Abstract
Brain endothelial cells play an essential role as structural and functional components of the blood–brain barrier (BBB). Increased BBB breakdown and brain injury are associated with neuroinflammation and are thought to trigger mechanisms involving matrix metalloproteinase upregulation. Emerging evidence also indicates that cyclooxygenase (COX) inhibition limits BBB disruption, but the mechanisms linking metalloproteinase to COX remain unknown. In this study, we sought to investigate the nuclear factor-kappa B (NF-κB) signaling pathway, a common pathway in both the regulation of matrix metalloproteinase-9 (MMP-9) and COX-2 expression, and the inhibitory properties of several chemopreventive flavonoids. Human brain microvascular endothelial cells were treated with a combination of phorbol 12-myristate 13-acetate (PMA), a carcinogen documented to increase MMP-9 and COX-2 through NF-κB, and several naturally occurring flavonoids. Among the molecules tested, we found that fisetin, apigenin, and luteolin specifically and dose-dependently antagonized PMA-induced COX-2 and MMP-9 gene and protein expressions as assessed by qRT-PCR, immunoblotting, and zymography respectively. We further demonstrate that flavonoids impact on IκK-mediated phosphorylation activity as demonstrated by the inhibition of PMA-induced IκB phosphorylation levels. Our results suggest that BBB disruption during neuroinflammation could be pharmacologically reduced by a specific class of flavonoids acting as NF-κB signal transduction inhibitors.
Keywords: blood–brain barrier, flavonoids, neuroinflammation, NF-κB signal transduction inhibitors
Introduction
Tumor-associated angiogenesis, a fundamental process in tumor growth and metastasis, consists of recruiting endothelial cells (EC) toward an angiogenic stimulus.1 The cells subsequently proliferate and differentiate to form endothelial tubes and capillary-like structures in order to deliver nutrients and oxygen to the tumor and to remove the products of its metabolism. In recent years, several pathways have, in addition to stimulation of tumor angiogenesis, been suggested to contribute to the cell metabolic adaptations required for carcinogenesis, which include decreased tumoral apoptosis, increased invasion and metastasis, immune suppression, and tumor-associated inflammation.2,3 An interesting link between overexpression of the proinflammatory marker cyclooxygenase (COX)-2 and tumor angiogenesis was recently described as one such metabolic adaptive phenotype.4–6 This is supported by the fact that in a normal cerebral cortex, COX-2 is only present in neurons but absent from vascular EC.7,8 It is however still unknown whether the EC-associated COX-2 correlates with high malignancy. Furthermore, little is known about the molecular events that dictate metabolic adaptation of EC in response to procarcinogenic stimuli. It is tempting to suggest that specific inhibition of metabolic pathways may offer a novel therapeutic approach that would simultaneously inhibit tumor-induced angiogenesis and inflammatory phenotypes.9,10
While human brain microvascular endothelial cells (HBMEC) play an essential role as structural and functional components of the blood–brain barrier (BBB), its disruption by the brain tumor-secreted matrix metalloproteinase-9 (MMP-9) is believed to favor tumor invasion.11,12 Recent studies delineated a unique brain endothelial phenotype in which MMP-9 secretion by HBMEC was increased upon treatment with the tumor-promoting agent phorbol 12-myristate 13-acetate (PMA).13,14 Inhibition of MMP-9 secretion was demonstrated to reduce both in vitro invasion and angiogenesis in human microvascular EC.15 Among strategies developed to inhibit extracellular matrix (ECM) degradation and inflammation processes, the design, synthesis, and evaluation of flavonoid derivatives has recently emerged as a potent strategy to target neurodegenerative disorders including different forms of dementia, as well as Alzheimer’s disease.16 In fact, a large number of mechanisms of action have been attributed to flavonoids commonly found in fruits, vegetables, wine, or tea as they can act as potent antioxidants and free radical scavengers.17,18 The rationale underlying the selection of flavones and related compounds was herein dictated by the known activity of apigenin against several forms of cancer, including leukemia, due in part to its action through JAK/STAT and PI3K/PKB signaling pathways.19 Thus, apigenin, luteolin, and fisetin were considered potential good candidates possessing polyphenolic functions capable of counteracting oxidative mechanisms. Indeed, the three compounds are lacking hydrophilic sugar or sugar-like moieties, and consequently, they are more hydrophobic and bear drug-like properties that characterize leads in drug discovery. Quercitrin was also chosen because it has a very similar structure to that of fisetin and yet exposes a rhamnoside residue which renders it more closely related to the other family of tested compounds (chlorogenic acid, arbutin, salicin, phlorizin, and coniferin), hence filling the gap for our QSAR profiles. These phenolic glycosides, while possessing some structurally related features, do not have the key flavone backbone.
Among the signaling pathways, NF-κB signaling is the one that enables the control of both MMP-9 and COX-2 inflammation marker expression.20,21 This current study therefore focuses on flavonoids as potential signal transduction inhibitors of carcinogen-mediated induction of the NF-κB pathway in a brain EC model. Eight flavonoids were evaluated: flavonol, fisetin; flavones, apigenin and luteolin; flavonol-glycoside, quercitrin; chlorogenic acid; and a few phenolic glucosides – arbutin, salicin, phlorizin, and coniferin. The aim of the study was to relate the structural differences of the flavonoids to their potency to inhibit PMA-induced MMP-9 and COX-2 expression in HBMEC.
