Abstract
Expression of the breast cancer susceptibility tumor-suppressor protein BRCA2, a protein potentially involved in DNA recombination repair, is tightly regulated throughout development. We have identified a transcriptional silencer at the distal end of the human BRCA2 gene promoter. This silencer is involved in the negative regulation of the expression of this gene in breast cell lines tested but not in HeLa or HepG2 cells. The 221-base-pair silencer region is characterized by a full-length Alu-repeat. Presence of specific BRCA2 silencer-binding proteins in the breast cell extracts indicates the potential regulation of BRCA2 gene expression by these proteins.
The second breast cancer gene, BRCA2, encodes a nuclear protein of 3814 amino acids (1). This steroidregulated protein has unique structural features, directly interacts with the repair protein RAD51, and has domains that implicate it as a possible transcriptional transactivator (1, 2). Along with BRCA1, germline mutations in the BRCA2 gene are found to be responsible for majority of hereditary breast cancers (1, 2). Both BRCA1 and BRCA2 gene products are involved in DNA repair and recombination processes and appear to play key roles in monitoring and/or repairing DNA lesions (1). Mutations in either of these genes may cause relaxation of this monitoring event and the unrepaired DNA lesions may lead to the accumulation of mutations and ultimately to cancer (1).
The role played by BRCA2 protein in sporadic breast and ovarian cancer is not known as mutations in this gene are rarely seen is such cases. If inactivation of BRCA2 associated functions contributes to sporadic malignancies, it might be achieved by epigenetic events. Other proteins involved in the BRCA2 pathway may determine the net concentration and activity of BRCA2 protein in a cell. Our study is aimed at understanding the possible mechanism of down-regulation of human BRCA2 gene. To this effect, we have found an Alu-repeat containing silencer at the upstream of BRCA2 gene promoter. We propose that protein(s) binding to this silencer may regulate BRCA2 gene expression in human breast cells.
MATERIALS AND METHODS
Cells
Cell lines used in this study include MCF-10F, MDA-MB- 231, MDA-MB-435S, BT-549, MCF-7, HeLa, and HepG2. Most of these cells were originally procured from American Type Culture Collection (ATCC, Manassas, VA) and maintained in the recommended (by ATCC) growth medium under the specified conditions. MDA-MB-435S and BT-549 cells (3) were obtained from J. Ochieng of Meharry Medical College and maintained in Ham’s F-12 media (3).
Cloning of the BRCA2 upstream sequence
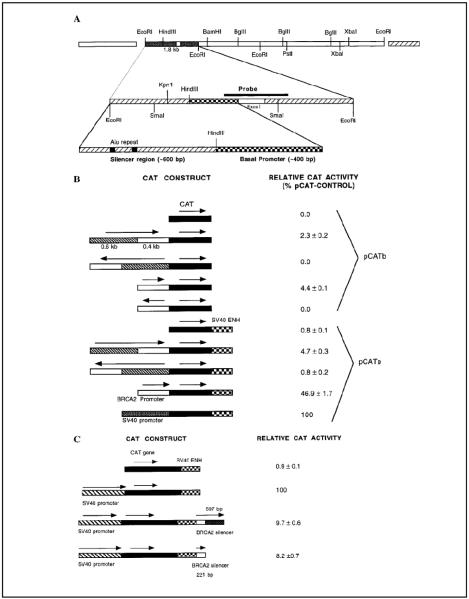
A human genomic library made in λFIXII (Stratagene, La Jolla, CA) vector was screened (4) with RT-PCR-amplified (primers 5′-GCCAACACTGAGAAAATACCCG- 3′ and 5′-AAATACAGCGTGCCCAGCAG-3′) 668 bp human BRCA2 exon 1 fragment as probe to obtain three clones potentially containing the BRCA2 upstream sequence. Southern hybridization (4) of the EcoRI-digest of one of the clones revealed a 1.8-kb fragment containing the exon I. Anchor PCR (4) with the pBLuescript KS(1) clone of this 1.8 kb DNA using T7 and exon I-terminal (5′-CCTAGTTTCAGAAGCTCGC-3′) primers and Pfu DNA polymerase (Stratagene) yielded the 1015-bp upstream sequence. Nucleotide sequencing (4) confirmed the identity of this fragment. Figure 1 summarizes this cloning scheme.
FIG. 1.
Cloning of the human BRCA2 gene silencer. (A) Sketch showing the promoter and the silencer regions in the context of the upstream sequence of human BRCA2 gene. (B) Relative CAT activity in cell extracts obtained from MCF-10F cells transfected with different pCATbasic and pCATenhancer constructs. (C) Relative CAT activity in cell extracts obtained from MCF-10F cells transfected with different pCATcontrol constructs having the silencer sequence. Results are means ± SE (n = 6).
