Abstract
Members of the Culex pipens mosquito group including C. quinquefasciatus are responsible for the transmission of Bancroftian filarisis as well as West Nile Virus (WNV) in the United States. As is the case for other mosquitoes, the host preference of this disease vector relies on olfaction and accordingly mediated via G-protein coupled signal transduction pathways. Here, we identify and characterize CqOR7, the first candidate member of the odorant receptor gene family from C. quinquefasciatus. CqOR7 displays extremely high primary amino acid conservation with other apparent orthologs including AaOR7, from the Dengue virus vector mosquito Aedes aegypti, AgOR7 from the malaria vector Anopheles gambiae and DOr83b from the fruit fly Drosophila melanogaster that form an essential non-conventional odorant receptor sub-family. CqOR7 transcripts can be detected in adult chemosensory tissues and during several pre-adult stages of C. quinquefasciatus, and the CqOR7 protein is localized to characteristic olfactory tissues such as the antennae and maxillary palps as well as the proboscis, a typically gustatory appendage. These results suggest that CqOR7 and its orthologs are likely to play a role in the chemosensory processes of Culicine and other mosquitoes that underlie their vectorial capacity.
Keywords: Olfaction, Culex, Mosquito, Odorant receptor, West Nile Virus, Vector
1. Introduction
West Nile Virus (WNV) has been spreading across North America since it was first recognized in New York City during 1999. By 2003, at least 9862 cases of WNV human infections were reported resulting in 264 deaths. In addition, WNV poses a significant threat to birds as well as economically important domestic animals such as cattle and horses. It has been established that WNV transmission is driven by the requirement for a vertebrate blood meal by female mosquitoes in order to complete their gonotrophic reproductive cycle. Olfactory signals provide important sensory inputs that a female mosquito uses to locate and attack a blood meal host (Takken and Knols, 1999) and, accordingly, shapes the negative impact of these and many other insects of economic and medical importance (Zwiebel and Takken, 2004). Therefore, a deeper understanding of the mosquito olfaction system may facilitate the development of methods that can interfere with the interaction of insect vectors with their host organisms.
Here, we report the identification and characterization of CqOr7 that represents the first, albeit non-conventional, candidate member of the odorant receptor (OR) family of proteins from C. quinquefasciatus. As is the case for other members of this group of non-conventional ORs, CqOr7 is widely expressed in olfactory appendages of both immature and adult stages, and shares great similarity with apparent orthologs from several other insects. These include Anopheles gambiae (AgOr7) (Hill et al., 2002); Aedes aegypti (AaOr7) (Melo et al., 2004); Drosophila melanogaster (DOr83b) (Clyne et al., 1999b; Gao and Chess, 1999; Vosshall et al., 1999); Heliothis virescens (HvirR2)(Krieger et al., 2003), as well as Apis mellifera (AmelR2) (Krieger et al., 2003). The high conservation level across species and the wide expression in chemosensory tissues of C. quinquefasciatus suggests that this receptor and its orthologs represent an OR sub-family that may play an important role in the chemosensory signal transduction in this mosquito and other insects.
The best-studied member of this non-conventional OR sub-family, Drosophila DOr83b has been shown to act as a nearly essential dimerization partner for other, conventional ORs in heterozygous systems (Neuhaus et al., 2005). Furthermore, DOr83b mutant flies manifest abnormal cytoplasmic aggregation of other co-expressed ORs and display dramatically impaired electrophysiological responses to some odorants (Larsson et al., 2004). As such, members of this particular gene sub-family (which we propose to hereafter designate the OR 83b sub-family) may prove useful as targets for disruption of the insect olfactory signal transduction pathway. Indeed, the study of this unique candidate OR sub-type may lead to novel approaches designed to reduce olfactory sensitivity and, therefore, the vectorial capacity of mosquitoes by disrupting vector/host interactions.
