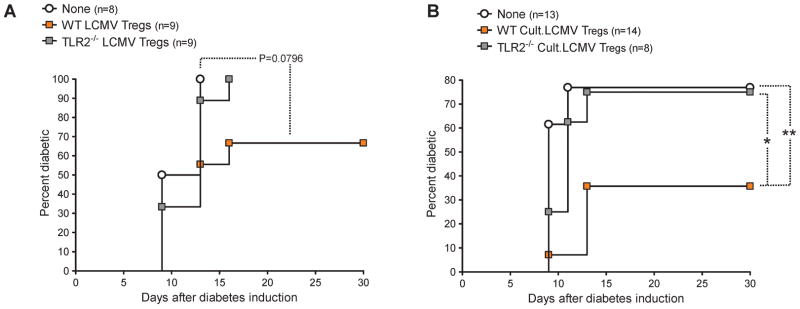
Based on these results, we assessed whether (i) similar to NOD mice CD4+CD25+ Tregs from LCMV-immune B6 mice might show a gain of function in autoimmune diabetes [12], and (ii) whether this phenomenon might be dependent on TLR2. To this aim, We used B6 RIP-GP mice [5, 6], which express the LCMV glycoprotein (GP) selectively in their pancreatic β cells and develop T1D following infection with LCMV. CD4+CD25+ T cells were purified from the spleen of LCMV-immune WT B6 mice and adoptively transferred into B6 RIP-GP mice in which autoimmune diabetes was triggered simultaneously by LCMV infection. Although the results we obtained did not reach statistical significance (P=0.0796), they showed a trend toward a protective effect of Tregs when virally modulated in WT but not TLR2-deficient mice (Figure 7A). Based on the importance of DCs in inducing T cell responses to LCMV [37] and enhancing Tregs in T1D [35, 36], we attempted to increase the effect of CD4+CD25+ Tregs by further exposing them in vitro to DCs from LCMV-infected mice. CD4+CD25+ Tregs purified from LCMV-immune mice were exposed in vitro to DCs obtained from mice recently challenged with LCMV, which we and others found to harbor an activated phenotype and carry LCMV particles [data not shown, and [38]]. After 6 days in culture, the Tregs were separated from the DCs and adoptively transferred into B6 RIP-GP mice in which autoimmune diabetes was triggered simultaneously by LCMV infection. While the capacity of LCMV-exposed, WT CD4+CD25+ T cells to protect B6 RIP-GP mice from T1D was enhanced after culture with DCs from WT LCMV-infected mice (Figure 7B), TLR2−/− Tregs cultured with TLR2−/− DCs had no effect on disease development. These results indicated that LCMV-induced Treg enhancement could be conferred by DCs and depended on TLR2.
Figure 7. LCMV confers CD4+CD25+ Tregs with protective capacity in type 1 diabetes via TLR2 and DCs.
(A) Cumulative diabetes incidence over time in B6 RIP-GP mice infected with LCMV and left untreated (None) or simultaneously injected with 1.5×106 CD4+CD25+ T cells purified from WT (WT LCMV Tregs) or TLR2−/− B6 mice (TLR2−/−LCMV Tregs) infected 21 days prior with LCMV. (B) CD4+CD25+ T cells purified from WT or TLR2−/− B6 mice infected 21 days prior with LCMV were cultured for 6 days with DCs purified from WT or TLR2−/− B6 mice, respectively, infected 48 h prior with LCMV (Cult.LCMV Tregs). Shown is cumulative diabetes incidence over time in B6 RIP-GP mice infected with LCMV and left untreated (None) or simultaneously injected with 5×105 Tregs from WT (WT Cult.LCMV Tregs) or TLR2−/− (TLR2−/− Cult.LCMV Tregs) cultures.
Discussion
Our observations indicate that triggering of TLR2 in a naïve context or upon viral infection confers protection from autoimmune diabetes by promoting the expansion of invigorated CD4+CD25+ Tregs, possibly via DCs. Since P3C-induced signaling occurs through heterodimerization of TLR2 with TLR1, further studies should assess the contribution of TLR1 in induction of immunoregulation and protection from type-1 diabetes. We did not observe Treg enhancement after treatment of NOD mice with Pam2CSK4 (data not shown), thus excluding a role for TLR6-TLR2 heterodimerization in this phenomenon.