Transformation of cells and reporter gene assay (4–6)
The following plasmid vectors, procured from Promega (Madison, WI), were employed in our study: pCATbasic, pCATenhancer, pCATcontrol, pSV-b-galactosidase, pGL3-basic, pGL3-enhancer, pGL3-control and pRL-TK (5). DNA fragments were cloned at the multiple cloning sites of the reporter plasmids in positive or reverse orientation with respect to the promoter to create experimental constructs. Nested deletions of the cloned inserts were performed using Promega’s Erase-a-Base reagents and protocol (5). Plasmids pSV-b-galactosidase and pRL-TK (5) were used as transformation controls for the CAT-plasmids and the luciferase-plasmids, respectively. Cells were transfected (4) with the plasmid DNA with the Lipofectamine plus reagent (GIBCO-BRL). The plates were incubated at 37°C in a CO2- incubator for 24–48 h. For CAT assay, transfected cells were lysed by scrapping with a rubber policeman in Reporter Lysis Buffer (Promega, 500 μl/well). The cleared and inactivated lysate (200 μl) was assayed for CAT activity in a mix of 250 mM Tris–HCl, pH 7.5, 0.4 mM butyryl coenzyme A, and 125 nCi of [14C]chloramphenicol (50 mCi/mmol, NEN-Dupont) in a final volume of 250 μl (6). The reaction mix was incubated at 37°C for 20 h, extracted twice with 250 μl of ethyl acetate, and dried. The pellet was resuspended in 50 μl of ethyl acetate and loaded on thin-layer chromatography (TLC) plates. The TLC plates were developed for 2 h at room temperature in chloroform/ methanol (95/5, v/v) and exposed to X-ray film or AMBIS Radioanalytic system for analysis, as described (6). Beta-galactosidase was assayed with the Promega reagent kit following the recommended protocol (5). Each CAT activity result was normalized with respect to b-galactosidase activity. Normalized CAT activity was expressed relative to that obtained with pCAT-control vector (5). For luciferase assay, transfected cells were lysed with Passive Lysis Buffer (Promega, 100 μl/well) at room temperature for 15 min on a rocker platform. The clear lysate (20 μl) was added to firefly luciferase assay reagent (LAR II containing beetle luciferin and coenzyme A (Promega Dual Luciferase Assay System, 100 μl), and luminescence was assayed for 10 sec (after 2 sec pre-measurement delay) using a Turner Designs Model 20/20 Luminometer. Renilla luciferase assay reagent (Promega, 100 μl, diluted in Stop & Glo solution) was added to the same reaction tube, vortexed for 2 sec and luminescence was again assayed for 10 s. The data were computed automatically to yield the ratio of firefly luciferase activity over Renilla luciferase activity for each sample. There were 6–12 replicate wells per test plasmid DNA. Proteins in cleared lysates were assayed using modified Bradford reagents (Bio-Rad).
Electrophoretic mobility shift assay (EMSA)
The 221 bp Alurepeat containing sequence from the silencer segment was PCR amplified using Pfu DNA polymerase (Stratagene), identity confirmed by nucleotide sequencing and this fragment was used for EMSA (4, 6) as probe after end-labeling with T4 polynucleotide kinase and [γ-32P]ATP (4, 6). Nuclear extracts were prepared from different cultured cells following standard protocols (4) and 5 μg of the nuclear proteins was used per incubation with the probe at 4°C for 30 min. The DNA-protein complexes were separated from unbound probe by polyacrylamide gel electrophoresis, gel was dried and autoradiographed. Some reactions (20 μl each) contained 1 μg pBluescript KS (1) DNA as quencher of nonspecific DNA binding proteins. Specificity of binding was tested by pre-incubating (10 min) the proteins with 200 ng of unlabeled probe DNA.
Statistical analysis (7)
Data are expressed as mean 6 SE where appropriate. Results were statistically analyzed performing the Student’s t test (7). Differences were considered statistically significant when P was <0.05.
