Abstract
Shigella dysenteriae type 1 is the causative agent of the most severe form of bacillary dysentery, which occurs as epidemics in many developing countries. We isolated a bacteriophage from surface water samples from Bangladesh that specifically lyses strains of S. dysenteriae type 1. This phage, designated SF-9, belongs to the Podoviridae family and has a 41-kb double-stranded DNA genome. Further screening of water samples for the prevalence of the phage revealed 9 of 71 (12.6%) water samples which were positive for the phage. These water samples were also positive in PCR assays for one or more S. dysenteriae type 1-specific genes, including ipaBCD and stx1, and live S. dysenteriae type 1 was isolated from three phage-positive samples. The results of this study suggest that phage SF-9 may have epidemiological applications in tracing the presence of S. dysenteriae type 1 in environmental waters.
Bacillary dysentery caused by Shigella dysenteriae type 1 is a major public health problem in many developing countries, including Bangladesh (8, 15, 19). There are four known species of Shigella which are pathogenic, and infection with S. dysenteriae type 1 usually progresses to the most severe form of dysentery with life-threatening complications (8). The most common underlying cause of death in fatal shigellosis is severe colitis, and the immediate associated causes are septicemia and pneumonia (20). Infection with S. dysenterieae type 1 can occur in an epidemic form, and Shigella-contaminated food and drink are often the source of epidemic spread. Very little was known about the presence of the pathogen in the aquatic environment until a recent study indicated that Shigella can survive and persist in surface waters (10). This suggested that environmental monitoring for the presence of the pathogen and its possible spread through environmental waters may provide new insights regarding the epidemiology of Shigella infection.
Shigella is ordinarily identified in stool specimens by in vitro culture of the organism and by several biochemical tests and agglutination assays. DNA probes and PCR assays directed against the large invasion plasmid have also been used to detect Shigella in stools (12, 18). However, identification of Shigella in environmental samples, in which the number of organisms is likely to be small, is limited mainly by the lack of a suitable enrichment technique. Shigellae have previously been identified in surface waters by a combination of PCR and culture techniques (10). For effective environmental monitoring, there is a need to develop more convenient and inexpensive alternative techniques. The presence of specific bacteriophages in water sources may serve as an indicator for the presence of the host bacteria, and this approach has been used in a previous study for epidemiological surveillance of Vibrio cholerae (16). Thus, detection of phages specific for Shigella strains may be a useful tool for predicting the presence of Shigella in environmental waters. Identification and characterization of specific phages are prerequisites for establishing phage-based methods of environmental monitoring. In this study, we isolated and characterized a bacteriophage specific for S. dysenteriae type 1 and studied its distribution in environmental waters in Bangladesh. Furthermore, we analyzed the phage-positive surface water samples for the presence of S. dysenterieae type 1.
MATERIALS AND METHODS
The S. dysenteriae type 1-specific bacteriophage SF-9 was initially isolated from a sample of river water collected in Dhaka City, Bangaldesh. To study the prevalence of the phage in the environment, 71 water samples collected from 10 different sites in two major rivers and a lake in Dhaka were tested. Samples were collected during a 6-month period between March and September 2002. All water samples were obtained in sterile containers, and the initial processing of the samples for detection of S. dysenteriae type 1-specific phages, PCR, and culture of Shigella was done within 3 h of collection.
Detection and isolation of bacteriophage.
Aliquots (20 ml) of water samples were centrifuged at a low speed (1,000 × g) to precipitate debris, and the supernatants were filtered through 0.22-μm-pore-size filters (Millipore Corporation, Bedford, Mass.) to exclude bacteria. Twenty-five different Shigella strains (Table 1) were used as potential recipients to detect the presence of possible Shigella phages. Logarithmic-phase cells (500 μl) of each bacterial strain in nutrient broth (Difco, Detroit, Mich.) were mixed with 3.5-ml aliquots of soft agar (nutrient broth containing 0.8% Bacto Agar [Difco]), and the mixtures were overlaid on nutrient agar plates. Aliquots (10 μl) of each bacterium-free filtrate were inoculated onto the plates. Six samples of filtrates were inoculated per plate, and the plates were incubated for 16 h at 37°C. A sample was positive for phage when a lytic or lysogenic plaque type was observed on the plates. Negative samples were retested after concentration of possible phage particles as follows. Aliquots (10 ml) of the sterile filtrates were mixed with 2.5 ml of a solution containing 20% polyethylene glycol 6000 and 10% NaCl and centrifuged at 12,000 × g. The precipitates were dissolved in 100 μl of SM buffer (100 mM NaCl, 8.1 mM MgSO4, 0.05 mM Tris-Cl [pH 7.5], 0.01% gelatin). These concentrated preparations were then retested for the presence of the phage as described above.