TLR2 was previously shown to promote rather than hinder T1D, notably by inducing TNF-α production by APCs [18]. On the other hand, a requirement for TLR2 in the development of T1D was not supported by a recent study [32]. Such opposing roles of TLR2 in this disease might reflect the importance of β cell antigen release concomitant to TLR signaling for autoimmunity to develop. TLR stimulation indeed causes autoimmune diabetes when triggered in the presence of β cell antigens [16, 17], but otherwise prevents the disease [24–27]. Our previous [12] and present findings suggest that this might be due to the capacity of immunostimulatory factors to enhance immunoregulation. Another, possibly related, important aspect might be the timing at which TLRs, and subsequent release of inflammatory cytokines, are triggered during the prediabetic phase [39]. In this regard, previous studies by us and others have shown that TNF-α differentially affects the outcome of T1D depending on the time of action [10, 40, 41]. TNF-α may also have opposing effects on CD4+CD25+ Tregs [41–43], which play a crucial role in T1D. Other inflammatory cytokines such as interferons can also differentially affect autoimmune processes in T1D, as supported by our previous work [12]. Finally, while TLR2 delivers pro-inflammatory signals, its engagement also causes the release of anti-inflammatory/immunoregulatory cytokines such as IL-10 [44, 45]. In sum, innate immune receptors like TLR2 can promote both autoimmunity and immunoregulation, and whether one or the other prevails determines the outcome of T1D.
In sum, modulation of the balance between autoimmunity and immunoregulation, and thus subsequent induction or prevention of T1D, might rely on the dual function of the innate immune players involved in the disease. Depending on timing and whether β cell antigens are present, TLR-mediated effects will differentially affect the development of autoimmunity. The opposing roles of infections in T1D, which also depend on timing and vary in terms of damage to β cells [2], may thus be accounted for by the capacity of viruses to differentially affect such innate immune factors depending on the context. For instance, TLR2 signaling, and subsequent activation of APCs/T cells and production of inflammatory cytokines may promote autoimmune processes when β cell antigens are present, but also appear to counter autoimmunity by enhancing and invigorating CD4+CD25+ Tregs and conferring DCs with tolerogenic properties.
Previous work has shown that TLR2 signaling enhances the function of CD4+CD25+ Tregs [22] and regulates their expansion and activity [29, 30]. TLR2 was proposed to control antimicrobial immunity by transiently limiting the function of natural Tregs (thus permitting T cell immunity) while enhancing their number (thus participating in terminating it). Accordingly, we found that acute anti-LCMV immunity coincided with ineffective activity of CD4+CD25+ Tregs (data not shown) but resulted in their increased frequency and function. TLR2 might thus act to regulate antiviral immunity, by enhancing the number and function of Tregs to control it, but impairing these cells as long as the invading virus is present. Intriguingly, to date, there is no evidence that LCMV particles can bind to TLR2. But while TLR2 is responsible for sensing components from micro-organisms, it can also recognize molecular motifs from certain endogenous ligands. In this regard, the chaperone HSP60 was shown to enhance the function of CD4+CD25+ Tregs through TLR2 signaling [22]. It is thus possible that viral infection triggers the release of molecules such as HSPs which promote the direct enhancement of CD4+CD25+ Tregs via TLR2. This might constitute a means to recognize and control potentially harmful immune processes through innate immunity. Such absence of antigenic specificity could enable control of immunity to infection not only by viruses but also bacteria or other pathogens. In particular, in the hygiene hypothesis it is proposed that a number of different infections in early life contribute to reduced susceptibility to T1D [46]. The capacity of the immune system to control immunopathology independent of antigen may thus account for the ability of numerous infections or non-infectious pro-inflammatory agents to protect from T1D in experimental models for this disease [13].