2. Materials and methods
2.1. Mosquito rearing
C. quinquefasciatus were reared as-described (Fox et al., 2001). For stock propagation, 4- to 5-days-old female mosquitoes were blood-fed for 30–45 min on anesthetized mice following the guidelines set by Vanderbilt Institutional Animal Care and Use Committee.
2.2. Molecular cloning
Primary amino acid sequences of the following genes were retrieved from GenBank: Drosophila melanogaster Or83b (NM079511), Anopheles gambiae Or7 (AY363725, AY363726), Aedes aegypti Or7 (AY582943). Blocks of sequences were generated using BlockMaker (http://blocks.fhcrc.org/blocks/make_blocks.html) and oligonucleotide primers for PCR amplification were designed from blocks using CODEHOP algorithm (http://blocks.fhcrc.org/blocks/codehop.html). Three primers were used in subsequent PCR amplifications: Deg 5′2:
-
CATCGCCCTGGCCAARATGMGNAA; Deg 3′1 :
CGGAGCCGTCGTACCARTGRCA; Deg3′2 :
GGTAGCCGATCACGGTGAAGSCRTANACRTT.
PCR templates were prepared from hand-dissected antennae from 1000 female C. quinquefasciatus mosquitoes that were used to generated total RNA with RNeasy (Qiagen, Valencia, CA) protocols followed by the preparation of and adaptor-ligated cDNAs using the Marathon cDNA Construction reagents (BD Biosciences Clontech, Palo Alto, CA). PCR reactions were carried out with a 1:10 dilution of antennal cDNAs and CODEHOP primers in a PTC-200 (MJ Research, Waltham, MA) thermal-cycler for 35 cycles with an optimal annealing temperature of 55 °C along with appropriate control reactions. All experimental-specific PCR products were gel-purified using QIAquick gel extraction reagents (Qiagen, Valencia, CA), cloned into the pCRII-TOPO cloning vector (Invitrogen, Carlsbad, CA) and subsequently sequenced in the DNA Core Facility at Vanderbilt University. Full length CqOr7 cDNA were obtained using RACE amplifications in a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA) under conditions as-described in Marathon kit manual with Adaptor primer 1 (AP1, Marathon cDNA kit, BD Biosciences Clontech, Palo Alto, CA) and CqOr7 specific RACE primers—RACE primer 1: AAGGTACCGCTTCTCGCAAATCAGGTCA and RACE primer 2: CAGGTACCTGTGCACGGTTGCATCGGA. PCR products were further cloned into the pCRII-TOPO cloning vector (Invitrogen, Carlsbad, CA) and sequenced as-described above. The complete CqOr7 nucleotide sequence has been deposited to Genbank where it has been assigned the accession number DQ231246.
2.3. RNA expression
Total RNA was isolated from the following C. quinquefasciatus tissues using the RNeasy reagents and protocols (Qiagen, Valencia, CA) as-described above—early instar larvae (2–4 days old), late instar larvae (10–14 days old), pupae, or adult tissues (4–6 days old). First strand cDNA synthesis was carried out by using Superscript II reverse transcriptase (Invitrogen Inc., Carlsbad, CA) and 0.5 µg of C. quinquefasciatus RNA according to the manufacturer’s instructions. In order to control for any genomic DNA contamination, all subsequent PCR reactions were carried out using the following intron-spanning (based on partial genomic sequencing, data not shown) primers—CqOR7 5′1: CACATGCTGACCTCGACCAT and CqOR7 3′1: CAGCTGCACCAACTCCATGAA for 30 cycles with an optical temperature of 60 °C. All RT–PCR reactions were replicated at least eight times. Furthermore, the Culex homolog for the ribosomal protein S7 gene (CqRPS7, Genbank accession AF272670) was amplified in tandem as a control for cDNA integrity by using the primers CqRPS7a: CTGGAGATGAACTGGACCT and CqRPS7b: CTTGTACACCGACGTGAAGG. PCR products were gel-purified, subcloned into the pCRII-TOPO cloning vector and sequenced as described above.