Materials and methods
Reagents
Sodium dodecylsulfate (SDS) and bovine serum albumin were purchased from Sigma (Oakville, ON). Electrophoresis reagents were purchased from Bio-Rad (Mississauga, ON). The enhanced chemiluminescence reagents were from Perkin Elmer (Waltham, MA). Micro bicinchoninic acid protein assay reagents were from Pierce (Rockford, IL). The polyclonal antibodies against IκB and phospho-IκB were purchased from Cell Signaling (Danvers, MA). The monoclonal antibody against GAPDH was from Advanced Immunochemical, Inc (Long Beach, CA). Horseradish peroxidase-conjugated donkey anti-rabbit and anti-mouse IgG secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). All other reagents were from Sigma-Aldrich Canada. Eight flavonoids were evaluated: flavonol, fisetin (2-(3,4-dihydroxyphenyl)-3,7-dihydroxychromen-4-one); flavones, apigenin (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) and luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromenone); the flavonol-glycoside quercitrin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-2-tetrahydro-pyranyl]oxy}-4-chromenone); chlorogenic acid ((1S,3R,4R,5R)-3-{[(2Z)-3-(3,4-dihydroxyphenyl) prop-2-enoyl]oxy}-1,4,5-trihydroxycyclohexanecarboxylic acid); as well as a few phenolic glucosides – arbutin ((2R,3S,4S,5R,6S)-2-hydroxymethyl-6-(4-hydroxyphenoxy) oxane-3,4,5-triol), salicin ((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[2-(hydroxymethyl)phenoxy]oxane-3,4,5-triol), phlorizin (1-{2,4-dihydroxy-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-phenyl}-3-(4-hydroxyphenyl)propan-1-one), and coniferin (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-{4-[(E)-3-hydroxy-prop-1-enyl]-2-methoxyphenoxy}oxane-3,4,5-triol. The phenolic flavonoid derivatives were either purchased from Sigma-Aldrich (St Louis, MO) or donated from the personal collection of Prof Ragai K Ibrahim from the University of Concordia (Montreal, QC).
Cell culture
Human brain microvascular endothelial cells (HBMEC) were characterized and generously provided by Dr Kwang Sik Kim of the Johns Hopkins University School of Medicine (Baltimore, MD). These cells were positive for factor VIII-Rag, carbonic anhydrase IV, and Ulex europaeus Agglutinin I; they took up fluorescently labeled, acetylated low-density lipoprotein and expressed gamma glutamyl trans-peptidase, demonstrating their brain EC-specific phenotype.18 HBMEC were immortalized by transfection with simian virus 40 large T antigen, and maintained their morphological and functional characteristics for at least 30 passages.22 HBMEC were maintained in RPMI 1640 (Gibco, Burlington, ON) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (iFBS) (HyClone Laboratories, Logan, UT), 10% (v/v) NuSerum (BD Bioscience, Mountain View, CA), modified Eagle’s medium nonessential amino acids (1%) and vitamins (1%) (Gibco), sodium pyruvate (1 mM), and EC growth supplement (30 μg/mL). Culture flasks were coated with 0.2% type-I collagen to support the growth of HBMEC monolayers. Cells were cultured at 37°C under a humidified atmosphere containing 5% CO2. All experiments were performed using passages 3 to 28.
Gelatin zymography
Gelatin zymography was used to assess the extent of proMMP-9 activity as previously described.23 Briefly, an aliquot (20 μL) of the culture medium was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in a gel containing 0.1 mg/mL gelatin. The gels were then incubated in 2.5% Triton X-100 and rinsed in nanopure distilled H2O. Gels were further incubated at 37°C for 20 hours in 20 mM NaCl, 5 mM CaCl2, 0.02% Brij-35, 50 mM Tris-HCl buffer, pH 7.6, then stained with 0.1% Coomassie Brilliant Blue R-250, and destained in 10% acetic acid, 30% methanol in H2O. Gelatinolytic activity was detected as unstained bands on a blue background.
Immunoblotting procedures
Proteins from control and treated cells were separated by SDS-PAGE. After electrophoresis, proteins were electrotransferred to polyvinylidene difluoride membranes which were then blocked for 1 hour at room temperature with 5% nonfat dry milk in Tris-buffered saline (150 mM NaCl, 20 mM Tris-HCl, pH 7.5) containing 0.3% Tween-20 (TBST). Membranes were further washed in TBST and incubated with the primary antibodies (1/1,000 dilution) in TBST containing 3% bovine serum albumin and 0.1% sodium azide, followed by a 1 hour incubation with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (1/2,500 dilution) in TBST containing 5% nonfat dry milk. Immunoreactive material was visualized by enhanced chemiluminescence (Amersham Biosciences, Baie d’Urfée, QC).
Total RNA isolation, cDNA synthesis, and real-time quantitative RT-PCR
Total RNA was extracted from cell monolayers using TriZol reagent (Life Technologies, Gaithersburg, MD). For cDNA synthesis, 2 μg of total RNA were reverse-transcribed using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). cDNA was stored at −80°C prior to PCR. Gene expression was quantified by real-time quantitative PCR using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). DNA amplification was carried out using an Icycler iQ5 (Bio-Rad), and product detection was performed by measuring binding of the fluorescent dye SYBR Green I to double-stranded DNA. The QuantiTect primer sets were provided by Qiagen (Valencia, CA): MMP-9 (QT00040040), COX-2 (QT00040586), β-Actin (QT01136772). GAPDH primer sets were synthesized by Biocorp (Dollard-des-Ormeaux, QC) with the following sequences: forward CCATCACCATCTTCCAGGAG and reverse CCTGCT-TCACCACCTTCTTG. The relative quantities of target gene mRNA compared against two internal controls, GAPDH and β-Actin mRNA, were measured by following a ΔCT method employing an amplification plot (fluorescence signal vs cycle number). The difference (ΔCT) between the mean values in the triplicate samples of target gene and those of GAPDH and β-actin mRNAs were calculated by iQ5 Optical System Software (v 2.0; Bio-Rad), and the relative quantified value (RQV) was expressed as 2−ΔCT.