While the mechanism by which TLR2 signaling conferred DCs with anti-diabetogenic properties remains to be determined, viral infection or P3C exposure may have enabled these cells to amliorate the function of CD4+CD25+ Tregs. Activated DCs present antigens normally not presented via MHC molecules under non-inflammatory conditions, e.g. in the absence of infection. This might notably be the case for self antigens released in the inflammatory milieu physiologically or upon immune-mediated tissue damage. A possible candidate in this regard is HSP60, which can enhance the function of CD4+CD25+ Tregs directly [22], but whose immunodominant peptide p277 bears tolerogenic properties in T1D, such that it is now evaluated in clinical studies to treat this disease [47, 48]. As discussed above, endogenous molecules like HSP60 may thus be released during viral infection and confer CD4+CD25+ Treg enhancement directly via TLR2, but also indirectly via antigenic presentation by DCs. In addition, presentation of other self antigens by DCs under inflammatory conditions might promote the recruitment of diabetogenic CD8+ T cells in the vicinity of DCs and their subsequent impairment by these cells. Such a phenomenon could occur for example through the PD-L1/PD-1 pathway, as suggested by our previous study [12]. In this regard, our present results and data not shown indicate that lymphoid cells stimulated through TLR2 in vitro or in vivo acquire high PD-L1 expression. In sum, it is possible that the contribution of DCs in TLR2-mediated prevention of T1D is to promote Treg function while curbing autoreactive responses.
A promising alternative to the therapeutic induction or enhancement of Tregs in vivo to treat T1D is their expansion in vitro for cell-based therapy. Our results suggest that stimulation via TLR2 might be well-suited for this purpose. Strategies exist to grow human CD4+CD25+ Tregs in large numbers in culture [49], and effort is currently undertaken to develop this approach in clinical trials [50]. A number of strategies consist of expanding Tregs polyclonally through stimulation via the TCR, along with co-stimulation (e.g. anti-CD3 and anti-CD28). While such expanded Tregs exhibit good preventive capacity in autoimmune diabetes, they seem to show rather limited efficacy in the reversion (as opposed to prevention) of new-onset disease. This may be due in part to their non-antigen-specific nature, but also notably to the inability of TCR-restricted stimulation to augment their suppressive function. Our results indicate that stimulation through TLR2 could be used as a means to not only increase the number of CD4+CD25+ Tregs in vitro, but also ameliorate their in vivo tolerogenic property in T1D.
We identify here a mechanism by which innate immunity, namely TLR2 stimulation, promotes immunoregulation and controls autoimmune processes in T1D. Therefore, it appears that similar phenomena account for both development and prevention of autoimmune diabetes. This suggests that the recurring occurrence of infectious events during early life might promote autoimmunity but will also drive the immune system to build increased immunoregulatory force. In this way, even in the case where autoimmune diabetes would be caused by an infection, previous encounters with other infectious agents might still prevent its development. Our results thus provide a novel mechanistic basis reconciling previous opposite observations in the field of infections and T1D. In addition, our finding that stimulation through TLR2 constitutes a well-suited means to expand CD4+CD25+ Tregs while ameliorating their tolerogenic function in T1D opens new possibilities for therapy of this disease and possibly other autoimmune disorders.
Materials and methods
Mice and virus
NOD/ShiLtJ mice, and WT or TLR2−/− C57BL/6J (B6) mice were purchased from the Jackson Laboratory. C57BL/6-RIP-GP (B6 RIP-GP) transgenic mice was described previously [5, 6]. For infection, a single dose of 104 PFU LCMV Armstrong 53b was given intraperitoneally. Blood glucose was monitored using OneTouch Ultra system (LifeScan), and mice exhibiting values greater than 300 mg/dl were considered diabetic. Animal work in all studies was approved by the LIAI Animal Care Committee.
Treatments
All injections were performed intraperitoneally in 200 μl volume. Tregs, DCs and mouse anti-mouse TLR2 mAb (Invivogen) were injected in PBS, and Pam3CSK4 (P3C, EMC Microcollections) was injected in DMEM (Invitrogen).
Insulitis scoring
Pancreas was collected and snap-frozen at the indicated time point after treatment. Frozen sections were stained with hematoxylin and eosin, and insulitis was scored blinded, as follows: (0) No insulitis, (1) peri-insulitis with no islet destruction, (2) severe peri-insulitis and some infiltrating insulitis, (3) infiltrating insulitis and some islet destruction, (4) infiltrating insulits and extensive islet destruction (or islet destroyed).