TABLE 1.
Susceptibility test results for various bacteria with Shigella phage SF-9 isolated from a river water sample in Bangladesh
| Organism | No. of isolates | Source of isolates | No. of isolates susceptible to phage SF-9 |
|---|---|---|---|
| S. dysenteriae type 1 | 9 | Patient, Bangladesh | 9 |
| S. dysenteriae type 1 | 2 | Surface water, Bangladesh | 2 |
| S. dysenteriae type 1 | 5 | Patient, India | 5 |
| S. dysenteriae type 2 | 3 | Patient, Bangladesh | 0 |
| S. dysenteriae type 3-10 | 4 | Patient, Bangladesh | 0 |
| S. flexneri type 1a | 2 | Patient, Bangladesh | 0 |
| S. flexneri type 1b | 2 | Patient, Bangladesh | 0 |
| S. flexneri type 2a | 6 | Patient, Bangladesh | 0 |
| S. flexneri type 2b | 3 | Patient, Bangladesh | 0 |
| S. flexneri type 3a | 2 | Patient, Bangladesh | 0 |
| S. flexneri type 3b | 1 | Patient, Bangladesh | 0 |
| S. flexneri type 3c | 1 | Patient, Bangladesh | 0 |
| S. flexneri type 4a | 1 | Patient, Bangladesh | 0 |
| S. flexneri type 5 | 1 | Patient, Bangladesh | 0 |
| S. flexneri type 6 | 2 | Patient, Bangladesh | 0 |
| S. flexneri type X | 1 | Patient, Bangladesh | 0 |
| S. flexneri type Y | 1 | Patient, Bangladesh | 0 |
| Schigella boydie type 1-6 | 4 | Patient, Bangladesh | 0 |
| S. boydie type 7-11 | 3 | Patient, Bangladesh | 0 |
| S. boydie type 12-15 | 4 | Patient, Bangladesh | 0 |
| Shigella sonnei | 3 | Patient, Bangladesh | 0 |
| V. cholerae O1 | 15 | Patient, Bangladesh | 0 |
| V. cholerae O139 | 12 | Patient, Bangladesh | 0 |
| Enterotoxigenic E. coli | 20 | Patient, Bangladesh | 0 |
| Enteropathogenic E. coli | 18 | Patient, Bangladesh | 0 |
| Providencia alcalifaciens | 3 | Patient and surface water, Bangladesh | 0 |
| STEC | 2 | Patient, Bangladesh | 0 |
| E. coli HB101 | 1 | Laboratory collection | 0 |
Phage production and test for host specificity.
A single discrete plaque was purified three times by the soft agar (0.7%) overlay method (17) with control S. dysenteriae type 1 strain SD33891 (13). To grow the phage in liquid medium, an overnight culture of strain SD33891 was diluted 1:100 in fresh nutrient broth and grown at 37°C for 4 h. The culture was then inoculated with phage from a single plaque. The bacterium-phage culture was incubated at 37°C for 16 h, when lysis of most of the bacteria occurred. The culture was centrifuged at 10,000 × g for 20 min, and chloroform (final volume, 1%) was added to the supernatant to kill any unlysed bacteria. The supernatant was kept at 4°C for 20 h, and the chloroform was evaporated. The number of phage particles was determined by testing serial dilutions of the supernatant by the soft agar overlay method with propagating strain SD33891. The host range of the phage was tested at a titer of 103 PFU/ml.
Stability of phage.
The effects of temperature, pH, and salinity on the stability of phage SF-9 were assessed as described previously (11). Briefly, defined numbers of phage particles were added to SM buffer preadjusted to different salinity and pH values and were incubated at different temperatures. The titer of phage particles remaining after 6 h was expressed as a percentage of the original titer.
PCR assays.