2.4. Scanning electron microscopy
Adult heads were fixed in 4% paraformaldehyde, 0.1% Triton X-100 in PBS followed by dehydration in ethanol series from 50–100% (10% increments) and ethanol:hexamethyldisilazane (HMDS) at (v/v) 75:25, 50:50, 25:75 and 0:100. The heads were then dried in a fume hood, mounted onto aluminum pins with colloidal silver paint and sputter-coated for 30 s with gold–palladium. The images were captured by using Hitachi S-4200 scanning electron microscope with Quartz PCI image acquisition software version 6.0 (Quartz Imaging Corp. Vancouver, BC).
2.5. Immunocytochemistry
Immunocytochemistry was performed using a rabbit polyclonal peptide antisera derived against amino acid residues 268–281 of the AgOR7 sequence (Pitts et al., 2004) with the sole modification of incubating the secondary antibody reaction overnight at 4 °C. Confocal images were captured by using LSM 510 META system attached to an Axioplan fluorescence microscope (Zeiss). Other images were captured by using DP70 charge-coupled device camera attached to a BX-60 fluorescent microscope (Olympus Inc., Bethpage, NY).
3. Results
3.1. CqOR7 transcripts
Degenerate primers were synthesized with the assistance of the CODEHOP web server based on a multiple sequence alignment of 83b family members from An. gambiae, A. aegypti and D. melanogaster. These primers were used to amplify a partial sequence of CqOr7 from a Marathon cDNA library prepared from C. quinquefasciatus antennae and confirmed by sequencing. Based on this, additional gene-specific primers were designed and used in RACE reactions to yield both N-terminal and C-terminal CqOr7 sequences.
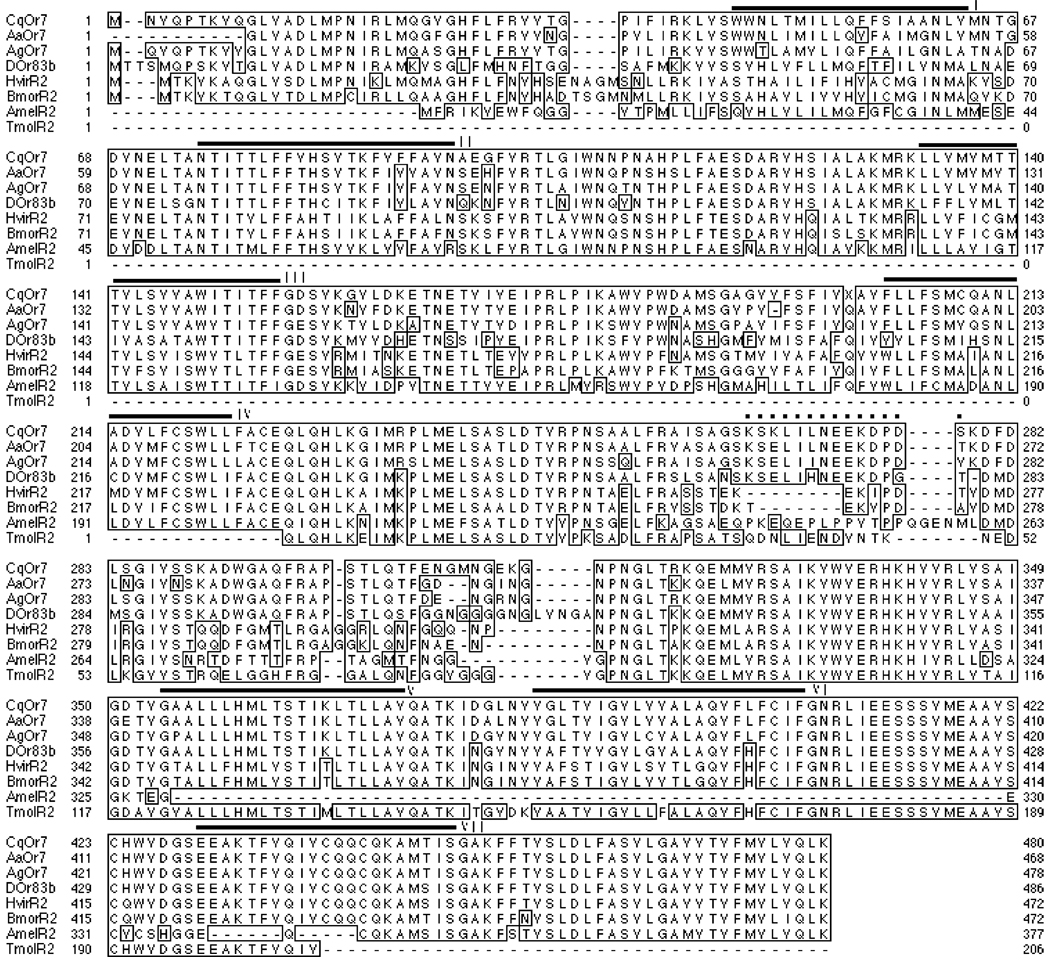
The predicted amino acid sequence of CqOR7 was aligned with those of other 83b sequences from An. gambiae, A. aegypti, D. melanogaster, H. virescens, A. mellifera, Bombyx mori and Tenebrio molitor. As shown in Fig. 1, the eight sequences have >80% similarity and >60% identity. Moreover, the 150 amino acids that comprise the C-terminal region shows extremely high conservation at levels that approach >90% identity. Specifically, CqOR7 shares 90% identity and 93% similarity in the terminal 230 amino acids with its most evolutionarily related homolog from A. aegypti, AaOR7. Compared with other insects’ orthologs, CqOr7 shares a higher level of conservation with AgOr7 and AaOr7.