Flow cytometry
Cells were stained with fluorescently labeled monoclonal antibodies (mAbs) (BD Biosciences, eBioscience, BioLegend, Caltag) as described previously [12]. Samples were processed on a LSRII or FACScalibur (BD Biosciences) and results analyzed using FlowJo (Tree Star). Non-specific binding was blocked using unlabeled anti-FcγR (BD Biosciences). Intracellular Foxp3 expression was assessed using a Foxp3 detection kit (eBioscience). For intracellular staining of cytokines, CD4+CD25+ T cells were stimulated with PMA and ionomycin (10 ng/ml and 0.5 μg/ml, respectively) or anti-CD3 (5 μg/ml) in Brefeldin A (Sigma-Aldrich) buffer prior to mAb staining.
CD4+CD25+ T cell purification
Female mice were euthanized 21 days after P3C treatment or LCMV infection, at which point virus was cleared from lymphoid tissue (data not shown). Cell suspensions were prepared from pooled spleens, mesenteric, inguinal and pancreatic LN of 10–25 mice per group, and CD4+CD25+ T cells were purified as described previously [12]. Briefly, CD4+ T cells negatively selected by magnetic separation using sheep anti-rat Dynabeads (Dynal) were stained with biotinylated anti-CD25 mAb, and CD4+CD25+ cells were purified by magnetic separation using anti-streptavidin MACS microbeads (Miltenyi Biotec). Cell purity was measured by flow cytometry and always greater than 95%.
DC purification
Mesenteric, inguinal and pancreatic LNs were collected and pooled from 5–25 female mice. After disruption by incubation at 37°C for 30 min in HBSS (Invitrogen) containing 0.5 mg/ml collagenase D (Roche), DCs were purified by magnetic separation using anti-CD11c MACS microbeads. Non-specific binding was blocked using unlabeled anti-FcγR (BD Biosciences). Cell purity was assessed by flow cytometry and always greater than 92%.
Treg culture
For P3C cultures, CD4+CD25+ T cells purified from naïve female NOD mice were cultured for 6 days with 2 μg/ml P3C and DCs purifed from naïve female NOD mice, at a ratio of 1 DC:3 Tregs, in RPMI 1640 supplemented with 10% FCS, 2 mM L-glutamine and 50 μM 2-mercaptoethanol (Complete RPMI), and 10 U/ml rhIL-2. For viral cultures, the CD4+CD25+ T cells were purified from female B6 mice infected 21 days prior with LCMV, and cultured for 6 days with DCs purifed from female B6 mice infected 48 h prior with LCMV, at a ratio of 1 DC:3 Tregs, in Complete RPMI. At the end of the cultures, the Tregs were negatively selected using rat anti-mouse MHC class II mAbs (BD Biosciences) and Sheep anti-rat Dynabeads (Dynal).
Statistical analyses
Statistical significance was determined using a logrank test (for T1D assessment) or an unpaired, two-tailed t-test. In all experiments, differences were considered significant when P<0.05. Statistical significance is displayed in each figure for the indicated groups as follows: * P<0.05, ** P<0.005, *** P<0.001.
Supplementary Material
Signaling through TLR2 provides DCs and CD4+CD25+ Tregs with increased IL-10 production and expand Tregs. (A) IL-10 and IL-6 production by CD11c+ DCs cultured overnight with media alone (None) or 2 μg/ml P3C, measured by cytokine multiplex (MSD) in the culture supernatants. (B) CD4+CD25+ T cells were purified and cultured for 6 days as described in Figure 4 with either media alone (None), P3C (P3C), DCs alone (DCs None), DCs and P3C (DCs + P3C), or DCs preincubated overnight (and washed) with P3C (DCs pre-P3C). At the end of the culture, the Tregs were separated from the DCs by negative selection of MHC class-II-expressing cells, counted, and cultured for 24 h in media alone. Shown is fold expansion of the Tregs over the 6-day culture period and IL-10 and IFN-γ production after the overnight culture, measured by cytokine multiplex (MSD) in the culture supernatants (from duplicate samples).