PCR primers used in this study have been described previously (10) and were based on previously published sequences of the relevant genes (22, 23). Aliquots (20 ml) of water were centrifuged at a low speed to precipitate debris, and the supernatants containing bacterial cells were used to isolate total nucleic acids by previously described methods (10) One to five microliters of the extracted nucleic acids was used in a multiplex PCR assay for the genes encoding invasive plasmid antigens (ipaBCD) and Shiga toxin (stx1). All PCR primers were synthesized commercially by Oswel DNA Service (University of Edinburgh, Edinburgh, United Kingdom). PCR reagents and kits were purchased from Perkin-Elmer Corporation (Norwalk, Conn.) and were used in accordance with the manufacturer's instructions. The thermocycling parameters for the PCR assays were denaturation at 94°C for 2 min, annealing of primers at 55°C for 2 min, and primer extension at 72°C for 3 min. Each PCR was conducted for 36 cycles, and the sizes of the PCR amplicons were ascertained by agarose gel electrophoresis.
Culture of environmental samples.
Water samples were analyzed for the presence of Shigella by previously described methods (6, 10). Briefly, 50-ml samples of water were filtered as described above, and the filters with residue were incubated in nutrient broth for 4 h at 37°C with shaking. Aliquots of each suspension were streaked onto MacConkey agar plates (Difco, Becton Dickinson and Company) and xylose lysine deoxycholate agar plates (Plasmatec Laboratory Products Ltd., Bridport, Dorset, United Kingdom). Suspected colonies were picked and subjected to biochemical and serological tests to identify Shigella. Culture-confirmed isolates were analyzed further by PCR for the presence of Shigella-specific virulence genes.
Electron microcopy of phage particles.
A high-titer phage preparation (∼1010 PFU/ml) was obtained by using the plate lysis procedure as described previously (4). The phage particles were negatively stained with 2% uranyl acetate and were examined with a Philips transmission electron microscope (model 420T) as described previously (4).
Isolation and analysis of phage nucleic acid.
For isolation and analysis of phage nucleic acid, culture supernatants containing phage particles were filtered through 0.22-μm-pore-size filters (Millipore). Each filtrate was mixed with 0.25 volume of a solution containing 20% polyethylene glycol 6000 and 10% NaCl and centrifuged at 12,000 × g to precipitate the phage particles. The precipitate was dissolved in a solution containing 20 mM Tris-Cl (pH 7.5), 60 mM KCl, 10 mM MgCl, and 10 mM NaCl and was digested with pancreatic DNase I (100 U/ml) and RNase A (50 μg/ml) at 37°C for 2 h. The solution was extracted with phenol-chloroform, and the total nucleic acids were precipitated with ethanol. The phage nucleic acid was digested with restriction endonucleases (Invitrogen Corporation, Carlsbad, Calif.) and analyzed by agarose gel electrophoresis by using standard procedures.
RESULTS AND DISCUSSION
Phage SF-9 was initially isolated from a sample of river water from Dhaka, Bangladesh. This phage produced clear plaques with a diameter of approximately 1 mm on a lawn of an S. dysenteriae type 1 strain. When grown in nutrient broth with control host strain SD33891 (10), the phage produced had a titer of ∼108 PFU/ml. The specificity of the phage was examined by using a panel of strains belonging to different species or serogroups (Table 1). Only S. dysenteriae type 1 strains were susceptible to the phage, and all other strains tested were clearly resistant. Previously described Shigella phages infected a broader spectrum of Shigella strains belonging to different species or serotypes (5, 14). Important examples include phages associated with serotype conversion of Shigella flexneri and phages encoding Shiga-like toxins (1, 5, 14). Shiga toxin-converting phages have been found to be distributed widely among Shigella and Escherichia coli strains (9, 14). The stx prophages carried by Shiga toxin-producing E. coli (STEC) strains can also multiply and lyse their bacterial hosts upon induction and are able to infect recipient strains. Although phage-specific DNA sequences are also found in the regions adjacent to stx genes in the chromosomes of S. dysenteriae type 1 strains, this prophage is assumed to be defective, and intact Shiga toxin-converting phages are not produced by S. dysenteriae type 1 (9).