Endothelial cell morphogenesis assay
Tubulogenesis was assessed using Matrigel aliquots of 50 μL, plated into individual wells of 96-well tissue culture plates (Costar, Amherst, MA) and allowed to polymerize at 37°C for 30 minutes. After brief trypsinization, HBMEC were washed and resuspended at a concentration of 106 cells/mL in serum-free medium. Twenty-five μL of cell suspension (25,000 cells/well) and 75 μL of medium with serum were added into each culture well. Cells were allowed to form capillary-like tubes at 37° C in 5% CO2/95% air for 20 hours in the presence or absence of 30 μM of the tested molecules. The formation of capillary-like structures was examined microscopically and images (10×) were recorded using a Retiga 1300 camera (QImaging, Surrey, BC) and a Nikon Eclipse TE2000-U microscope (Tokyo, Japan). The extent to which capillary-like structures formed in the gel was quantified by analysis of digitized images to determine the thread length of the capillary-like network, using a commercially available image analysis program (Northern Eclipse, Mississauga, ON) as described and validated previously.24,25 For each experiment, four randomly chosen areas were quantified by counting the number of tubes formed. Tubulogenesis data are expressed as a mean value derived from at least three independent experiments.
Statistical data analysis
Data are representative of three or more independent experiments. Statistical significance was assessed using Student’s unpaired t-test. Probability values of less than 0.05 were considered significant and an asterisk identifies such significance in the figures.
Results
Fisetin, apigenin, and luteolin inhibit HBMEC in vitro capillary-like structure formation
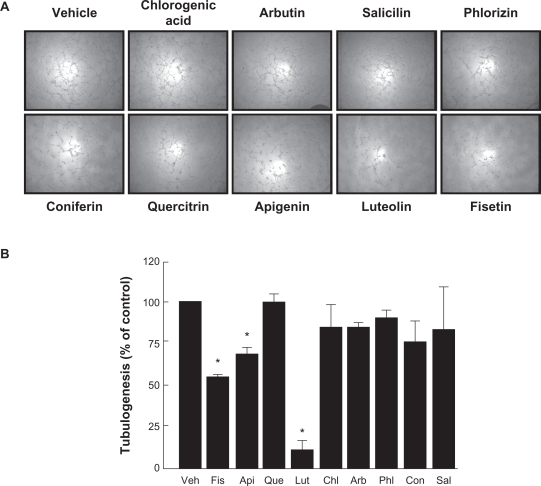
The effects of chlorogenic acid and of eight structurally related phenolic derivatives (Figure 1), all natural molecules present in plants, were tested on HBMEC. Matrigel induced tubulogenesis assay was used to assess the effect of flavonoids and phenolic derivatives on capillary-like structure formation in HBMEC. As described in the Methods section, cells were seeded on top of Matrigel and left to adhere. Several tested compounds were then added and capillary formation left to proceed for 18 hours. We found that, in vehicle-treated cells as well as in chlorogenic acid-, arbutin-, salicin-, phlorizin-, coniferin-, and quercitrin-treated cells, capillary-like formation was well-defined (Figure 2A) in comparison to cells exposed to 30 μM of the nonglycosidic flavonoids apigenin, luteolin, or fisetin, which had their structures significantly disrupted (Figure 2B).
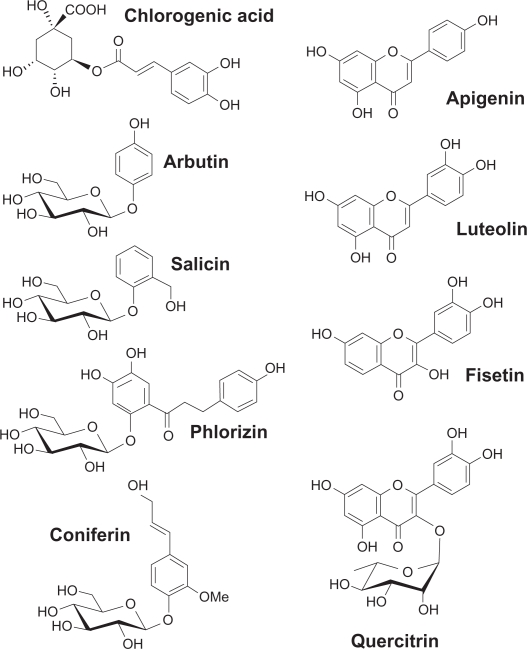
Figure 1.
Chemical structures of the flavonoids used to antagonize carcinogen-induced MMP-9 and COX-2 expressions. The different moieties of these compounds are indicated.
Abbreviations: COX-2, cyclooxygenase-2; MMP-9, matrix metalloproteinase-9.
Figure 2.
Inhibition of in vitro capillary-like structure formation by flavonoids in HBMEC. In order to assess the potential anti-angiogenic properties of flavonoids, in vitro tubulogenesis assay was performed with HBMEC seeded on top of Matrigel as described in the Materials and Methods section. A) Molecules (30 μM) were added 30 minutes after seeding of the cells on top of Matrigel. Structure formation was monitored after 18 hours. Representative phase contrast pictures were taken. B) The extent of three-dimensional capillary-like structure formation (tubulogenesis) was assessed as described in the Materials and Methods section. The length of the tube network was quantitated using Northern Eclipse software. Data are representative of three independent experiments.
Note: *Significant at P < 0.05.
Abbreviation: HBMEC, human brain microvascular endothelial cells.
