Abstract
[Pyridine, 2,6-bis[(4R)-4,5-dihydro-4-(2-phenylethyl)-2-oxazolyl]-]
1. Procedure
A. (S)-2-Amino-4-phenylbutan-1-ol (1)
An oven-dried, 250-mL, two-necked, round-bottomed flask equipped with a condenser fitted with an argon inlet and a magnetic stirbar was purged with argon for 10 min. Anhydrous THF (200 mL) (Note 1) was added into the flask by syringe through the open neck under a positive pressure of argon. Next, lithium aluminum hydride (3.98 g, 105 mmol, 1.50 equiv) (Note 2) was added through the open neck under a positive pressure of argon, and the solution was stirred at rt until bubbling had ceased. l-Homophenylalanine (12.5 g, 70.0 mmol, 1.00 equiv) (Note 3) was added in portions (500 mg per portion) over 10 min through the open neck under a positive pressure of argon, during which time the solution gently refluxed. Upon completion of the addition, the open neck was capped, and the heterogeneous gray reaction mixture was heated at reflux in an oil bath for 24 h. Next, the mixture was allowed to cool to rt, THF (100 mL) was added, and the solution was cooled to 0 °C in an ice bath. Water (10 mL) was added dropwise over 10 min (Note 4), and then a solution of NaOH (2.5 M, 20 mL) was added in one portion. The resulting mixture was heated at reflux for 20 min until the color of the precipitate changed from gray to white. The warm solution was filtered through a Büchner funnel that contained a bed of celite (1.0 cm height), and the precipitate was washed with warm THF (100 mL). The filtrate was concentrated under reduced pressure (20 torr) to remove the THF and most the water. The residue was dried azeotropically with toluene (two 50-mL portions) by rotary evaporation (20 torr) and then under high vacuum (0.5 torr) for 2 h, which yielded the desired β-amino alcohol 1 as a white solid (10.6-11.0 g, 92-95% yield) (Note 5) that was used in the next step without further purification.
B. (R)-2-Amino-4-phenylbutan-1-ol (2)
An oven-dried, 250-mL, two-necked, round-bottomed flask equipped with a condenser fitted with an argon inlet and a magnetic stirbar was purged with argon for 10 min. Anhydrous THF (200 mL) (Note 1) was added into the flask by syringe through the open neck under a positive pressure of argon. Next, lithium aluminum hydride (4.56 g, 120 mmol, 1.50 equiv) (Note 2) was added over 5 min through the open neck under a positive pressure of argon. The solution was stirred at rt for 5 min, and then d-homophenylalanine ethyl ester hydrochloride (19.5 g, 80.0 mmol, 1.00 equiv) (Note 6) was added in portions (500 mg per portion) over 10 min through the open neck under a positive pressure of argon, during which time the solution gently refluxed. Upon the completion of the addition, the open neck was capped, and the heterogeneous gray reaction mixture was heated at reflux in an oil bath for 24 h. Next, the mixture was allowed to cool to rt, THF (100 mL) was added, and the solution was cooled to 0 °C in an ice bath. Water (10 mL) was added dropwise over 10 min (Note 4), and then a solution of NaOH (2.5 M, 20 mL) was added in one portion. The resulting mixture was heated at reflux for 20 min until the color of the precipitate changed from gray to white. The warm solution was filtered through a Büchner funnel that contained a bed of celite (1.0 cm height), and the precipitate was washed with warm THF (100 mL). The filtrate was concentrated under reduced pressure (20 torr) to remove the THF and most of the water. The residue was dried azeotropically with toluene (two 50-mL portions) by rotary evaporation (20 torr) and then under high vacuum (0.5 torr) for 2 h, which yielded the desired β-amino alcohol 2 as a white solid (12.5-13.0 g, 94-98% yield) (Note 7) that was used in the next step without further purification.
C. 2,6-Bis[(4R)-4,5-dihydro-4-(2-phenylethyl)-2-oxazolyl]pyridine (3)
(Note 8) An oven-dried, 250-mL, two-necked, round-bottomed flask equipped with a condenser fitted with an argon inlet and a magnetic stirbar was purged with argon for 10 min. 2,6-Pyridinedicarbonitrile (2.58 g, 20.0 mmol, 1.00 equiv) (Note 9), anhydrous toluene (120 mL; added by syringe) (Note 10), and zinc trifluoromethanesulfonate (363 mg, 1.00 mmol, 0.050 equiv) (Note 11) were added through the open neck under a positive pressure of argon, and the mixture was stirred at rt for 5 min. A solution of (R)-2-amino-4-phenylbutan-1-ol (2) (6.60 g, 40.0 mmol, 2.00 equiv) in toluene (30.0 mL) was added, the open neck was capped, and the reaction mixture was heated at reflux in an oil bath for 24 h. Next, the reaction mixture was allowed to cool to rt, and it was diluted with ethyl acetate (300 mL). The solution was washed with a saturated aqueous solution of NaHCO3 (200 mL) and brine (200 mL), and then it was dried over anhydrous MgSO4 (20 g) and filtered through a Büchner funnel that contained a bed of celite (1.0 cm height). The filtrate was concentrated under reduced pressure on a rotary evaporator (20 torr), and the residue was purified by column chromatography on silica gel (10 cm diameter × 20 cm height), eluting with ethyl acetate:hexanes:Et3N (1:1:0.02) (Note 12), which afforded the desired pyridine derivative 3 (6.89-7.06 g, 81-83% yield, >99% ee) as a white solid (Note 13 and Note 14).