Electron microscopic examination of phage SF-9 revealed that the phage particles had hexagonal heads and short tails and thus belonged to the family Podoviridae (2) (Fig. 1). Analysis of nucleic acids derived from the phage preparation by using a number of restriction enzymes revealed that the genome of the phage consisted of approximately 41 kb of double-stranded DNA. The genome of SF-9 was considerably smaller than the genomes of lamboid phages containing genes for Shiga-like toxins (9).
FIG. 1.
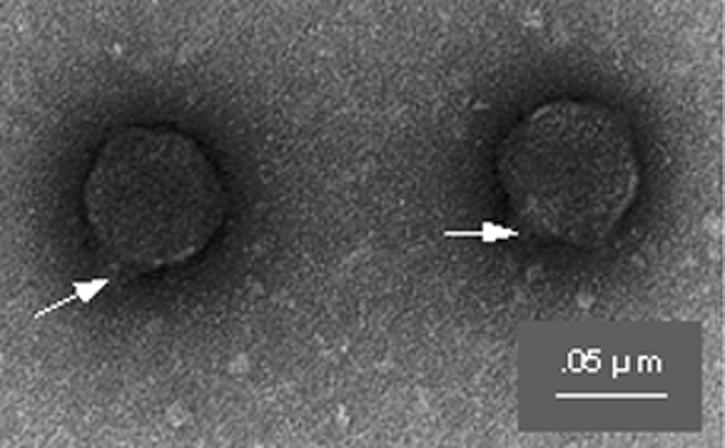
Electron micrograph showing the morphology of S. dysenteriae type 1-specific phage SF-9. Note the hexagonal head and short tail (arrows). Magnification, ×180,000.
The phage was fairly stable at pHs ranging from 6.0 to 9.0 and at temperatures below 37°C. As determined after 6 h of incubation, 65 to 82% of the original phage particles remained infectious. At temperatures above 45°C, however, the majority of the phage particles were rapidly inactivated. Phage particles remained infectious for more than 4 weeks when they were stored at room temperature in SM buffer containing at least 0.5% NaCl. These findings suggest that SF-9 particles may persist in the aquatic environment as infectious agents depending on these and possibly other parameters.
SF-9 was detected in 9 of 71 water samples (12.6%) tested for the presence of the phage. The phage isolates were first tested for host specificity and later were compared by using the restriction endonuclease cleavage patterns of the phage DNA. Phage isolates from the nine different positive samples produced similar DNA restriction patterns, confirming that the phage isolates were the same strain (Fig. 2). The same water samples were also analyzed by Shigella-specific PCR and by conventional culture.
FIG. 2.
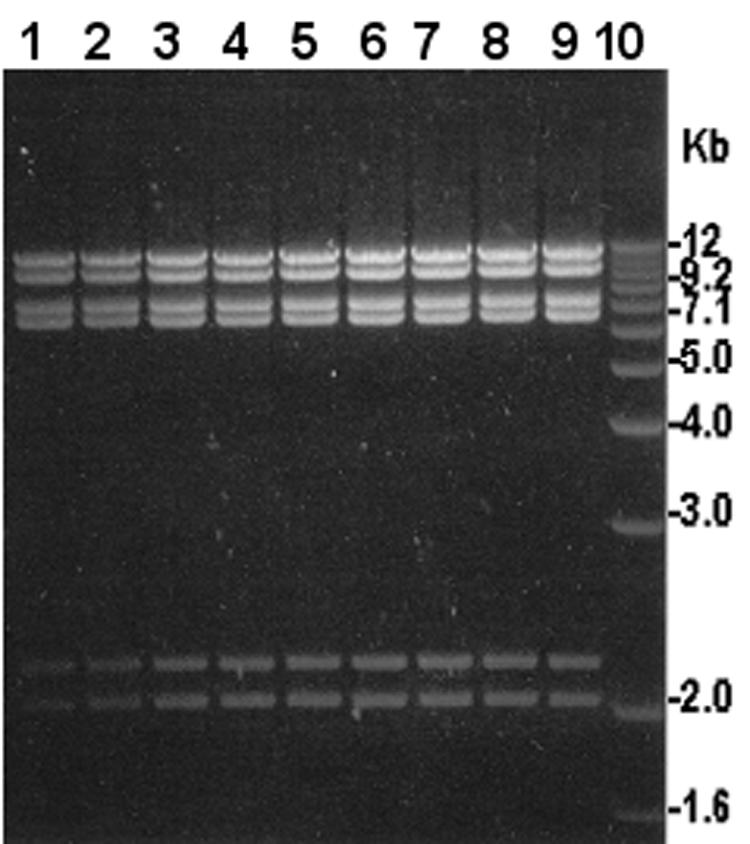
Restriction analysis of DNA isolated from SF-9 phage isolated from different water samples. Lanes 1 through 9, MluI restriction profiles of phage DNA from different samples; lane 10, 1-kb Plus DNA ladder (Invitrogen) used as a molecular size marker.