Flavonoids inhibition of carcinogen-induced MMP-9 gene expression and protein secretion
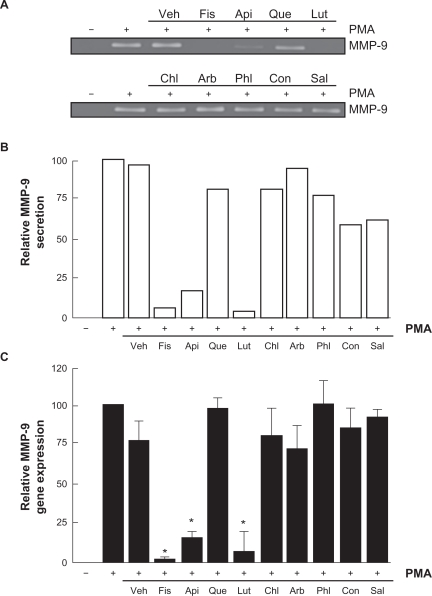
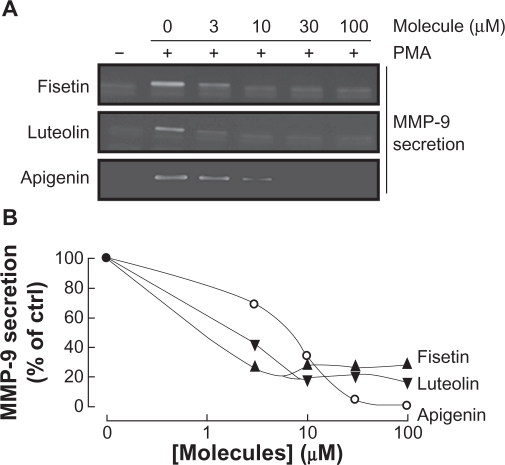
Among the secreted enzymes involved in ECM degradation, matrix metalloproteinases (MMP) are well-documented as being involved in cell migration and tubulogenesis.13,26 MMP-9, an enzyme involved in the degradation of the ECM, is secreted by a variety of cells and its presence was shown to be increased upon carcinogen promoting agents such as the phorbol ester PMA.27–29 HBMEC were treated for 18 hours with the above mentioned flavonoids in serum-free medium. Gelatin zymography (Figure 3A) was then used to measure MMP-9 levels, which were significantly increased upon PMA treatment in comparison to vehicle-treated cells (Figure 3B). Addition of the nonglycosidic flavonoids fisetin, apigenin, or luteolin to PMA-treated cells resulted in inhibition of MMP-9 activity (Figure 3B). It was found that PMA also increased MMP-9 gene expression while the presence of fisetin, apigenin, or luteolin inhibited this increase, suggesting transcriptional regulation of the MMP-9 gene (Figure 3C). The anti-MMP-9 effects of fisetin, apigenin, and luteolin were also found to be dose-dependent as assessed by zymography (Figure 4A), with a Ki of 1.6 μM, 2.2 μM, and 8.3 μM respectively for fisetin, luteolin, and apigenin (Figure 4B).
Figure 3.
Flavonoids inhibition of carcinogen-induced MMP-9 gene expression and protein secretion. HBMEC were serum-starved in the presence of various flavonoids (30 μM) in combination with vehicle or 1 μM PMA for 18 hours. A) Conditioned media were then harvested and gelatin zymography was performed in order to detect PMA-induced proMMP-9 and hydrolytic activity as described in the Materials and Methods section. B) Scanning densitometry was used to quantify the extent of proMMP-9 gelatinolytic activity in treated cells. Data shown is representative of two independent experiments. C) Total RNA isolation and quantitative reverse transcription-polymerase chain reaction were performed as described in the Materials and Methods section to assess MMP-9 gene expression in the above-described conditions. Data are representative of three independent qPCR experiments.
Note: *Significant at P < 0.05.
Abbreviations: HBMEC, human brain microvascular endothelial cells; MMP-9, matrix metalloproteinase-9; PMA, phorbol 12-myristate 13-acetate; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
Figure 4.
Dose-dependent inhibition of MMP-9 secretion by fisetin, luteolin, and apigenin. HBMEC were serum-starved in the presence of various concentrations of fisetin, luteolin, and apigenin in combination with vehicle or 1 μM PMA for 18 hours. A) Conditioned media were then harvested and gelatin zymography was performed in order to detect PMA-induced proMMP-9 and hydrolytic activity as described in the Materials and Methods section. B) Scanning densitometry was used to quantify the extent of proMMP-9 gelatinolytic activity in treated cells. Data shown is representative of two independent experiments.
Abbreviations: HBMEC, human brain microvascular endothelial cells; MMP-9, matrix metalloproteinase-9; PMA, phorbol 12-myristate 13-acetate.
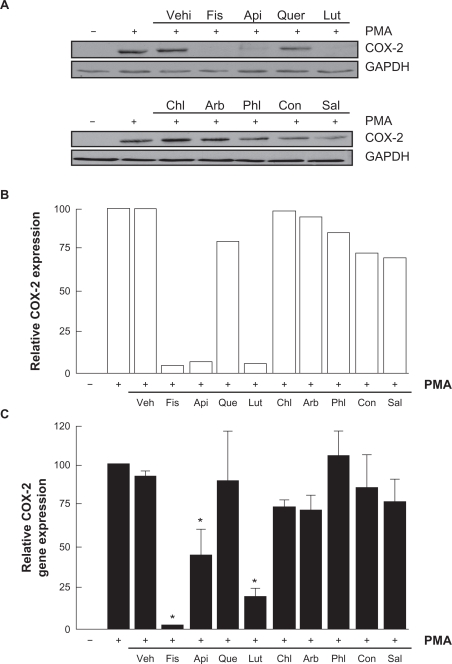
Flavonoids inhibition of carcinogen-induced COX-2 gene and protein expression
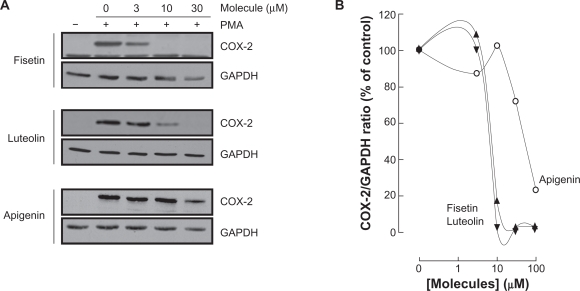
Various molecular mechanisms mediate inflammatory processes and angiogenesis, one of which is reflected by increased expression of the inflammatory biomarker COX-2.30 In order to investigate the effect of flavonoids on HBMEC-associated inflammation, we tested the effects of the flavonoids on PMA-induced cell signaling in HBMEC by Western blotting. Cells were therefore treated with 1 μM of PMA in the presence of 30 μM of the flavonoid for 18 hours and COX-2 expression was evaluated in cell lysates by Western Blotting (Figure 5A). We found that the nonglycosidic derivatives fisetin, apigenin, and luteolin significantly inhibited COX-2 protein (Figure 5B) and gene (Figure 5C) expression in the presence of PMA, whereas PMA-induced COX-2 expression was not affected by the other molecules. Further experiments were performed by treating HBMEC with various concentrations of fisetin, apigenin, or luteolin in the presence of PMA for 18 hours (Figure 6A). These molecules were found to inhibit the COX-2 protein expression in a dose-dependent manner (Figure 6B).