2. Notes
THF (99+%) was purchased from J.T. Baker (water content: 24 ppm) and purified by passage through activated alumina under argon.
Lithium aluminum hydride (97%) was purchased from Alfa Aesar and used as received.
l-Homophenylalanine (98+%) was purchased from CNH Technologies, Inc. and used as received.
Quenching excess lithium aluminum hydride with water is a highly exothermic process that produces H2. Dropwise addition of water is recommended, and care should be taken to efficiently cool and stir the reaction mixture. The quenching should be conducted in an efficient fume hood in order to safely vent the H2 gas.
The purity of compound 1 (95%) was determined by HPLC analysis (tr = 4.21 min) with an Agilent 1100 Series HPLC system equipped with an Eclipse XDB-C18 column (length 150 mm, I.D. 4.6 mm, particle size 5 μm), using a 2% → 98% MeOH/(0.2% AcOH in water) gradient for 3 min, then 98% MeOH/(0.2% AcOH in water) for 5 min, with a flow rate of 0.8 mL/min. Compound 1 can be purified via column chromatography on silica gel (see Note 7 for conditions and characterization data). [α]21D = +0.76 (c = 1.0, CHCl3).
d-Homophenylalanine ethyl ester hydrochloride (≥98%) was purchased from Fluka and used as received.
The purity of compound 2 (93%) was determined by HPLC analysis, using the same conditions as for compound 1. Compound 2 can be purified via column chromatography on silica gel, eluting with MeOH:CH2Cl2:Et3N (5:95:2). Rf = 0.4 (MeOH:CH2Cl2,1:9). Compound 2 has the following properties: 1H NMR (CDCl3, 500 MHz) δ: 7.27 (t, J = 8.0 Hz, 2H), 7.16-7.18 (m, 3H), 3.58 (dd, J = 10.5, 3.5 Hz, 1H), 3.31 (dd, J = 10.5, 7.5 Hz, 1H), 2.82-2.86 (m, 1H), 2.70-2.76 (m, 1H), 2.61-2.66 (m, 1H), 1.92 (br s, 3H), 1.71-1.77 (m, 1H), 1.54-1.60 (m, 1H); 13C NMR (CDCl3, 125 MHz) δ: 141.9, 128.6, 128.4, 126.1, 66.4, 52.4, 35.9, 32.5; IR (film) 3352, 2924, 1601, 1495, 1454, 1367, 1055, 748, 699 cm−1; LRMS (ESI) calcd for C10H16NO (M+H) 166.1; found 166.1; [α]21D = −0.78 (c = 1.05, CHCl3); mp 40-43 °C.
2,6-Bis[(4S)-4,5-dihydro-4-(2-phenylethyl)-2-oxazolyl]pyridine (97%) is available from Aldrich.
2,6-Pyridinedicarbonitrile (97%) was purchased from Aldrich and used as received.
Toluene (99+%) was purchased from J.T. Baker (water content: <0.01%) and purified by passage through activated alumina under argon.
Zinc trifluoromethanesulfonate (98%) was purchased from Strem and used as received.
Column chromatography was performed on Sorbent Technologies 60 Å silica gel.
By conducting the reaction with anhydrous zinc chloride as the catalyst (10%) and chlorobenzene as the solvent at 120 °C for 24 h, the pybox ligand can be generated in comparable yield.2
Compound 3 has the following properties: 1H NMR (CDCl3, 500 MHz) δ: 8.21 (d, J = 8.0 Hz, 2H), 7.89 (t, J = 8.0 Hz, 1H), 7.20-7.31 (m, 10H), 4.59 (t, J = 8.5 Hz, 2H), 4.33-4.39 (m, 2H), 4.16 (t, J = 8.5 Hz, 2H), 2.84-2.89 (m, 1H), 2.76-2.81 (m, 1H), 2.05-2.11 (m, 1H), 1.90-1.96 (m, 1H);13C NMR (CDCl3, 125 MHz) δ: 162.5, 146.9, 141.6, 137.5, 128.5, 128.4, 126.1, 125.9, 73.3, 66.5, 37.6, 32.4; IR (film) 3438, 2926, 2238, 1643, 1575, 1496, 1454, 1359, 1248, 1171, 1108, 1076, 975, 910, 735, 701 cm−1; LRMS (ESI) calcd for C27H28N3O2 (M+H) 426.2; found 426.2; [α]21D = +141 (c = 1.15, CHCl3); mp 86-88 °C. The enantiomeric excess of 3 was determined by supercritical fluid chromatography (SFC) analysis on a Berger SFC MiniGram system: Chiralcel OD-H column (length 250 mm, I.D. 4.6 mm); solvent system: 20% MeOH, 3.0 mL/min; retention times: 19.00 min ((R)-enantiomer), 21.73 min ((S)-enantiomer).