RESULTS AND DISCUSSION
Functional identification of the basal promoter for human BRCA2 gene
We have cloned a 1015-bp sequence (Fig. 1A) from the transcription start site of human BRCA2 gene. The DNA fragment was cloned in front of chloramphenicol acetyl transferase (CAT)- or luciferase genes in reporter plasmids, and breast cells were transiently transfected to evaluate whether this upstream sequence can drive the expression of these reporter genes. The 1015 bp upstream sequence of human BRCA2 gene could promote the expression of CAT gene in transfected MCF-10F cells (Fig. 1B). This property was dependent on the orientation of the upstream sequence with respect to the reporter gene, suggesting that unlike BRCA1 promoter, which is bidirectional (8), BRCA2 promoter is unidirectional. The promoter activity is enhanced when the 603-bp fragment from the distal end was removed (Fig. 1B). The conclusions drawn from our study have been verified independently using both CAT and luciferase reporter assay systems. The degree of enhancement was dependent on the presence or absence of SV40 enhancer in the reporter plasmid (Fig. 1B). The enhancement of activity was about 2-fold with pCATb plasmid construct whereas with pCATe plasmid construct, the enhancement was about 10 fold (Fig. 1B). This observation may suggest that, in addition to the silencer elements, there may be some enhancer elements present in the deleted 603-bp fragment. These elements may be common with the SV40 enhancer elements; thus, their absence in the pCATb-0.4 kb construct was compensated in the pCATe-0.4 kb construct. We are in the process of identification of such possible elements in this DNA segment.
Identification of an Alu-repeat-containing silencer at the upstream sequence
Removal of a 603 bp fragment from the distal end of the 1015-bp upstream sequence enhanced the promoter activity of this upstream sequence (Fig. 1B), suggesting the presence of silencer elements in the 603-bp DNA. We have tested whether this silencer can also work with SV40 late promoter/ enhancer system. We have following three conclusions from this study: (i) besides BRCA2 promoter, the silencer can also negatively regulate SV40 promoter (Fig. 1C); (ii) the ~250-bp region from the proximal end of the silencer is not necessary for this negative regulation of the SV40 promoter activity (Figs. 1C and 2A); and (iii) as revealed by Pustell DNA matrix analysis, the silencer has direct repeat in its sequence (Figs. 2B and 2C) and the repeat is that of a conserved Alu sequence (Figs. 2B and 2C).
FIG. 2.
Role of Alu-sequence in the silencer for the inhibition of promoter activities. (A) Mutational analysis of the silencer. The CAT activity was obtained in the extracts from MCF-10F cells transfected with different pCATcontrol constructs having the silencer sequence deletion mutant fragments. Alu sequences are shown by solid bars. Results are means ± SE (n = 6). (B) Nucleotide sequence of the 221 bp silencer region. The Alu-repeats are underlined. The consensus elements known to bind to Alu-binding protein are bold-faced. (C) Pustell DNA matrix analysis of the 221-bp DNA (with itself). The presence of direct repeat sequence in this DNA is indicated by two parallel lines above and below the identity diagonal.
Nested deletion mutagenesis of the 603 bp silencer fragment
To further understand the gross structure– function relationship in the silencer sequence and particularly to understand whether Alu-repeat is necessary for this negative regulatory property of the silencer, we performed nested deletion mutagenesis of the cloned silencer fragment. The preliminary results obtained with the nested deletion mutants of the silencer fragment are summarized in Fig. 2A. Alu elements in this segment appeared to be critical for negatively regulating the activity of SV40 promoter/enhancer system of pCATcontrol vector in transfected MCF-10F cells (Fig. 2A).
BRCA2 silencer (221 bp) is active in different human female breast cells but not in non-breast cells
To test whether the activity of the BRCA2 silencer element is active in breast cells in general, we transiently transfected four different lines of human breast cells and two lines of human non-breast cells with pGL3 plasmid constructs with or without the silencer (Fig. 3A) and assayed for luciferase activity in the extracts of the transfected cells. In addition to the non-malignant breast cells, MCF-10F, the silencer was also active in malignant breast cells like MDA-MB-231, MCF-7, BT- 549, and MDA-MB-435S cells. Similar to that in the MCF-10F cells, the activity of the silencer was independent of the orientation or the position of the elements with respect to the promoter (data not shown). The silencer was inactive in the non-breast cells like HeLa or HepG2 cells (Fig. 3B). The activity of SV40 promoter/enhancer system of the test plasmid (pLC) in these cells was 100- to 200-fold higher than that in the breast cells. Whether this high activity of the promoter in the non-breast cells contributed to the inactivity of the silencer needs to be determined. However, the cloned 400 bp BRCA2 promoter (Fig. 1A), whose activity in these non-breast cells was similar to that in the breast cells, also could not be silenced by the BRCA2 silencer in HepG2 or HeLa cells (data not shown). It is possible that the absence of breast cell specific BRCA2 silencer binding proteins in these non-breast cells (see below) was responsible for the inactivity of the silencer in these cells.