Figure 5.
Flavonoids inhibition of carcinogen-induced COX-2 gene and protein expression. A) HBMEC were serum-starved in the presence of various flavonoids (30 μM) in combination with vehicle or 1 μM PMA for 18 hours. Lysates were isolated, electrophoresed via SDS-PAGE, and immunodetection of COX-2 and GAPDH performed as described in the Materials and Methods section. B) Scanning densitometry of COX-2 expression was only performed in PMA-treated cells since no COX-2 was detectable in vehicle-treated cells. Densitometric data of a representative blot is shown. C) Total RNA isolation, RT-PCR, and qPCR were performed as described in the Materials and Methods section to assess COX-2 gene expression in the above-described conditions. Data are representative of three independent qPCR experiments.
Note: *Significant at P < 0.05.
Abbreviations: COX-2, cyclooxygenase-2; HBMEC, human brain microvascular endothelial cells; PMA, phorbol 12-myristate 13-acetate; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Figure 6.
Dose-dependent inhibition of COX-2 expression by fisetin, luteolin, and apigenin. HBMEC were serum-starved in the presence of various concentrations of fisetin, luteolin, and apigenin in combination with vehicle or 1 μM PMA for 18 hours. A) Lysates were isolated, electrophoresed via SDS-PAGE, and immunodetection of COX-2 and GAPDH performed as described in the Materials and Methods section. B) Scanning densitometry of COX-2 expression was only performed in PMA-treated cells since no COX-2 was detectable in vehicle-treated cells. Densitometric data of a representative blot out of three is shown.
Abbreviations: COX-2, cyclooxygenase-2; HBMEC, human brain microvascular endothelial cells; PMA, phorbol 12-myristate 13-acetate; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
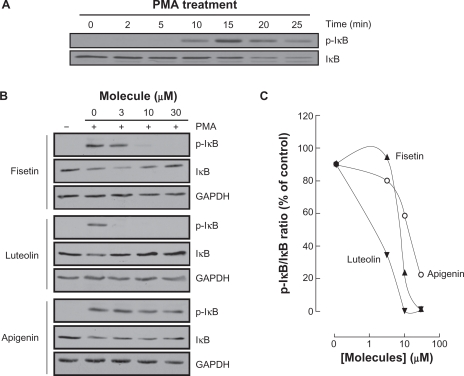
Carcinogen-induced IκB phosphorylation is inhibited by fisetin, luteolin, and apigenin
Among MMP-9 expression regulators, the nuclear factor-kappaB (NF-κB) signaling pathway has been demonstrated to link cancer to inflammatory diseases.31 We therefore assessed whether this signaling was activated upon PMA treatment and whether it was reflected in IkappaB (IκB) degradation. HBMEC were serum-starved then treated with 1 μM PMA up to 25 minutes, lysates were isolated and IκB phosphorylation was assessed through Western Blotting (Figure 7A, upper panel). PMA signaling led to the phosphorylation of IκB at 15 minutes, followed by a decrease in IκB expression (Figure 7A, lower panel).32 Inhibition of PMA-mediated phosphorylation of IκB was next assessed in order to demonstrate whether this mechanism contributes to the anti-MMP-9 and anti-COX-2 inhibitory activities of the best three flavonoids identified above. Preincubation with fisetin, luteolin, or apigenin followed by a 15 minute PMA treatment led to IκB phosphorylation and to a concomitant dose-dependent decrease in IκB for apigenin only (Figure 7B). Inhibition of IκB phosphorylation by fisetin and luteolin led to reappearance of IκB. The ratios of phosphorylated IκB over total IκB expression were quantified by scanning densitometry and represented (Figure 7C).
Figure 7.
Carcinogen-induced IκB phosphorylation is inhibited by fisetin, luteolin, and apigenin. A) HBMEC were serum-starved for 30 minutes then treated with 1 μM PMA for the indicated time. Lysates were isolated, electrophoresed via SDS-PAGE and immunodetection of phosphorylated IκB (P-IκB) and IκB proteins was performed as described in the Materials and Methods section. B) HBMEC were serum-starved for 30 minutes in the presence of either vehicle or 30 μM fisetin, luteolin, and apigenin. Cells were then incubated for 15 minutes with 1 μM PMA. Lysates were isolated, electrophoresed via SDS-PAGE and immunodetection of phosphorylated IκB (P-IκB), IκB, and of GAPDH proteins was performed as described in the Materials and Methods section. C) Quantification was performed by scanning densitometry of the autoradiograms. Data were expressed as the percent of basal P-IκB/IκB ratios in vehicle pretreated cells. Densitometric data of a representative blot out of three is shown.