Acknowledgments
We thank Malina McClure for mouse colony maintenance, Yang Chen and Tom Wolfe for technical help, and Priscilla Colby for administrative assistance. This work was supported by an NIH P01 grant AI58105-03 with the NIAID for M.G.vH, and fellowships from the JDRF and FRM for C.M.F. We gratefully acknowledge support from the Brehm Coalition.
Non-standard abbreviations
- CVB
Coxsackievirus B
- LCMV
lymphocytic choriomeningitis virus
- P3C
Pam3CSK4
- PD-1
programmed death-1
- PD-L1
programmed death-ligand 1
- T1D
type 1 diabetes
References
- 1.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008;57:2863–2871. doi: 10.2337/db07-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foulis AK, Jackson R, Farquharson MA. The pancreas in idiopathic Addison’s disease--a search for a prediabetic pancreas. Histopathology. 1988;12:481–490. doi: 10.1111/j.1365-2559.1988.tb01968.x. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz MS, Ilic A, Fine C, Balasa B, Sarvetnick N. Coxsackieviral-mediated diabetes: induction requires antigen-presenting cells and is accompanied by phagocytosis of beta cells. Clin Immunol. 2004;110:134–144. doi: 10.1016/j.clim.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 6.Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 7.von Herrath M, Holz A. Pathological changes in the islet milieu precede infiltration of islets and destruction of beta-cells by autoreactive lymphocytes in a transgenic model of virus-induced IDDM. J Autoimmun. 1997;10:231–238. doi: 10.1006/jaut.1997.0131. [DOI] [PubMed] [Google Scholar]
- 8.von Herrath MG, Oldstone MB. Interferon-gamma is essential for destruction of beta cells and development of insulin-dependent diabetes mellitus. J Exp Med. 1997;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seewaldt S, Thomas HE, Ejrnaes M, Christen U, Wolfe T, Rodrigo E, Coon B, Michelsen B, Kay TW, von Herrath MG. Virus-induced autoimmune diabetes: most beta-cells die through inflammatory cytokines and not perforin from autoreactive (anti-viral) cytotoxic T-lymphocytes. Diabetes. 2000;49:1801–1809. doi: 10.2337/diabetes.49.11.1801. [DOI] [PubMed] [Google Scholar]
- 10.Christen U, Wolfe T, Mohrle U, Hughes AC, Rodrigo E, Green EA, Flavell RA, von Herrath MG. A dual role for TNF-alpha in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J Immunol. 2001;166:7023–7032. doi: 10.4049/jimmunol.166.12.7023. [DOI] [PubMed] [Google Scholar]
- 11.Christen U, Benke D, Wolfe T, Rodrigo E, Rhode A, Hughes AC, Oldstone MB, von Herrath MG. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest. 2004;113:74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippi CM, Estes EA, Oldham JE, von Herrath MG. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J Clin Invest. 2009;119:1515–1523. doi: 10.1172/JCI38503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, Mathis D, Rossini AA, Campbell SE, Kahn R, Kreuwel HT. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 15.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 16.Moriyama H, Wen L, Abiru N, Liu E, Yu L, Miao D, Gianani R, Wong FS, Eisenbarth GS. Induction and acceleration of insulitis/diabetes in mice with a viral mimic (polyinosinic-polycytidylic acid) and an insulin self-peptide. Proc Natl Acad Sci U S A. 2002;99:5539–5544. doi: 10.1073/pnas.082120099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, Odermatt B, Conrad C, Ittner LM, Bauer S, Luther SA, Uematsu S, Akira S, Hengartner H, Zinkernagel RM. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 20.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 21.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 22.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Zhou S, Kurt-Jones EA, Mandell L, Cerny A, Chan M, Golenbock DT, Finberg RW. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35:822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- 24.Karumuthil-Melethil S, Perez N, Li R, Vasu C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J Immunol. 2008;181:8323–8334. doi: 10.4049/jimmunol.181.12.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serreze DV, Hamaguchi K, Leiter EH. Immunostimulation circumvents diabetes in NOD/Lt mice. J Autoimmun. 1989;2:759–776. doi: 10.1016/0896-8411(89)90003-6. [DOI] [PubMed] [Google Scholar]
