Abstract
We describe a rare case of adult T-cell leukemia characterized by an expansion of CD4+ CD8+ double-positive lymphocytes associated with human T-lymphotropic virus type 1 (HTLV-1) and a complex karyotype in a 43-year-old Caribbean male who was initially admitted to our hospital with significant lethargy, visual disturbances, dysphagia, right facial palsy and numbness in both feet for 3 days. He was found to have severe hypercalcemia (15.6 mg/dl). Peripheral blood smear showed multilobulated clover-shaped nuclei. Bone marrow and CSF flow cytometries revealed abnormal monoclonal expansion of T cells positive for CD4, CD5, CD8 and CD25 but negative for CD7, CD20, CD56, CD68 and terminal deoxynucleotidyl transferase. The polymerase chain reaction analysis showed a distinct band of the T-cell receptor γ gene, revealing T-cell clonal integration of the proviral DNA of HTLV-1, thus confirming the diagnosis of acute adult T-cell leukemia/lymphoma. Cytogenetic study revealed a male karyotype with monosomy 12, unbalanced translocation 5q and 13q and additional material on 5q, 7q, 14q and 17q. The patient underwent prednisone (EPOCH) chemotherapy followed by autologous transplantation with BEAM regimen. Although patients with a rare mixed CD4+ CD8+ immunophenotype usually present with an aggressive clinical course and have a poor prognosis, our patient was able to survive for 2.5 years.
Key Words: Acute T-cell leukemia, Cyclophosphamide, Doxorubicin, Etoposide, Human T-lymphotropic virus type 1 (HTLV-1), Prednisone, Vincristine
Background
Adult T-cell leukemia/lymphoma (ATLL) is an aggressive type of leukemia/lymphoma associated with the human T-cell lymphotropic virus type 1 (HTLV-1) that is characterized by a short survival time and a poor response to chemotherapy [1]. There are several clinical subtypes of ATLL, namely an acute, lymphomatous, chronic, smoldering, and a rare cutaneous type [2, 3]. The disease is most frequently associated with a mature CD4+ CD8- T-cell phenotype. However, rare cases of an unusual form of immunophenotype characterized by co-expression of CD4+ CD8+ double-positive cells have been reported [4, 5, 6, 7]. Isolated case reports have suggested a median survival time of about 6–8 months despite intensive treatment. Characteristic clinical features include high white-cell counts, skin lesions, hepatosplenomegaly, lymphadenopathy and hypercalcemia. Here, we report a rare case of dual CD4+ CD8+ double-positive ATLL with complex cytogenetic characteristics and discuss the clinical presentation and the course of this rare disease.
Case Report
A 43-year-old Caribbean male presented with a 3-day history of generalized body aches, weakness, myalgia, visual disturbances, dysphagia, constipation, and numbness in both feet. His past medical history was significant for hypertension and childhood infection of strongyloidiasis. Physical examination disclosed significant lethargy, malaise, uveitis in both eyes and, interestingly, right facial palsy. Routine laboratory test revealed a white blood cell count of 7,300/mm3, hemoglobin 10.4 mg/dl, hematocrit 31.3%, platelet 477,000/mm3, absolute neutrophil count 2,560 L, lymphocytes 5,500/mm3, serum calcium 15.6 mg/dl, serum albumin 3.9 g/dl, blood urea nitrogen 26 mg/dl, and serum creatinine 2.1 mg/dl. Other laboratory tests, including liver function tests, urine analysis, parathyroid hormone (PTH), parathyroid hormone related peptide (PTHrp), vitamin D3, angiotensin converting enzyme, serum protein electrophoresis, urine protein electrophoresis, serum immunoglobulins and immunofixation were within normal limits. Neuroimaging was unremarkable. Examination of peripheral smear revealed presence of abnormal lymphocytes with multilobulated clover-shaped nuclei. Computed tomography scans of the abdomen and pelvis showed a multifocal mass and enlargement of the liver and spleen. No enlargement of the periaortic, iliac or inguinal lymph nodes was observed. Serum antibodies against HTLV-1 (gp19, gp21) were found to be positive and negative for HIV. Bone marrow flow cytometric analysis also revealed 49% abnormal clones of T cells positive for CD2, CD4, CD5, CD8, CD25, CD38 and CD45 and