Abbreviations: HBMEC, human brain microvascular endothelial cells; PMA, phorbol 12-myristate 13-acetate; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Discussion
The adaptive mechanisms responsible for EC survival under procarcinogenic conditions remain poorly documented. EC are believed to be metabolically robust and to adapt to procarcinogenic paracrine stimulation and conditions such as those encountered within the hypoxic tumor microenvironment.33,34 Although increasing interest has been manifested towards cancer therapies that target cell metabolism, very few studies have specifically assessed the combined impact of targeting the EC angiogenic and inflammatory phenotype. In our study, we induced in vitro procarcinogenic stimulation of brain microvascular endothelium using PMA in combination with naturally occurring flavonoids and phenolic glycosides in order to assess their anti-angiogenic and anti-inflammatory properties. In vitro tubulogenesis, PMA-induced MMP-9 secretion, and expression of COX-2 were therefore assessed in order to provide a metabolic and adaptive link between endothelial inflammation and angiogenesis.
Our study highlights the combined anti-angiogenic and anti-inflammatory effects of nonglycosidic flavonoids against carcinogen-stimulated HBMEC, as demonstrated by decreased MMP-9 and COX-2 expression biomarkers. We showed that fisetin, apigenin, and luteolin, upon procarcinogenic stimulation with PMA, efficiently inhibited both MMP-9 secretion and COX-2 expression in HBMEC, an inhibitory effect that we believe to be mediated through the common NF-κB signaling pathway that regulates both biomarkers’ gene and protein expression. Our findings support those obtained in several other cell models. In fact, fisetin’s anti-inflammatory effects were found to suppress lipopolysaccharide-induced NF-κB activation in macrophage and dendritic cell maturation,35 and in tumor necrosis factor-induced NF-κB activation in human lung adenocarcinoma cells.36 Since fisetin and quercitrin have analogous aglyconic structures, the lack of activity of the latter points toward the aglycons as the pharmacophoric entity. This is somewhat clearly illustrated in comparison with the phenolic glycosides, which have phenols in common but are missing the flavonoid skeleton. As for apigenin, several studies reported suppression of PMA-induced tumor cell invasion,37 of PMA-induced COX-2 transcriptional activity,38 and inhibition of inflammatory mediators release in human mast cell lines.39 Finally, luteolin was found to suppress phorbol ester TPA (a mimic of diacylglycerol and PKC activator)-induced MMP-9 activation in a glioblastoma cell line model.40 Altogether, our findings not only support the potential anti-inflammatory properties of those three molecules reported in tumoral and immune compartments, they now further highlight important anti-angiogenic effects on the vascular compartment as reflected through the inhibition of in vitro tubulogenesis and carcinogen-induced MMP-9.
To date, only few reports documented an association between COX-2 expression and ECM degradation consequent to procarcinogenic stimulation. Among the numerous signaling pathways triggered by procarcinogenic culture conditions, NF-κB is at the crossroads of both MMP-9 and COX-2 regulation by PMA, but the intracellular players still remain undefined, at least within the anti-angiogenic effects we report herein. Among the intracellular events that could link PMA-induced signaling to COX-2 induction, NF-κB can contribute to regulate the expression of COX-2 through endoplasmic reticulum (ER) stress and, in part, through induction of the ER chaperone GRP78/BiP, which is expressed at high levels in a variety of tumors and which confers drug resistance to both proliferating and dormant cancer cells.16 Importantly, it was recently demonstrated that partial reduction of GRP78 substantially reduced tumor microvessel density.17 On the other hand, moderate activity of the ER stress response system exerts an anti-apoptotic function and supports tumor cell survival and chemoresistance, whereas more severe aggravation may exceed the protective capacity of this system and turn on its pro-apoptotic module.41 In a recent study, we further demonstrated in vitro through the combination of two pharmacologic approaches that increased COX-2 expression only occurs within those EC which possess low intracellular ATP levels and which are cultured under procarcinogenic conditions.42 Noteworthy, luteolin was shown to change ATP levels and trigger ER stress-induced cell death.43
Several flavonoids have also been reported to interfere with the oxidative damage activity of inducible nitric oxide synthase activity, and to play a nitric oxide scavenging role in the therapeutic effects of flavonoids.44 Nitric oxide is produced by several different types of cells, including EC and macrophages. When flavonoids are used as antioxidants, free radicals are scavenged and therefore can no longer react with nitric oxide, resulting in less damage. Selected phenolic compounds were also shown to inhibit both the COX and 5-lipoxygenase pathways.45 Moreover, the anti-inflammatory ability of flavonoids to inhibit eicosanoid biosynthesis, such as prostaglandins which are the end products of the COX and lipoxygenase pathways, has also been reported.46 The exact mechanism by which flavonoids inhibit these enzymes is not clear.
In summary, the present study has allowed the identification and molecular characterization of three specific flavonoids to act as inhibitors of EC-mediated tubulogenesis, and as signal transduction inhibitors against carcinogen-mediated induction of COX-2 and MMP-9, while phenolic glycosides were shown to be inactive including quercitrin, a closely related rhamnosylated analog of the above flavonoids. Since fisetin and quercitrin have analogous aglyconic structures, the lack of activity of the latter points toward the aglycons as the pharmacophoric entity. This is somewhat clearly illustrated in comparison with the phenolic glycosides, which have phenols in common but are missing the flavonoid skeleton. Moreover, we provide evidence that the NF-κB pathway may be inhibited through the targeting of IκK phosphorylation capacity that ultimately may reduce both the acquisition of a proinflammatory phenotype, as reflected by decreased COX-2 expression, and the acquisition of pro-angiogenic phenotype, as reflected by a decrease in MMP-9. Our results therefore suggest that BBB disruption during neuroinflammation could be pharmacologically reduced by a specific class of flavonoids acting as NF-κB signal transduction inhibitors.