- 26.Iguchi M, Inagawa H, Nishizawa T, Okutomi T, Morikawa A, Soma GI, Mizuno D. Homeostasis as regulated by activated macrophage. V. Suppression of diabetes mellitus in non-obese diabetic mice by LPSw (a lipopolysaccharide from wheat flour) Chem Pharm Bull (Tokyo) 1992;40:1004–1006. doi: 10.1248/cpb.40.1004. [DOI] [PubMed] [Google Scholar]
- 27.Quintana FJ, Rotem A, Carmi P, Cohen IR. Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: modulation of spontaneous 60-kDa heat shock protein autoimmunity. J Immunol. 2000;165:6148–6155. doi: 10.4049/jimmunol.165.11.6148. [DOI] [PubMed] [Google Scholar]
- 28.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G, Zhao Y. Toll-like receptors and immune regulation: their direct and indirect modulation on regulatory CD4(+) CD25(+) T cells. Immunology. 2007;122:149–156. doi: 10.1111/j.1365-2567.2007.02651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008 doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 34.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth BF, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Probst HC, van den Broek M. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J Immunol. 2005;174:3920–3924. doi: 10.4049/jimmunol.174.7.3920. [DOI] [PubMed] [Google Scholar]
- 38.Homann D, McGavern DB, Oldstone MB. Visualizing the viral burden: phenotypic and functional alterations of T cells and APCs during persistent infection. J Immunol. 2004;172:6239–6250. doi: 10.4049/jimmunol.172.10.6239. [DOI] [PubMed] [Google Scholar]
- 39.Filippi CM, von Herrath MG. Islet beta-cell death - fuel to sustain autoimmunity? Immunity. 2007;27:183–185. doi: 10.1016/j.immuni.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Yang XD, McDevitt HO. Role of TNF-alpha in the development of autoimmunity and the pathogenesis of insulin-dependent diabetes mellitus in NOD mice. Circ Shock. 1994;43:198–201. [PubMed] [Google Scholar]
- 41.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci U S A. 2002;99:12287–12292. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF down-modulates the function of human CD4+CD25hi T regulatory cells. Blood. 2006 doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF Receptor Type 2 Promotes Expansion and Function of Mouse CD4+CD25+ T Regulatory Cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 44.Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- 45.Sing A, Reithmeier-Rost D, Granfors K, Hill J, Roggenkamp A, Heesemann J. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc Natl Acad Sci U S A. 2005;102:16049–16054. doi: 10.1073/pnas.0504728102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filippi C, von Herrath M. How viral infections affect the autoimmune process leading to type 1 diabetes. Cell Immunol. 2005;233:125–132. doi: 10.1016/j.cellimm.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Elias D, Cohen IR. Peptide therapy for diabetes in NOD mice. Lancet. 1994;343:704–706. doi: 10.1016/s0140-6736(94)91582-2. [DOI] [PubMed] [Google Scholar]
- 48.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 50.Bluestone JA. Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol. 2005;5:343–349. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Signaling through TLR2 provides DCs and CD4+CD25+ Tregs with increased IL-10 production and expand Tregs. (A) IL-10 and IL-6 production by CD11c+ DCs cultured overnight with media alone (None) or 2 μg/ml P3C, measured by cytokine multiplex (MSD) in the culture supernatants. (B) CD4+CD25+ T cells were purified and cultured for 6 days as described in Figure 4 with either media alone (None), P3C (P3C), DCs alone (DCs None), DCs and P3C (DCs + P3C), or DCs preincubated overnight (and washed) with P3C (DCs pre-P3C). At the end of the culture, the Tregs were separated from the DCs by negative selection of MHC class-II-expressing cells, counted, and cultured for 24 h in media alone. Shown is fold expansion of the Tregs over the 6-day culture period and IL-10 and IFN-γ production after the overnight culture, measured by cytokine multiplex (MSD) in the culture supernatants (from duplicate samples).