Fig. 1.
Alignment of CqOr7 ortholog peptides using the single amino acid code. Identical residues are shaded and boxed. Transmembrane domains I–VII are indicated with black bars. Dotted line indicates peptide used for generating OR7 antiserum. For a list of genes and accession numbers see materials and methods.
3.2. RNA expression
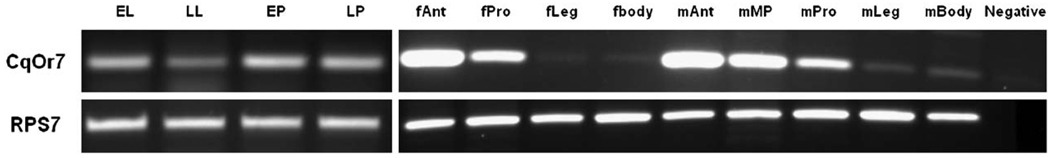
Odorant receptors are expressed in sensory neurons in olfactory appendages of insects, including the antennae and maxillary palps (Vosshall, 2000). We examined the expression pattern of CqOr7 transcripts by non-quantitative RT-PCR analyses of tissues isolated from several developmental stages and adult tissues (Fig. 2). In order to control for artifactual amplification from genomic DNA contamination, an intron-spanning primer set that results in diagnostic products from either cDNA (215 bp) or genomic DNA (523 bp) templates was designed. PCR products with an expected size of 215 bp can be observed with cDNA templates from different tissues, and the intensity of the products (relative to a constitutively expressed internal control) suggests variable expression levels in different tissues. For example, in addition to a significant expression throughout larval and pupal pre-adult life stages CqOr7 mRNA is robustly expressed in antennae from both male and female adult mosquitoes. CqOr7 expression is also observed in male and female mouthparts. CqOr7 transcripts are undetectable in these assays from female legs, while RNA derived from male legs display relatively weak CqOr7 expression as compared with antennae, maxillary palps and proboscis. Overall, as expected, CqOr7 expression is observed in the major olfactory tissues of C. quinquefasciatus comprising the antennae and maxillary palps, as well as in traditionally non-olfactory appendages such as the proboscis and legs from adults. In developmental studies, CqOr7 is first found in the early larvae stage and can be readily detectable throughout all developmental stages.
Fig. 2.