Acknowledgments
BA and RR respectively hold a Canada Research Chair in Molecular Oncology from the Canadian Institutes of Health Research, and a Canada Research Chair in Medicinal Chemistry from the Natural Sciences and Engineering Research Council of Canada. ET is a Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT) awardee. This study was funded by a team grant from FQRNT awarded to BA and RR. We are also thankful to Prof RK Ibrahim from the University of Concordia (Montreal, QC) for a donation of his natural product collection as well as to Mrs Amira Moheb for the sample identification.
Footnotes
Disclosure
No conflicts of interest were declared in relation to this paper.
References
- 1.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 2.Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenesis. 2010;31:334–341. doi: 10.1093/carcin/bgp322. [DOI] [PubMed] [Google Scholar]
- 3.Hayes A. Cancer, cyclo-oxygenase and nonsteroidal anti-inflammatory drugs – can we combine all three? Vet Comp Oncol. 2007;5:1–13. doi: 10.1111/j.1476-5829.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- 4.Costa C, Soares R, Reis-Filho JS, et al. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55:429–434. doi: 10.1136/jcp.55.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fournier LS, Novikov V, Lucidi V, et al. MR monitoring of cyclooxygenase-2 inhibition of angiogenesis in a human breast cancer model in rats. Radiology. 2007;243:105–111. doi: 10.1148/radiol.2431050658. [DOI] [PubMed] [Google Scholar]
- 6.Müller-Decker K, Fürstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog. 2007;46:705–710. doi: 10.1002/mc.20326. [DOI] [PubMed] [Google Scholar]
- 7.Breder CD, Saper CB. Expression of inducible cyclooxygenase mRNA in the mouse brain after systemic administration of bacterial lipopolysaccharide. Brain Res. 1996;713:64–69. doi: 10.1016/0006-8993(95)01474-8. [DOI] [PubMed] [Google Scholar]
- 8.Yamagata K, Andreasson KI, Kaufmann WE, et al. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 9.Cascante M, Boros LG, Comin-Anduix B, et al. Metabolic control analysis in drug discovery and disease. Nat Biotechnol. 2002;20:243–249. doi: 10.1038/nbt0302-243. [DOI] [PubMed] [Google Scholar]
- 10.Boros LG, Cascante M, Lee WN. Metabolic profiling of cell growth and death in cancer: applications in drug discovery. Drug Discov Today. 2002;7:364–372. doi: 10.1016/s1359-6446(02)02179-7. [DOI] [PubMed] [Google Scholar]
- 11.Lakka SS, Gondi CS, Rao JS. Proteases and glioma angiogenesis. Brain Pathol. 2005;15:327–341. doi: 10.1111/j.1750-3639.2005.tb00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonoiu A, Mahajan SD, Ye L, et al. MMP-9 gene silencing by a quantum dot-siRNA nanoplex delivery to maintain the integrity of the blood brain barrier. Brain Res. 2009;1282:142–155. doi: 10.1016/j.brainres.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annabi B, Rojas-Sutterlin S, Laroche M, et al. The diet-derived sulforaphane inhibits matrix metalloproteinase-9-activated human brain microvascular endothelial cell migration and tubulogenesis. Mol Nutr Food Res. 2008;52:692–700. doi: 10.1002/mnfr.200700434. [DOI] [PubMed] [Google Scholar]
- 14.Roomi MW, Monterrey JC, Kalinovsky T, et al. Distinct patterns of matrix metalloproteinase-2 and -9 expression in normal human cell lines. Oncol Rep. 2009;21:821–826. [PubMed] [Google Scholar]
- 15.Jadhav U, Chigurupati S, Lakka SS, et al. Inhibition of matrix metalloproteinase-9 reduces in vitro invasion and angiogenesis in human microvascular endothelial cells. Int J Oncol. 2004;25:1407–1414. [PubMed] [Google Scholar]
- 16.Craggs L, Kalaria RN. Revisiting dietary antioxidants, neurodegeneration and dementia. Neuroreport. 2001;22:1–3. doi: 10.1097/WNR.0b013e328342741c. [DOI] [PubMed] [Google Scholar]
- 17.Rice-Evans C, Packer L, editors. Flavonoids in Health and Disease. 2nd ed. New York: Marcel Dekker Inc; 2003. Occurrence and Analysis; pp. 1–63. [Google Scholar]
- 18.Rusak G, Gutzeit HO, Ludwig-Muller J. Structurally related flavonoids with antioxidative properties differentially affect cell cycle progression and apoptosis of human acute leukemia cells. Nutr Res. 2005;25:143–155. [Google Scholar]
- 19.Ruela-de-Sousa RR, Fuhler GM, Blom N, Ferreira CV, Aoyama H, Peppelenbosch MP. Cytotoxicity of apigenin on leukemia cell lines: implications for prevention and therapy. Cell Death Dis. 2010;1:e19. doi: 10.1038/cddis.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luqman S, Pezzuto JM. NFkappaB: a promising target for natural products in cancer chemoprevention. Phytother Res. 2010;24:949–963. doi: 10.1002/ptr.3171. [DOI] [PubMed] [Google Scholar]
- 21.Tergaonkar V. NFkappaB pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell Biol. 2006;38:1647–1653. doi: 10.1016/j.biocel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Greiffenberg L, Goebel W, Kim KS, et al. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun. 1998;66:5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sina A, Lord-Dufour S, Annabi B. Cell-based evidence for aminopeptidase N/CD13 inhibitor actinonin targeting of MT1-MMP-mediated proMMP-2 activation. Cancer Lett. 2009;279:171–176. doi: 10.1016/j.canlet.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin N, Annabi B, Sik Kim K, et al. The response to brain tumor-derived growth factors is altered in radioresistant human brain endothelial cells. Cancer Biol Ther. 2006;5:1539–1545. doi: 10.4161/cbt.5.11.3459. [DOI] [PubMed] [Google Scholar]