Waste Disposal Information
All toxic materials were disposed of in accordance with “Prudent Practices in the Laboratory” National Academy Press: Washington, DC, 1995.
3. Discussion
Chiral pyridine-2,6-bisoxazolines (pybox) form tridentate complexes with a variety of metals, and they have proved to be highly effective ligands in asymmetric catalysis. 3 We have reported several examples of enantioselective Negishi cross-couplings of secondary alkyl halides with organozinc reagents that are catalyzed by Ni/pybox complexes.4,5 When α-bromo amides4a and benzylic bromides4b are employed as electrophiles, commercially available i-Pr-Pybox is the ligand of choice. However, for Negishi couplings of allylic chlorides, CH2CH2Ph-pybox is the optimal ligand from the standpoints of enantioselectivity and yield.4c Herein, we describe procedures for the synthesis of CH2CH2Ph-pybox.
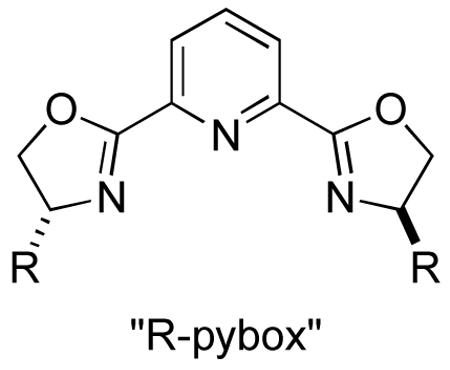
The procedure is based on a general route developed by Pires and coworkers.6 (R)- and (S)-2-Amino-4-phenylbutan-1-ol are readily available by reduction of homophenylalanine or an ester derivative.
Appendix
Chemical Abstracts Nomenclature; (Registry Number)
l-Homophenylalanine: Benzenebutanoic acid, α-amino-, (αS)-; (943-73-7)
d-Homophenylalanine ethyl ester hydrochloride: Benzenebutanoic acid, α-amino-, ethyl ester, hydrochloride (1:1), (αR)-; (90940-54-8)
Lithium aluminum hydride: Aluminate(1-), tetrahydro-, lithium (1:1), (T-4)-; (16853-85-3)
(S)-2-Amino-4-phenylbutan-1-ol: Benzenebutanol, β-amino-, (βS)-; (27038-09-1)
(R)-2-Amino-4-phenylbutan-1-ol: Benzenebutanol, β-amino-, (βR)-; (761373-40-4)
2,6-Pyridinedicarbonitrile; (2893-33-6)
Zinc trifluoromethanesulfonate: Methanesulfonic acid, 1,1,1-trifluoro-, zinc salt (2:1); (54010-75-2)
2,6-Bis[(4R)-4,5-dihydro-4-(2-phenylethyl)-2-oxazolyl]pyridine: Pyridine, 2,6-bis[(4R)-4,5-dihydro-4-(2-phenylethyl)-2-oxazolyl]-
References
- 2.Witte H, Seeliger W. Angew. Chem. Int. Ed. 1972;11:287–288. See also: Chelucci G, Deriu S, Pinna GA, Saba A, Valenti R. Tetrahedron: Asymmetry. 1999;10:3803–3809.
- 3.For leading references to the use of oxazolines as ligands in asymmetric catalysis, see: Hargaden GC, Guiry PJ. Chem. Rev. 2009;109:2505–2550. doi: 10.1021/cr800400z. McManus HA, Guiry PJ. Chem. Rev. 2004;104:4151–4202. doi: 10.1021/cr040642v. Desimoni G, Faita G, Quadrelli P. Chem. Rev. 2003;108:3119–3154. doi: 10.1021/cr020004h.
- 4.(a) Fischer C, Fu GC. J. Am. Chem. Soc. 2005;127:4594–4595. doi: 10.1021/ja0506509. [DOI] [PubMed] [Google Scholar]; (b) Arp FO, Fu GC. J. Am. Chem. Soc. 2005;127:10482–10483. doi: 10.1021/ja053751f. [DOI] [PubMed] [Google Scholar]; (c) Son S, Fu GC. J. Am. Chem. Soc. 2008;130:2756–2757. doi: 10.1021/ja800103z. [DOI] [PubMed] [Google Scholar]; (d) Smith SW, Fu GC. J. Am. Chem. Soc. 2008;130:12645–12647. doi: 10.1021/ja805165y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lundin PM, Esquivias J, Fu GC. Angew. Chem. Int. Ed. 2009;48:154–156. doi: 10.1002/anie.200804888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For an overview, see: Rudolph A, Lautens M. Angew. Chem. Int. Ed. 2009;48:2656–2670. doi: 10.1002/anie.200803611.
- 6.Cornejo A, Fraile JM, García JI, Gil MJ, Martínez-Merino V, Mayoral JA, Pires E, Villalba I. Synlett. 2005:2321–2324. [Google Scholar]