Expression of CqOR7 in pre-adult and adult C. quinquefasciatus. Lane as follows—early larvae (EL), late larvae (LL), early pupae (EP), late pupae (LP), female antennae (fAnt), female proboscis (fPro), female leg (fLeg), female body (fBody), male antennae (mAnt), male maxillary palp (mMP), male proboscis (mPro), male leg (mLeg), male body (mBody), negative control. RPS7 acts as positive control.
3.3. Protein expression
We used a rabbit polyclonal antisera that was raised against a 14 amino acid polypeptide of the deduced amino acid sequence of AgOR7 (Pitts et al., 2004) to examine the localization of the CqOr7 protein (Fig. 1, dotted line). Although there are two amino acid substitutions in the corresponding peptide sequence of CqOR7, the AgOR7 polyclonal antibody specifically labeled neurons throughout proximal, intermediate and distal segments of the female C. quinquefasciatus antenna (Figs. 3(C), (D), and (G)). Through systematic analyses of overlapping immunolabeled sections, each consisting of between five to eight consecutive antennal segments, we were able to observe CqOr7-specific labeling in all 13 flagellar segments in C. quinquefasciatus. Since the thicker and shorter proximal segments can be reasonably distinguished from the longer and thinner distal segments, we conclude that CqOr7-specific labeling is present in all flagellar segments.
Fig. 3.
Localization of CqOR7 protein in female C. quinquefasciatus antennae. Red is anti-AgOR7 marked with Cy3-labelled secondary antibody. Green is anti-horseradish peroxidase conjugated to FITC. (A) SEM of the first antenna segment ST—sensillum trichodica (scale bar is 40 µm). (B) SEM of the second antenna segment GP—grooved pegs, (scale bar is 40 µm). (C) CqOr7 labeling of the first antenna segment, arrows shows the labeling of a neuron cell body, (scale bar is 20 µm). (D) CqOr7 labeling of the second antenna segment, (scale bar is 20 µm). (E) control reaction using pre-immune serum as primary antibody, arrow shows an unlabeled sensillum trichodica (scale bar is 40 µm). (F) No CqOr7 labeling is observed in grooved peg (scale bar is 5 µm). (G) CqOr7 labeling of the first 6 antenna segments (scale bar is 40 µm).
As was the case for both AgOR7 (Pitts et al., 2004) and AaOR7 (Melo et al., 2004) homologs, CqOr7 labeling was observed within dendrites and cell bodies of sensilla trichodica in C. quinquefasciatus (Fig. 3D) where specific labeling can be detected in every sensillum examined. As a positive control, anti-horseradish peroxidase conjugated with FITC (HRP) was used to label neuronal cell bodies and axons (Jan and Jan, 1982; Sun and Salvaterra, 1995). In any one plane of section and with the exception of the first (proximal) antennal segment (Fig. 3C), approximately 20–30% of the HRP-positive neurons were labeled with AgOR7 antibody. Although overlapping of HRP:FITC (green) and AgOR7:Cy3 (red) signals were observed in many instances (Fig. 3), there were considerable sections where no obvious overlap was observed on the same cell body, suggesting these two antibodies may label the different sides of the membrane. This phenomenon is consistent with AgOR7 and AaOR7 staining (Melo et al., 2004; Pitts et al., 2004).
Dendrites from sensilla trichodica were in general strongly labeled with AgOR7 antisera (Fig. 3D), although in these instances HRP labeling was typically not observed, suggesting the lack of the HRP epitope in the dendrites of this type of sensillum. Interestingly, basiconic sensilla from C. quinquefasciatus antennae, which are sometimes referred to as grooved pegs, apparently express neither CqOr7 nor HRP epitopes (Fig. 3F) consistent with observations in of both An. gambiae and A. aegypti mosquitoes (Melo et al., 2004; Pitts et al., 2004).