- 25.Lamy S, Gingras D, Béliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62:381–385. [PubMed] [Google Scholar]
- 26.Abécassis I, Olofsson B, Schmid M, et al. RhoA induces MMP-9 expression at CD44 lamellipodial focal complexes and promotes HMEC-1 cell invasion. Exp Cell Res. 2003;291:363–376. doi: 10.1016/j.yexcr.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Annabi B, Currie JC, Moghrabi A, Béliveau R. Inhibition of HuR and MMP-9 expression in macrophage-differentiated HL-60 myeloid leukemia cells by green tea polyphenol EGCg. Leuk Res. 2007;31:1277–1284. doi: 10.1016/j.leukres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Tahanian E, Lord-Dufour S, Das A, Khosla C, Roy R, Annabi B. Inhibition of tubulogenesis and of carcinogen-mediated signaling in brain endothelial cells highlight the antiangiogenic properties of a mumbaistatin analog. Chem Biol Drug Des. 2010;75:481–488. doi: 10.1111/j.1747-0285.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 29.Annabi B, Vaillancourt-Jean E, Weil AG, Béliveau R. Pharmacological targeting of β-adrenergic receptor functions abrogates NF-κB signaling and MMP-9 secretion in medulloblastoma cells. Onco Targets Ther. 2010;3:219–226. doi: 10.2147/OTT.S14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neergheen VS, Bahorun T, Taylor EW, Jen LS, Aruoma OI. Targeting specific cell signaling transduction pathways by dietary and medicinal phytochemicals in cancer chemoprevention. Toxicology. 2010;278:229–241. doi: 10.1016/j.tox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Dong J, Jimi E, Zeiss C, Hayden MS, Ghosh S. Constitutively active NF-kappaB triggers systemic TNFalpha-dependent inflammation and localized TNFalpha-independent inflammatory disease. Genes Dev. 2010;24:1709–1717. doi: 10.1101/gad.1958410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signalling. Immunol Res. 2008;42:3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mertens S, Noll T, Spahr R, et al. Energetic response of coronary endothelial cells to hypoxia. Am J Physiol. 1990;258:H689–H694. doi: 10.1152/ajpheart.1990.258.3.H689. [DOI] [PubMed] [Google Scholar]
- 34.Shryock JC, Rubio R, Berne RM. Release of adenosine from pig aortic endothelial cells during hypoxia and metabolic inhibition. Am J Physiol. 1988;254:H223–H229. doi: 10.1152/ajpheart.1988.254.2.H223. [DOI] [PubMed] [Google Scholar]
- 35.Liu SH, Lin CH, Hung SK, et al. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. J Agric Food Chem. 2010;58:10831–10839. doi: 10.1021/jf1017093. [DOI] [PubMed] [Google Scholar]
- 36.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 37.Noh HJ, Sung EG, Kim JY, et al. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by apigenin via the inhibition of p38 mitogen-activated protein kinase-dependent matrix metalloproteinase-9 expression. Oncol Rep. 2010;24:277–283. doi: 10.3892/or_00000857. [DOI] [PubMed] [Google Scholar]
- 38.Yi Lau GT, Leung LK. The dietary flavonoid apigenin blocks phorbol 12-myristate 13-acetate-induced COX-2 transcriptional activity in breast cell lines. Food Chem Toxicol. 2010;48:3022–3027. doi: 10.1016/j.fct.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 39.Kang OH, Lee JH, Kwon DY. Apigenin inhibits release of inflammatory mediators by blocking the NF-κB activation pathways in the HMC-1 cells. Immunopharmacol Immunotoxicol. doi: 10.3109/08923973.2010.538851. In press. [DOI] [PubMed] [Google Scholar]
- 40.Lin CW, Shen SC, Chien CC, Yang LY, Shia LT, Chen YC. 12-O-tetradecanoylphorbol-13-acetate-induced invasion/migration of glioblastoma cells through activating PKCalpha/ERK/NF-kappaB-dependent MMP-9 expression. J Cell Physiol. 2010;225:472–481. doi: 10.1002/jcp.22226. [DOI] [PubMed] [Google Scholar]
- 41.Cho HY, Thomas S, Golden EB, et al. Enhanced killing of chemoresistant breast cancer cells via controlled aggravation of ER stress. Cancer Lett. 2009;282:87–97. doi: 10.1016/j.canlet.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Tahanian E, Peiro S, Annabi B. Low intracellular ATP levels exacerbate carcinogen-induced inflammatory stress response and inhibit in vitro tubulogenesis in human brain endothelial cells. J Inflamm Res. 2011;4:1–10. doi: 10.2147/JIR.S15880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HZ, Yang WH, Bao BY, Lo PL. Proteomic analysis reveals ATP-dependent steps and chaperones involvement in luteolin-induced lung cancer CH27 cell apoptosis. Eur J Pharmacol. 2010;642:19–27. doi: 10.1016/j.ejphar.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 44.Van Acker SA, Tromp MN, Haenen GR, van der Vijgh WJ, Bast A. Flavonoids as scavengers of nitric oxide radical. Biochem Biophys Res Commun. 1995;214:755–759. doi: 10.1006/bbrc.1995.2350. [DOI] [PubMed] [Google Scholar]
- 45.Laughton MJ, Evans PJ, Moroney MA, Hoult JR, Halliwell B. Inhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives. Relationship to antioxidant activity and to iron ion-reducing ability. Biochem Pharmacol. 1991;42:1673–1681. doi: 10.1016/0006-2952(91)90501-u. [DOI] [PubMed] [Google Scholar]
- 46.Damas J, Bourdon V, Remacle-Volon G, Lecomte J. Pro-inflammatory flavonoids which are inhibitors of prostaglandin biosynthesis. Prostaglandins Leukot Med. 1985;19:11–24. doi: 10.1016/0262-1746(85)90157-x. [DOI] [PubMed] [Google Scholar]