non-immunoreactive for CD7, CD20, CD34, CD56, CD117, HLA-DR and terminal deoxynucleotidyl transferase. Cerebral spinal fluid flow cytometry confirmed similar findings with presence of clonal T cells positive for CD2, CD3, CD4, CD5, CD8 and CD25 and negative for CD7. Bone marrow cytogenic study showed a complex male karyotype of 47,XY with several numeric and structural abnormalities involving the long arm of chromosomes 1, 3, 6, 7, 12, 13, and 14q32, as well as the short arm of chromosomes 10, 17, and 21. We also noticed monosomy of chromosome 12 and unbalanced translocation on the long arm of chromosomes 5 and 13, additional material on the long arm of chromosomes 5, 7, 12, 14 (14q+ chromosome) and 17, and on the short arm of 2 of the 3 copies of chromosome 4 as well as a marker chromosome in 16 of 20 metaphases, suggesting a partial trisomy or inactive genetic material. Finally, rearrangement of the T-cell receptor γ (TCRγ) gene located on chromosome 7 was tested to analyze the clonality in T-cell lymphoproliferations amplified by fluorescent polymerase chain reaction (PCR), which showed a distinct band of the TCRγ gene, revealing T-cell clonal integration of proviral DNA of HTLV-1, thus confirming the diagnosis of acute ATLL.
The patient was given a chemotherapy regimen of etoposide, vincristine, doxorubicin, cyclophosphamide and prednisone (EPOCH) along with aggressive management of hypercalcemia using hydration, bisphosphonates and calcitonin. Repeated flow cytometry after the 3rd cycle of the EPOCH regimen showed no abnormal phenotype in the bone marrow. However, after the 5th cycle of the EPOCH regimen, an Ommaya tap was done that revealed Candida species. The patient then underwent successful autologous stem cell transplantation with high-dose BEAM. Repeated bone marrow aspirate and biopsy 9 months after transplantation demonstrated no evidence of lymphoma and T-cell band, T-cell γ and T-cell σ, nor an abnormal phenotype on flow cytometry. However, after 13 months of autologous stem cell transplantation, the patient relapsed and was admitted to the hospital with refractory hypercalcemia. Salvage regimen which included gemcitabine and vinorelbine was started. Unfortunately, the response to the treatment was poor and a hyper-CVAD regimen (i.e., a high dose of methotrexate and a high dose of cytarabine) was introduced with rapid resolution of hypercalcemia and white cell count. However, disease recurred again with refractory hypercalcemia after 3 months, being complicated by disseminated infection requiring antibacterial, antiviral and antifungal agents. Overall, the patient survived for 25 months from the time of diagnosis and 17 months after transplantation.
Discussion
We have reported a rare case of expansion of a CD4+ CD8+ double-positive cell population in a Caribbean male with a complex male karyotype, infected with HTLV-1. The disease was first described in Kyushu, in southwestern Japan, and it occurs most frequently in endemic areas such as Japan, the Caribbean basin, West Africa, Brazil, Central and South America and northern Iran [2, 4]. The disease is considered a fatal malignant post-thymic lymphoproliferative disorder and is characterized by the presence of leukemic cells with highly convoluted nuclei (so-called 'flower-like cells'), lymphadenopathy, hepatosplenomegaly, skin lesions, and hypercalcemia.
The involvement of CD4+ cells in the development of ATLL is indisputable; however, few case reports have noticed an unusual phenotype of double-positive CD4+ and CD8+ expression in patients with an extremely aggressive course.
Kim et al. [8] reported an extremely aggressive course with poor survival in a patient with CD4+ CD8+ double-positive acute adult T-cell leukemia with skin manifestations. Kamihira et al. [7] reported a poorer prognosis with a median survival of 7.8 months as compared to patients with the typical CD4- CD8- phenotype. Ciminale et al. [9] describe a CD4+ CD8+ phenotype acute lymphoma in a Greek patient with an aggressive clinical course of refractory hypercalcemia. Several authors have implicated a high proliferation index (>18%), and large cells are suggestive of a poor prognosis in these patients [10].