FIG. 3.
Activity of BRCA2 silencer (221 bp) in different female breast cells. (A) Sketch showing two pGL3 constructs used in this study to test the activity of the BRCA2 silencer in different human female breast cells. Constructs similar to pLCR with the silencer sequence in the other orientation or cloned near the promoter, were also constructed and used. (B) Relative luciferase activity in different human female breast cells transfected with pLC or pLCR. pRL-TK was used as the reference plasmid. Two nonbreast human cell lines, HepG2 and HeLa, were used as controls. The relative luciferase activities in the cell extracts of the transfected HepG2 or HeLa cells were 100- to 200-fold higher than those in the breast cells. The lysates were diluted 100- and 200-fold for HeLa and HepG2 cells, respectively, before luciferase assay. Results are means ± SE (n = 12).
Identification of specific Alu-repeat binding proteins in breast cells
Our studies by EMSA with the 221 bp Alu-repeat containing BRCA2 gene silencer fragment as probe and breast cell nuclear extracts as source for the binding proteins identified at least three protein– DNA complexes (Fig. 4). As expected, the protein/DNA complexes disappeared when the competitor unlabeled 221 bp Alu-repeat containing DNA was present in excess in the reaction mixture (Fig. 4). Addition of similar concentrations of unrelated pBluescript KS(1) DNA fragment did not affect the appearance of the protein/ DNA complexes (Fig. 4), further suggesting that the complexes are probe-specific. Although Alu-elements are proposed to be involved in DNA replication, transcriptional regulation and nuclear transport of signal recognition particle RNA, their precise physiological roles are not known (9). Proteins that bind to Alu element and Alu RNA have been identified in human cells (10–15). In HeLa cells, two proteins of 120 and 35 kDa specifically bind to Alu-elements (10, 11). The 35-kDa protein is localized exclusively in the nucleus, while the 120-kDa protein is distributed between nucleus and cytoplasm (10, 11). The 35-kDa protein is regulated by phosphorylation. Upon dephosphorylation, its DNA binding activity is significantly enhanced (10, 11). A 60-kDa protein from human sperm nuclei that specifically binds to Alu DNA-repeats has also been purified (12). The specific DNA binding site of this protein within the Alu-sequence has been mapped by methylation interference assay and EMSA (12). This sperm Alu binding protein selectively protects Alu elements from methylation in vitro and was suggested to be responsible for the un-methylated state of Alu sequences in the male germ line resulting in a parent specific differential inheritance of Alu-methylation (12). Recent report indicates that there is no methylation of CpG dinucleotides within the promoter of the BRCA2 gene in breast cells (16). Whether the silencer nuclear protein complexes, as shown in Fig. 4, are made up of multimers of the same protein or are complexes of different proteins needs to be determined.
FIG. 4.

Binding of nuclear proteins with the Alu-sequence. Autoradiogram of an EMSA gel showing specific binding of proteins from the nuclear extracts of MCF7 (lanes 1–4) and MDA-MB-231 cells (lanes 5–8) with the 221-bp BRCA2 silencer sequence. Lanes 1 and 5, radiolabeled probe only; lanes 2 and 6, radiolabeled probe incubated with nuclear extract (1 μg protein); lanes 3 and 7, nuclear extract preincubated for 10 min with 50-fold excess unlabeled silencer DNA before addition of the labeled probe; lanes 4 and 8, nuclear extract preincubated for 10 min with 50- fold excess unlabeled linearized pBluscript KS (1) DNA before addition of the labeled probe. The specific DNA–protein complexes are shown by arrows.
Possible mode of action of the BRCA2 gene silencer
Silencers are functionally defined as cis-acting regulatory DNA elements that down regulate gene transcription (17). They generally exhibit activity in either orientation, may be either position-dependent or -independent, and may or may not effect heterologous promoters (17). Some of the transcriptional silencers so far discovered were found to have conserved Alu-repeats (17–19). Alu-repeats are members of the SINE (Short Interspersed Element) family of human repetitive DNA sequences (9). They are approximately 300 bp long and interspersed throughout the human genome with an average spacing of 5 kb. The number of Alu-repeats is estimated at ~500,000 copies in the haploid human genome (9). The precise involvement of Alu-repeats in cellular metabolism is unclear, but they have been frequently identified in the transcriptional regulatory regions of a number of genes (9). Alu repeats have been associated with regulatory elements (in both silencers and enhancers) of a number of genes, including keratin 18, erythropoietin, CD8a, theta-1 globin, and the gamma chain Fc/T cell receptor genes (see Ref. 18). The mechanisms by which Alu-repeats are believed to modulate transcription are diverse. In some instances, Alu-repeat sequences mediate repression through transcriptional interference mechanisms, as demonstrated with epsilon-globin (20). Transcriptional interference results from transcription initiated at an internal RNA polymerase III promoter that is contained in a subset of Alu sequences (20). One cautionary note is that although our results strongly support the conclusion of our study, the data generated with the artificial constructs made with the BRCA2 silencer sequences may not reflects the real in vivo role of the silencer elements when it is in the chromosomal context. Further studies are underway to elucidate the role of BRCA2 silencer in the regulation of BRCA2 gene expression in human breast cells.