The most important components of virulence in Shigella infection include adherence, invasiveness, and toxigenicity (19). The genes for these virulence factors reside either on plasmids, on the bacterial chromosome, or on lysogenic bacteriophages. The major virulence genes of S. dysenteriae type 1 include plasmid-encoded genes for invasiveness and intracellular movement, whose products are referred to as invasive plasmid-associated antigens (Ipa), and the gene for Shiga toxin, which is encoded by a chromosomally integrated phage genome (22, 23). In the present study, all nine water samples that were positive for the SF-9 phage were also positive for one or more Shigella-specific genes in PCR assays (Table 2). Two of these samples were positive for both the ipaBCD genes encoding invasive plasmid antigens and the stx1 gene encoding Shiga toxin, whereas the remaining seven samples were positive for stx1 and negative for ipaBCD. PCR assays specific for stx1 failed to produce an amplicon from SF-9 phage DNA, indicating that SF-9 did not carry the stx1 gene. One possible reason for the presence of stx1 alone in these seven samples is that S. dysenteriae type 1 strains lost the large plasmid encoding the ipaBCD genes. It has previously been demonstrated that Shigella strains in the environment tend to lose the plasmid-encoded virulence genes, whereas the chromosomal genes are more conserved (10). The presence of STEC strains could also be a reason for positive stx1 and negative ipaBCD results in the PCR assay. Although S. dysenteriae type 1 produces the prototypical Shiga toxin, different variants of Shiga toxin are known to be produced by STEC strains, which carry temperate lambdoid phages encoding Shiga toxin (21). However, previous epidemiological surveillance has shown that diarrhea due to STEC strains is extremely rare in Bangladesh (3, 7). Since all seven water samples were positive for phage SF-9, which was shown to be specific for S. dysenteriae type 1 and failed to lyse all other strains tested, including STEC strains (Table 1), the stx1 PCR product was most likely derived from S. dysenteriae type 1 strains which had lost the plasmid encoding ipaBCD. This conclusion was further supported by the isolation of live S. dysenteriae type 1 strains from three SF-9 phage-positive water samples. PCR analysis confirmed the presence of the stx1 gene but the absence of the ipaBCD genes in two of these isolates (data not shown). All S. dysenteriae type 1 isolates from environmental water samples were also found to be susceptible to SF-9 phage in subsequent assays.
TABLE 2.
Analysis of 71 surface water samples for the presence of S. dysenteriae type 1-specific phage, SF-9 and S. dysenteriae type 1
| No. of samplesa | Presence of SF-9 phageb | PCR assay for:
|
Isolation of Shigellaspecies | |
|---|---|---|---|---|
| ipaBCD | stx1 | |||
| 1 | + | + | + | S. dysenteriae type 1 |
| 1 | + | + | + | Negative |
| 5 | + | − | + | Negative |
| 2 | − | − | − | Negative |
| 56 | − | − | − | Negative |
| 1 | − | + | − | S. flexneri |
| 3 | − | − | − | Negative |
| 2 | + | − | + | S. dysenteriae type 1 |
Samples were collected from 10 different sampling sites in two rivers and a lake in Dhaka city, Bangladesh.
All nine water samples which were found to be positive for phage SF-9 were from the two rivers.
It was demonstrated previously that a preliminary screening by PCR followed by culturing substantially improves the chance of isolating Shigella from environmental samples (10). The present study indicated that the presence of SF-9 phage in water may also be used as a possible indicator of the presence of S. dysenteriae type 1. However, it should be noted that live Shigella was not isolated from six of nine water samples which were positive for the phage, although the samples were positive for one or more Shigella-specific genes as determined by PCR assays. Nevertheless, the presence of the phage indicates the presence of live or dead organisms and may indicate possible fecal contamination of surface water by patients with shigellosis. In addition, since the SF-9 phage was found to be fairly stable at temperatures below 37°C, the presence of SF-9 may also indicate possible fecal contamination of water in the recent past.