Although HTLV-1 infects both CD4+ and CD8+ T cells, leukemogenic potential of the virus is restricted to the CD4+ subset. The dynamic relationship between the co-expansion of two cell populations of CD4+ and CD8+ in the course of the disease is still unclear. However, since the CD4/CD8 double-positive phenotype is characteristically associated with the majority of immature thymic precursors, it is hypothesized that the infection with HTLV-1 and expansion of CD8+ cells plays a helper role in the selection of specific CD4+ cell clones, and thus, during intrathymic ontogeny, immature CD4+/CD8+ thymocytes develop into functionally competent CD3+/CD4+/CD8- or CD3+/CD4-/CD8+ T cells after transient expression of the CD4/CD8 double-positive phenotype [11]. Sibon et al. [12] have shown that infected CD4+ and CD8+ cells displayed the same pattern of clonal expansion in vivo, and both subsets of T cells disseminated the virus at a proviral state, although the degree of clonal expansion was higher in CD4+ than in CD8+ lymphocytes, which accounted for the significantly higher proviral loads harbored by the CD4+ subset of T cells. Alternatively, the viral infection may alter the expression of interleukin-4, which can modulate the expression of CD8 on the surface of CD4+ T cells, resulting in a concomitant expression of both markers [13].
Another possible mechanism includes clonal expansion of a less differentiated cell population which is immortalized by HTLV-1 infection; however, the finding that terminal deoxynucleotidyl transferase, which is a marker for thymic T-cell precursors, is usually not expressed in the infiltrative tumor cells opposes this hypothesis [7, 11, 12, 13].
One of the distinct features of the disease is refractory hypercalcemia, which requires frequent hospitalization and aggressive hydration. This phenomenon has been studied previously by Prager et al. [14] and other study groups [15, 16, 17, 18, 19]. They have shown increased bone resorption mediated by parathyroid hormone-related protein (PTHrP), transactivated by the HTLV-1 tax and HTLV-11 tax proteins, which mediates its effects on PTHrP via cellular transcription factors activator protein (AP)-2 and AP-1. Transactivation via an AP-2 motif represents a novel interaction of tax with a cellular transcription factor or lymphokines such as interleukin 1 (IL-1), IL-2, IL-6 and tumor necrosis factor (TNF), and dysregulation of cellular gene transcription by viral proteins. Interestingly, the hypercalcemia in our patient occurred in the setting of normal PTHrP and low 1,25(OH)2D levels, supporting the role for cytokines in the development of HTLV-1-related hypercalcemia [20].
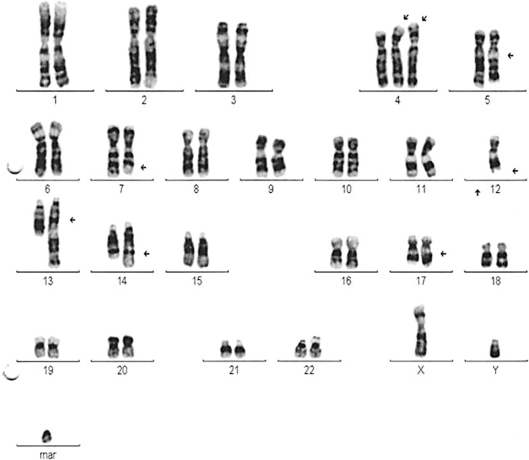
Another interesting feature are the clonal chromosome abnormalities along with clinical heterogeneity and a plethora of secondary abnormalities in our patient. Interestingly, we noticed a hyperdiploid male karyotype, monosomy of chromosome 12 and an unbalanced translocation on the long arm of chromosomes 5 and 13, additional material on the long arm of chromosomes 5, 7, 12, 14 (14q+ chromosome) and 17 and on the short arm of 2 of the 3 copies of chromosome 4, and a marker chromosome in 16 of 20 metaphases, suggesting a partial trisomy or inactive genetic material. Five of these metaphases also showed a second copy of the marker chromosome. Interestingly, only 4 metaphases showed a normal karyotype. Complex cytogenetic characteristics are presented in fig. 1.
Fig. 1.
Complex cytogenetic characteristics with multiple chromosomal aberrations.