Interestingly, CqOr7 labeling was restricted to only the distal part of the first antenna segment where clear cell body labeling could be observed (Fig. 3C). This result stands in contrast to A. aegypti, where AaOr7 was detected throughout the first antennal segment (Melo et al., 2004) and to An. gambiae, where the entire first antennal segment was devoid of AgOr7 labeling (Pitts et al., 2004). This likely reflects the fact that in C. quinquefasciatus, sensilla trichodea are only present in the distal part of the first antennal flagellum (Fig. 3A). Importantly, CqOr7 labeling was never observed within scales, microtrichia or sensilla chaetica (Fig. 3(C) and (D)).
Consistent with the localization of CqOr7 mRNA (Fig. 2) immunoreactivity was also observed in the maxillary palps of the female C. quinquefasciatus (Fig. 4B). While a total of four types of sensory hair structures are found on C. quinquefasciatus maxillary palps—non-innervated incrotrichia and scales as well as mechanosensory sensilla chaetica and thin walled captitate peg sensilla (Fig. 4A). CqOr7 labeling was restricted to the dendrites but, interestingly, not cell bodies of capitate peg sensilla (Fig. 4B). As was the case for antennae, HRP labeling was also observed in capitate pegs on the maxillary palp where it was localized to many dendrites. Some weak background staining, which was present in the pre-immune control (data not shown), was also observed on cell bodies. Once again, mechanosensory, microtrichia and scales remained unlabeled in these preparations (Fig. 4B).
Fig. 4.
Localization of CqOR7 protein in female C. quinquefasciatus maxillary palp and proboscis. Red is anti-AgOR7 marked with Cy3-labeled secondary antibody. Green is anti-horseradish peroxidase conjugated with FITC. (A) SEM of a female C. quinquefasciatus maxillary palp CP—capitate pegs (scale bar is 25 µm). (B) CqOr7 labeling of the capitate pegs (scale bar is 25 µm). (C) SEM of a female C. quinquefasciatus proboscis region, arrow shows a distinct type of sensillum (scale bar is 5 µm). (D) CqOr7 is labeled in a distinct type of sensilla shown by arrow (scale bar is 20 µm).
AgOR7 antisera also labeled a distinct subset of neuronal cells from the distal labellum of the proboscis of female C. quinquefasciatus (Fig. 4D), which has been characterized as the principal gustatory organ in mosquitoes. This is in agreement with similar data from An. gambiae and A. aegypti (Melo et al., 2004; Pitts et al., 2004), but in contrast to the localization of DOr83b whose adult expression is limited to the antennae and maxillary palps (Vosshall et al., 1999). In C. quinquefasciatus, clear cell body and dendrite were also labeled (Fig. 4D). When compared with antennae, however, far fewer cells were labeled in proboscis, although the labeling within those cells is very strong. From the SEM studies it appears that only a limited number of chemosensory sensilla are distributed across the labellum of the proboscis (Fig. 4C) and these sensilla are only found on the upper part of the labellum.
4. Discussion
We have identified and characterized CqOr7, a non-conventional member of the OR family of proteins from the WNV vector mosquito C. quinquefasciatus. As-expected, CqOr7 shares an extremely high conservation of its primary amino acid sequence with other 83b sub-family members from other insect species (Clyne et al., 1999a; Gao and Chess, 1999; Vosshall et al., 1999; Hill et al., 2002; Krieger et al., 2003; Melo et al., 2004). These genes are apparent orthologs based on both functional (Jones et al., 2005) and sequence conservation. Indeed, there is compelling evidence to suggest that members of this sub-family of non-conventional OR proteins do not themselves bind odorant ligands but rather form heterodimers with co-expressed “conventional” ORs (Neuhaus et al., 2005) and, moreover, these complexes are required for localization of ORs to dendrites (Larsson et al., 2004). As such, it is not surprising to note the extreme conservation displayed by members of this sub-family of ORs despite the general divergence and species-specific gene expansions that are characteristic of the evolution of other insect ORs (Hill et al., 2002).