ACKNOWLEDGMENTS
We thank Drs. L. Matrisian and J. Ochieng for their suggestions on the growth and maintenance of human breast cells and Ms. Laura M. Selenke for carefully reading the manuscript. This work was supported by NIH Grants P30CA4909509S1, SG12RR0303412, and 1F31CA79035-01.
REFERENCES
- 1.Feunteun J. Mol. Med. Today. 1998;6:263–267. doi: 10.1016/s1357-4310(98)01262-3. [DOI] [PubMed] [Google Scholar]
- 2.Kinzler KW, Vogelstein B. Nature. 1997;386:761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 3.Ochieng J, Warfield P, Johnson KN. Am. J. Obstet. Gynecol. 1997;176:S240–S245. doi: 10.1016/s0002-9378(97)70382-x. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel M, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. Wiley; New York: 1994. [Google Scholar]
- 5.Doyle K. The Source for Discovery: Protocols and Application Guide. Promega Corp.; Madison, WI: 1996. [Google Scholar]
- 6.Seay MB, Heard PL, Chaudhuri G. Infect. Immun. 1996;64:5129–5137. doi: 10.1128/iai.64.12.5129-5137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell MJ, Machin D. Medical Statistics: A Commonsense Approach. Wiley; New York: 1994. [Google Scholar]
- 8.Xu C-F, Chambers JA, Solomon E. J. Biol. Chem. 1997;272:20994–20997. doi: 10.1074/jbc.272.34.20994. [DOI] [PubMed] [Google Scholar]
- 9.Deininger PL. In: Mobile DNA. Berg DE, Howe MM, editors. Am. Soc. Microbiol.; Washington, DC: 1989. pp. 619–636. [Google Scholar]
- 10.Perelygina LM, Tomilin NV, Podgornaya OI. Mol. Biol. Rep. 1987;12:111–116. doi: 10.1007/BF00368878. [DOI] [PubMed] [Google Scholar]
- 11.Chiang Y, Vishwanatha JK. Mol. Cell Biochem. 1996;155:131–138. doi: 10.1007/BF00229310. [DOI] [PubMed] [Google Scholar]
- 12.Chesnokov IN, Schmid CW. J. Biol. Chem. 1995;270:18539–18542. doi: 10.1074/jbc.270.31.18539. [DOI] [PubMed] [Google Scholar]
- 13.Cox GS, Gutkin DW, Haas MJ, Cosgrove DE. Biochim. Biophys. Acta. 1998;1396:67–87. doi: 10.1016/s0167-4781(97)00175-9. [DOI] [PubMed] [Google Scholar]
- 14.Kropotov A, Sedova V, Ivanov V, Sazeeva N, Tomlin A, Krutilina R, Li Oei S, Griesenbeck J, Buchlow G, Tomlin N. Eur. J. Biochem. 1999;260:336–346. doi: 10.1046/j.1432-1327.1999.00162.x. [DOI] [PubMed] [Google Scholar]
- 15.Luk’yanov DV, Reshetnikova GF, Podgornaya OI. Biochemistry (Moscow) 1999;64:17–24. [PubMed] [Google Scholar]
- 16.Collins N, Wooster R, Stratton MR. Br. J. Cancer. 1997;76:1150–1156. doi: 10.1038/bjc.1997.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogbourne S, Antalis TM. Biochem. J. 1998;331:1–14. doi: 10.1042/bj3310001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewitt SM, Fraizer GC, Saunders GF. J. Biol. Chem. 1995;270:17908–17912. doi: 10.1074/jbc.270.30.17908. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Bensadoun A. Biochim. Biophys. Acta. 1999;1436:390–404. doi: 10.1016/s0005-2760(98)00148-9. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Grindlay GJ, Bushel P, Mendelsohn L, Allan M. Mol. Cell. Biol. 1990;10:1209–1216. doi: 10.1128/mcb.10.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]