Itoyama et al. [21] reveal cytogenetic findings in 50 cases of ATLL where multiple breaks (at least 6), abnormalities of chromosomes 1p, 1p22, 1q, 1q10-21, 2q, 3q, 3q10-12, 3q21, 14q, 14q32 and 17q, and partial loss of chromosomes 2q, 9p, 14p, 14q and 17q regions correlated with shorter survival [19]. The most frequent gains include trisomy 3, trisomy 8, trisomy 9 and trisomy 21. Monosomies involve chromosomes 4, 8, 10 and 22.
Several authors have also suggested that 14q32 translocations were the most common abnormalities and are consistently observed in human T-cell tumors [22, 23]. To date, this is the only report which showed monosomy of chromosome 12 and complex cytogenetic chromosomal abnormalities with a male karyotype in HTLV-1 CD4+ CD8+ double-positive lymphoma.
We believe that despite the aggressive course of the disease, the current treatment has allowed the patient to live longer. We used EPOCH chemotherapy with a 4-day infusion schedule of cyclophosphamide, vincristine, doxorubicin and etoposide, followed by antiretroviral therapy and successful autologous bone marrow transplantation with BEAM therapy. The patient remained in complete remission for 13 months after bone marrow transplantation with no evidence of recurrent HTLV-1 infection on PCR testing and on flow cytometry. However, salvage regimen which included gemcitabine and vinorelbine and, later on, hyper-cyclophosphamide, vincristine, adriamycin and dexamethasone resulted in a transient response after relapse.
In conclusion, we have shown that double-positive ATLL is an aggressive form of lymphoma associated with refractory hypercalcemia. Despite recent advances in treatment, information about this rare disease is still limited. In connection with the multiple chromosomal aberrations and complex cytogenetic characteristics observed in our patient, further investigation into the role of HTLV-1 in these lymphomas and clinical trials are warranted.
Disclosure Statement
The authors have no conflict of interest.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Kaplan JE, Osame M, Kubota H, et al. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J Acquir Immune Defic Syndr. 1990;3:1096–1101. [PubMed] [Google Scholar]
- 2.Dourado I, Alcantara LC, Barreto ML, et al. HTLV-I in the general population of Salvador, Brazil: a city with African ethnic and sociodemographic characteristics. J Acquir Immune Defic Syndr. 2003;34:527–531. doi: 10.1097/00126334-200312150-00013. [DOI] [PubMed] [Google Scholar]
- 3.Shimoyama M, Members of the Lymphoma Study Group Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma: a report from the Lymphoma Study Group (1984–1987) Br J Haematol. 1991;79:428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 4.Pombo De Oliveira MS, Loureiro P, Bittencourt A, et al. Geographic diversity of adult T-cell leukemia/lymphoma in Brazil. The Brazilian ATLL Study Group. Int J Cancer. 1999;81:1–8. doi: 10.1002/(sici)1097-0215(19991029)83:3<291::aid-ijc1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Tamura K, Unoki T, Sagawa K, et al. Clinical features of OKT4+/OKT8+ adult T-cell leukemia. Leuk Res. 1985;9:1353–1359. doi: 10.1016/0145-2126(85)90122-5. [DOI] [PubMed] [Google Scholar]
- 6.Tamura K, Makino S, Araki Y, et al. A case of CD4+/CD8- adult T-cell leukemia with good response to interferon-beta terminating as a CD4+/CD8+ adult T-cell lymphoma. Leuk Res. 1987;11:665–668. doi: 10.1016/0145-2126(87)90041-5. [DOI] [PubMed] [Google Scholar]
- 7.Kamihira S, Sohda H, Atogami S, et al. Phenotypic diversity and prognosis of adult T-cell leukemia. Leuk Res. 1992;16:435–441. doi: 10.1016/0145-2126(92)90168-7. [DOI] [PubMed] [Google Scholar]
- 8.Kim YJ, Hwang ES, Kim IH, et al. CD4/CD8 double-positive, acute type of adult T-cell leukemia/lymphoma with extensive cutaneous involvement. Int J Dermatol. 2006;45:1193–1195. doi: 10.1111/j.1365-4632.2006.02613.x. [DOI] [PubMed] [Google Scholar]