CqOr7 is expressed in the main chemosensory organs of the adult mosquito—antennae, maxillary palps, proboscis, legs, whilst expression in other parts of the adult body is largely undetectable. Furthermore, from a developmental standpoint, CqOr7 RNA is detectable in early larvae stages, which is in agreement with the expression pattern of its mosquito orthologs from An. gambiae and A. aegypti (Melo et al., 2004; Pitts et al., 2004). In D. melanogaster, DOr83b is one of 23 ORs found to be expressed in larval stages (Vosshall et al., 1999; Kreher et al., 2005). The expression of CqOr7 in the pre-adult stage suggests a chemosensory role of this gene during the early development of C. quinquefasciatus, whereby it is likely to play an important role in larval feeding and other behaviors.
A polyclonal antiserum directed against a highly conserved sequence of amino acid was used to localize CqOr7 protein in C. quinquefasciatus. While the peptide used to generate antisera is known to be unique to AgOR7 in An. gambiae, the absence of data on the C. quinquefasciatus proteome prevents similar exclusions. Nevertheless, the fact that AgOR7 peptide antiserum specifically labels olfactory sensilla and neuronal cell bodies in C. quinquefasciatus as well as An. gambiae (Pitts et al., 2004) and A. aegypti (Melo et al., 2004) strongly supports its utility as a general marker for OR7 family members in mosquitoes. The specificity of the antisera is further supported by a lack of labeling in pre-immune control in C. quinquefasciatus and other mosquitoes. This antibody labels CqOR7 in three kinds of tissues—antennae, maxillary palps and proboscis, where signals are restricted to three types of sensilla, of which two have been described to function in the perception of a variety of odorants and carbon dioxide (Bowen, 1996; Grant and O’Connell, 1996), while the third has been implicated in contact chemosensation and mechanosensation (Pappas and Larsen, 1976). Interestingly, mosquito-grooved peg sensilla are specifically not labeled with CqOR7, AgOR7 or AaOR7, although grooved pegs of An. gambiae and A. aegypti have been shown to be sensitive to a variety of odors, some of which are known kairomones for host seeking (Meijerink et al., 2001). Indeed, the absence of OR7 proteins in olfactory responsive mosquito-grooved peg sensillum suggests the presence of another pathway for olfactory signal transduction that is independent of OR7 function. Similarly, while there are significant effects on olfactory signaling in Drosophila DOr83b mutants (Larsson et al., 2004) or with RNA interference-mediated silencing of DOr83b (Neuhaus et al., 2005), it is important to note that in Drosophila not all olfactory neurons co-express both conventional and non-conventional OR proteins (Clyne et al., 1999b; Vosshall et al., 1999).
CqOr7 mRNA and protein is also found in the proboscis, which is usually viewed as a contact chemosensory appendage associated with gustation in mosquitoes (Pappas and Larsen, 1976). In D. melanogaster no ORs, including the widely expressed DOr83b, have been shown to be expressed in the fruitfly proboscis. While in An. gambiae and A. aegypti, AgOR7 and AaOR7 are both robustly expressed in proboscis (Melo et al., 2004; Pitts et al., 2004) as is the case forHvR2 from H. virescens (Krieger et al., 2002). Based on the essential role that DOr83b play in the localization and function of co-expressed DOr proteins (Larsson et al., 2004; Neuhaus et al., 2005), the expression of Or83b orthologs in the proboscis across these mosquito species strongly suggests the presence of cryptic olfactory inputs derived from these gustatory organs. Such olfactory responses derived from an appendage that typically comes into extremely close approximation to human skin volatiles, may play a critical role in the penultimate steps of blood-feeding behaviors. Further study of this non-conventional receptor will facilitate our understanding of chemosensation in mosquitoes and, ultimately, may facilitate the development of novel anti-malarial programs that target olfactory-based behaviors of vector mosquitoes.
Acknowledgements
This work gratefully acknowledges the contributions of Dr. Hugh Robertson, University of Illinois, Champagne/Urbana, for his efforts in the design of oligonucleotide primers used for CqOR7 cloning. We offer special thanks to Dr. Michael Rutzler and the Vanderbilt Imaging Core for assistance with confocal imaging, Mr. R. Jason Pitts for SEM imaging, and other members of the Zwiebel lab for critical reading of the manuscript. This investigation received financial support from Vanderbilt University’s Discovery Grant program, as well as the National Institutes of Health (DC04692 and AI056402 to L.J.Z.).