At least three periods of epidemic outbreaks of dysentery due to S. dysenteriae type 1 were recorded between 1972 and 1994 on the Indian subcontinent (8, 15, 19). However, the mechanism associated with periodic outbreaks of shigellosis and the factors associated with the emergence or decline of epidemics are not clear. Shigellae are generally believed to have only a human or primate host. Although Shigella-contaminated food and drink are often the source of epidemic spread, a recent study indicated that Shigella can persist and possibly spread through environmental waters (10). This finding has epidemiological significance, particularly since in developing countries with inadequate sanitation, fecal contamination of environmental waters by enteric pathogens is very common. Since phage SF-9 was isolated from a considerable proportion of water samples in the present study, we assume that the presence of S. dysenteriae type 1 in surface water is more common than previously appreciated. It is, therefore, important to monitor the presence of S. dysenteriae type 1 in the environment to better understand the epidemiology of shigellosis. In a previous study the presence of vibriophages in water was demonstrated to be a potential tool for predicting the presence of V. cholerae (16), which causes periodic epidemics of cholera in many developing countries. In view of inadequacies in convenient techniques to detect Shigella in environmental samples, monitoring for the presence of bacteriophages specific for Shigella may prove to be a useful epidemiological tool for predicting outbreaks and the spread of shigellosis. Detailed analysis of a large number of water samples for the presence of Shigella by conventional methods is also impractical. Although PCR is a sensitive detection technique, use of this technique in remote areas for environmental monitoring is not yet practical, particularly in developing countries with inadequate laboratory facilities. The phage-based detection technique, on the other hand, may be easier to perform in such situations and has the potential to be adapted to field settings. The identification of phage SF-9 in this study may contribute significantly to this effort.
Acknowledgments
This research was funded by a special research grant from the government of Japan to the International Centre for Diarrhoeal Disease Research, Bangladesh. The International Centre for Diarrhoeal Disease Research, Bangladesh, is supported by countries and agencies which share its concern for the health problems of developing countries. Current donors providing unrestricted support include the aid agencies of the governments of Australia, Bangladesh, Belgium, Canada, Japan, the Kingdom of Saudi Arabia, The Netherlands, Sweden, Sri Lanka, Switzerland, and the United States.
REFERENCES
- 1.Acheson, D. W. K., J. Reidl, X. Zhang, G. T. Keusch, J. J. Mekalanos, and M. K. Waldor. 1998. In vivo transduction with Shiga toxin 1-encoding phage. Infect. Immun. 66:4496-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann, H.-W. 1987. Bacteriophage taxonomy. Microbiol. Sci. 4:214-218. [PubMed] [Google Scholar]
- 3.Albert, M. J., A. S. G. Faruque, S. M. Faruque, R. B. Sack, and D. Mahalanabis. 1999. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J. Clin. Microbiol. 37:3458-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert, M. J., N. A. Bhuyan, A. Rahman, A. N. Ghosh, K. Hultenby, A. Weintraub, S. Nahar, A. K. M. G. Kibriya, M. Ansaruzzaman, and T. Shimada. 1996. Phage specific for Vibrio cholerae O139 Bengal. J. Clin. Microbiol. 34:1843-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allison, G. E., and N. K. Verma. 2000. Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri. Trends Microbiol. 8:17-23. [DOI] [PubMed] [Google Scholar]
- 6.American Public Health Association. Detection of pathogenic bacteria, p. 86-100. In A. E. Greenberg, L. S. Clesceri, and A. D. Eaton (ed.,), Standard methods for the examination of water and wastewater. American Public Health Association, Washington, D.C.