- 9.Ciminale V, Hatziyanni M, Felber BK, et al. Unusual CD4+CD8+ phenotype in a Greek patient diagnosed with adult T-cell leukemia positive for human T-cell leukemia virus type I (HTLV-I) Leuk Res. 2000;24:353–358. doi: 10.1016/s0145-2126(99)00193-9. [DOI] [PubMed] [Google Scholar]
- 10.Bittencourt AL, da Graças Vieira M, Brites CR, et al. Adult T-cell leukemia/lymphoma (ATL) in Bahia, Brazil: analysis of prognostic factors in a group of 70 patients. Am J Clin Pathol. 2007;128:875–882. doi: 10.1309/2YGD1P0QCVCWBLDX. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Nagata Y, Kamihira S, et al. IL-2-dependent ATL cell lines with phenotypes differing from the original leukemia cells. Leuk Res. 1991;15:619–625. doi: 10.1016/0145-2126(91)90031-n. [DOI] [PubMed] [Google Scholar]
- 12.Sibon D, Gabet AS, Zandecki M, et al. HTLV-1 propels untransformed CD4 lymphocytes into the cell cycle while protecting CD8 cells from death. J Clin Invest. 2006;116:974–983. doi: 10.1172/JCI27198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paliard X, Malefijt RW, de Vries JE, et al. Interleukin-4 mediates CD8 induction on human CD4+ T-cell clones. Nature. 1988;335:642–644. doi: 10.1038/335642a0. [DOI] [PubMed] [Google Scholar]
- 14.Prager D, Rosenblatt JD, Ejima E. Hypercalcemia, parathyroid hormone-related protein expression and human T-cell leukemia virus infection. Leuk Lymphoma. 1994;14:395–400. doi: 10.3109/10428199409049695. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T. HTLV-1-associated diseases. Int J Hematol. 1997;66:257–258. doi: 10.1016/s0925-5710(97)00077-7. [DOI] [PubMed] [Google Scholar]
- 16.Moseley JM, Danks JA, Grill V, et al. Immunocytochemical demonstration of PTHrP protein in neoplastic tissue of HTLV-1 positive human adult T cell leukemia/lymphoma: implications for the mechanism of hypercalcemia. Br J Cancer. 1991;64:745–748. doi: 10.1038/bjc.1991.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards CM, Edwards SJ, Bhumbra RP, et al. Severe refractory hypercalcemia in HTLV-1 infection. J R Soc Med. 2003;96:126–127. doi: 10.1258/jrsm.96.3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe T, Yamaguchi K, Takatsuki K, et al. Constitutive expression of parathyroid hormone-related protein gene in human T cell leukemia virus type 1 (HTLV-1) carriers and adult T cell leukemia patients that can be trans-activated by HTLV-1 tax gene. J Exp Med. 1990;172:759–756. doi: 10.1084/jem.172.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda K, Okazaki R, Inoue D, et al. Transcription of the gene for parathyroid hormone-related peptide from the human is activated through a cAMP-dependent pathway by prostaglandin E1 in HTLV-I-infected T cells. J Biol Chem. 1993;268:1174–1179. [PubMed] [Google Scholar]
- 20.Whang-Peng J, Bunn PA, Knutsen T, et al. Cytogenetic studies in human T-cell lymphoma virus (HTLV)-positive leukemia-lymphoma in the United States. J Natl Cancer Inst. 1985;74:357–369. [PubMed] [Google Scholar]
- 21.Itoyama T, Chaganti RS, Yamada Y, et al. Cytogenetic analysis and clinical significance in adult T-cell leukemia/lymphoma: a study of 50 cases from the human T-cell leukemia virus type-1 endemic area, Nagasaki. Blood. 2001;97:3612–3620. doi: 10.1182/blood.v97.11.3612. [DOI] [PubMed] [Google Scholar]
- 22.Mengle-Gaw L, Albertson DG, Sherrington PD, et al. Analysis of a T-cell tumor-specific breakpoint cluster at human chromosome 14q32. Proc Natl Acad Sci USA. 1988;85:9171–9175. doi: 10.1073/pnas.85.23.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukasaki K, Krebs J, Nagai K, et al. Comparative genomic hybridization analysis in adult T-cell leukemia/lymphoma: correlation with clinical course. Blood. 2001;97:3875–3881. doi: 10.1182/blood.v97.12.3875. [DOI] [PubMed] [Google Scholar]