References
- Bowen Q. Sensory aspects of host location in mosquitoes. In: Bock GR, Cardew G, editors. Olfaction in Mosquito-host Interactions. New York: Wiley; 1996. pp. 197–211. [Google Scholar]
- Clyne PJ, Certel SJ, et al. The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron. 1999a;22(2):339–347. doi: 10.1016/s0896-6273(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, et al. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999b;22(2):327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Fox AN, Pitts RJ, et al. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc. Natl. Acad. Sci. USA. 2001;98(25):14693–14697. doi: 10.1073/pnas.261432998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Chess A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 1999;60:31–39. doi: 10.1006/geno.1999.5894. [DOI] [PubMed] [Google Scholar]
- Grant A. Electrophysiological Responses from Receptor Neurons in Mosquito Maxillary Palp Sensilla. In: O’Connell R, editor. Ciba Foundation Symposium. Chichester: Wiley; 1996. [DOI] [PubMed] [Google Scholar]
- Hill CA, Fox AN, et al. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298(5591):176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- Jan L, Jan Y. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc. Natl. Acad. Sci. USA. 1982;79:2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Nguyen TA, et al. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr Biol. 2005;15(4):R119–R121. doi: 10.1016/j.cub.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kreher SA, Kwon JY, et al. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46(3):445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Krieger J, Raming K, et al. A divergent gene family encoding candidate olfactory receptors of the moth Heliothis virescens. Eur. J. Neurosci. 2002;16(4):619–628. doi: 10.1046/j.1460-9568.2002.02109.x. [DOI] [PubMed] [Google Scholar]
- Krieger J, Klink O, et al. A candidate olfactory receptor subtype highly conserved across different insect orders. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2003;189(7):519–526. doi: 10.1007/s00359-003-0427-x. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Meijerink J, Braks MAH, et al. Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J. Insect Physiol. 2001;47:455–464. doi: 10.1016/s0022-1910(00)00136-0. [DOI] [PubMed] [Google Scholar]
- Melo AC, Rutzler M, et al. Identification of a chemosensory receptor from the yellow fever mosquito, Aedes aegypti, that is highly conserved and expressed in olfactory and gustatory organs. Chem. Sens. 2004;29(5):403–410. doi: 10.1093/chemse/bjh041. [DOI] [PubMed] [Google Scholar]
- Neuhaus EM, Gisselmann G, et al. Odorant receptor hetero-dimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 2005;8(1):15–17. doi: 10.1038/nn1371. [DOI] [PubMed] [Google Scholar]
- Pappas LG, Larsen JR. Gustatory hairs on the mosquito, Culiseta inornata. J. Exp. Zool. 1976;196(3):351–360. doi: 10.1002/jez.1401960309. [DOI] [PubMed] [Google Scholar]
- Pitts RJ, Fox AN, et al. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector, Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2004;101:5058–5063. doi: 10.1073/pnas.0308146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Salvaterra PM. Characterization of nervana, a Drosophila melanogaster neuron-specific glycoprotein antigen recognized by anti-horseradish peroxidase antibodies. J. Neurochem. 1995;65:434–443. doi: 10.1046/j.1471-4159.1995.65010434.x. [DOI] [PubMed] [Google Scholar]
- Takken W, Knols BG. Odor-mediated behavior of Afrotropical malaria mosquitoes. Ann. Rev. Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- Vosshall LB. Olfaction in Drosophila. Curr. Opin. Neurobiol. 2000;10(4):498–503. doi: 10.1016/s0959-4388(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, et al. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96(5):725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Zwiebel LJ, Takken W. Olfactory regulation of mosquito-host interactions. Insect Biochem. Mol. Biol. 2004;34(7):645–652. doi: 10.1016/j.ibmb.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]