- 7.Baqui, A., R. B. Sack, R. E. Black, K. Haider, A. Hossain, A. R. M. A. Alim, M. Yunus, H. R. Chowdhury, and A. K. Siddique. 1992. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children <5 years of age. J. Infect. Dis. 166:792-796. [DOI] [PubMed] [Google Scholar]
- 8.Chen, L. C., M. Rahman, and A. M. Sarder. 1980. Epidemiology and causes of death among children in a rural area of Bangladesh. Int. J. Epidemiol. 9:25-33. [DOI] [PubMed] [Google Scholar]
- 9.Eckhard S., R. Lurz, and L Beutin. 2001. Characterization of a Shiga toxin-encoding temperate bacteriophage of Shigella sonnei. Infect. Immun. 69:7588-7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faruque, S. M., R. Khan, M. Kamruzzaman, S. Yamasaki, Q. S. Ahmad, T. Azim, G. B. Nair, Y. Takeda, and D. A. Sack. 2002. Isolation of Shigella dysenteriae type 1 and S. flexneri strains from surface waters in Bangladesh: comparative molecular analysis of environmental Shigella isolates versus clinical strains. Appl. Environ. Microbiol. 68:3908-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faruque. S. M., Asadulghani, M. M. Rahman, M. K. Waldor, and D. A. Sack. 2000. Sunlight-induced propagation of the lysogenic phage encoding cholera toxin. Infect. Immun. 68:4795-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frankel, G., L. Riley, J. A. Giron, J. Valmassoi, A. Friedman, N. Strockbine, S. Falkow, and G. Schoolnik. 1990. Detection of Shigella in feces using DNA amplification. J. Infect. Dis. 161:1252-1256. [DOI] [PubMed] [Google Scholar]
- 13.Haider, K., A. Chatkaeomorakot, B. A. Kay, K. A. Talukder, D. N. Taylor, P. Echeverria, and D. A. Sack. 1990. Trimethoprim resistance gene in Shigella dysenteriae 1 isolates obtained from widely scattered locations of Asia. Epidemiol. Infect. 104:219-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James, C. E., K. N. Stanley, H. E. Allison, H. J. Flint, C. S. Stewart, R. J. Sharp, J. R. Saunders, and A. J. McCarthy,. 2001. Lytic and lysogenic infection of diverse Escherichia coli and Shigella strains with a verocytotoxigenic bacteriophage. Appl. Environ. Microbiol. 67:4335-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz, S. L 1986. The burden of disease resulting from diarrhea, p. 159-169. In S. L. Katz (ed.), New vaccine development: establishing properties. Diseases of importance in developing countries, vol. 2. National Academy Press, Washington, D.C.
- 16.Madico, G., W. Checkley, R. H. Gilman, N. Bravo, L. Cabrera, M. Calderon, and A. Ceballos. 1996. Active surveillance for Vibrio cholerae O1 and vibriophages in sewage water as a potential tool to predict cholera outbreaks. J. Clin. Microbiol. 34:2968-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukerjee, S. 1978. Principles and practice of typing Vibrio cholerae. Methods Microbiol. 12:50-115. [Google Scholar]
- 18.Oberhelman, R. A., D. J. Kopecko, M. M. Venkatesan, E. Salazar-Lindo, E. Gotuzzo, A. Yi, E. Chea-woo, R. Ruiz, C. Fernandez-Prada, R. Leon-Barua, and R. B. Sack. 1993. Evaluation of alkaline phosphatase-labelled ipaH probe for diagnosis of Shigella infections. J. Clin. Microbiol. 31:2101-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronsmans, C., M. L. Bennish, and T. Wierzba. 1988. Diagnosis and management of dysentery by community health workers. Lancet ii:552-555. [DOI] [PubMed]
- 20.Speelman, P., I. Kabir, and M. Islam. 1984. Distribution and spread of colonic lesions in shigellosis: a colonoscopic study. J. Infect. Dis. 150:899-903. [DOI] [PubMed] [Google Scholar]
- 21.Strockbine, N. A., L. R. M. Marques, J. W. Newland, H. W. Smith, R. K. Holmes, and A. D. O'Brien. 1986. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 53:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unkmeir, A., and H. Schmidt. 2000. Structural analysis of phage-borne stx genes and their flanking sequences in Shiga toxin-producing Escherichia coli and Shigella dysenteriae type 1 strains. Infect. Immun. 68:4856-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatesan, M. M., J. M. Buysse, and D. J. Kopecko. 1988. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc. Natl. Acad. Sci. USA 85:9317-9321. [DOI] [PMC free article] [PubMed] [Google Scholar]


