Abstract
Background
In rodents, exposure to intermittent hypoxia (IH), a hallmark of obstructive sleep apnea (OSA), is associated with neurobehavioral impairments, increased apoptosis in the hippocampus and cortex, as well as increased oxidant stress and inflammation. Excessive NADPH oxidase activity may play a role in IH-induced CNS dysfunction.
Methods and Findings
The effect of IH during light period on two forms of spatial learning in the water maze and well as markers of oxidative stress was assessed in mice lacking NADPH oxidase activity (gp91phox _/Y) and wild-type littermates. On a standard place training task, gp91phox _/Y displayed normal learning, and were protected from the spatial learning deficits observed in wild-type littermates exposed to IH. Moreover, anxiety levels were increased in wild-type mice exposed to IH as compared to room air (RA) controls, while no changes emerged in gp91phox _/Y mice. Additionally, wild-type mice, but not gp91phox _/Y mice had significantly elevated levels of NADPH oxidase expression and activity, as well as MDA and 8-OHDG in cortical and hippocampal lysates following IH exposures.
Conclusions
The oxidative stress responses and neurobehavioral impairments induced by IH during sleep are mediated, at least in part, by excessive NADPH oxidase activity, and thus pharmacological agents targeting NADPH oxidase may provide a therapeutic strategy in sleep-disordered breathing.
Introduction
Obstructive Sleep Apnea (OSA), a clinical syndrome characterized by repeated episodes of upper airway obstruction during sleep, is now recognized as a significant and highly prevalent health problem, due not only to its cardiovascular and metabolic morbidity, but also because of the prominent cognitive and behavioral implications of the disease. The neuropsychological impairments are accompanied by increased levels of systemic markers of oxidative stress and inflammation in addition to gray matter loss in neural sites contributing to cognitive function [1]–[5]. The inordinate sensitivity of neuronal tissues to alterations in oxygen homeostasis has led to the hypothesis that the behavioral consequences and cellular losses observed in OSA patients are produced, at least in part, by the episodic hypoxia-reoxygenation cycles during sleep that characterize OSA. In support of this hypothesis, rodent models have demonstrated that chronic exposure to intermittent hypoxia during the rest period (IH), in the absence of significant sleep fragmentation, is accompanied by neurodegenerative changes, increased oxidant stress and inflammation, and impaired spatial learning in the Morris water maze [6]–[18], and that genetic or pharmacological manipulations of oxidative stress pathways attenuated IH-induced deficits [18], [19].
NADPH oxidase has been primarily studied in the context of its role in phagocyte oxidative burst [20]. This enzyme has however emerged as a major source of ROS generation in mammalian cells, including the CNS [21]–[24], and a role for NADPH oxidase in astrocyte function has been described [25]. Moreover, it has been demonstrated that NADPH oxidase is expressed in neurons [26], [23] and localized at synapses [26]. NADPH oxidase is composed of two membrane-bound subunits (gp91phox and p22phox) and three cytosolic subunits, which include p47phox, p67phox, and Rac [27]. The membrane-bound subunits form a heterodimer that stabilizes them within the membrane, whereas the cytosolic subunits are recruited to the membrane following stimulation. Complete complex assembly is necessary for full NADPH oxidase activity [28]. Mutations in the gp91phox and p47phox genes are the most common mutations that cause chronic granulomatous disease (CGD; [29]). These mutations disable the NADPH oxidase complex, thereby preventing the oxidation of NADPH and the subsequent production of superoxide [30], [31], which is required for pathogen destruction as well as most superoxide-dependent signal transduction in nonphagocytic cells [27], [32], [33]. gp91phox [34] and p47phox [35] mutant mice have been generated and can therefore be used to explore the putative role of NADPH oxidase in murine models of sleep apnea.
In the present study, we examined this hypothesis in NADPH oxidase mutant mice (gp91phox-/Y) and wild type littermates by exposing them to IH, and assessed changes in hippocampus-dependent learning and memory tasks. In addition, we also tested other behavioral paradigms for anxiety and depression, since such problems are frequently encountered in patients with sleep apnea [36]–[41].
Materials and Methods
Animals
Male hemizygous gp91phox-/Y (B6.129S-Cybbtm1Din/J) mice (20–22 grams) and C57BL/6J mice (20–22 grams) were purchased from Jackson Laboratories (Bar Harbor, Maine), housed in a 12 hr light/dark cycle (lights on from 7:00 am to 7:00 pm) at a constant temperature (26±1°C). Mice were housed in groups of four in standard clear polycarbonate cages, and were allowed access to food and water ad libitum. All behavioral experiments were performed during the light period (between 9:00 am and 12:30 pm). Mice were randomly assigned to either IH or room air (RA) exposures. The experimental protocols were approved by the Institutional Animal Use and Care Committee and are in close agreement with the National Institutes of Health Guide in the Care and Use of. All efforts were made to minimize animal suffering and to reduce the number of animals used. Figure 1 summarizes the timeline of the treatments and the behavioral experiments. After each behavioral paradigm the mice were immediately returned back to their respective exposures.
Figure 1. Schematic diagram on the sequence of behavioral experiments and exposures to either IH or RA in both wild type and NADPH oxidase knock-out mice.
Intermittent Hypoxia Exposures
Animals were maintained in 4 identical commercially-designed chambers (30″x20″x20″; Oxycycler model A44XO, BioSpherix, Redfield, NY) operated under a 12 hour light-dark cycle (7:00 am–7:00 pm) for 14 days prior to behavioral testing. Oxygen concentration was continuously measured by an O2 analyzer, and was changed by a computerized system controlling gas outlets, as previously described [6], [42], [43], such as to generate oxyhemoglobin nadir values in the 65–72% range every 180 seconds. Ambient temperature was kept at 22–24°C.
Behavioral Testing
The Morris water maze was used to assess spatial reference learning and memory, as well as working memory. The maze protocol is similar to that described by Morris [44] with modifications for mice. The maze consisted of a white circular pool, 1.4 m in diameter and 0.6 m in height, filled to a level of 35 cm with water maintained at a temperature of 21°C (Morris 1984). Pool water was made opaque by addition of 150 ml of non-toxic white tempera paint. A Plexiglas escape platform (10 cm in diameter) was positioned 1 cm below the water surface and placed at various locations throughout the pool. Extramaze cues surrounding the maze were located at fixed locations, and visible to the mice while in the maze. Maze performance was recorded by a video camera suspended above the maze and interfaced with a video tracking system (HVS Imaging, Hampton, UK).
Briefly, a standard place-training reference memory task was conducted on mice in the water maze following exposure to 14 days of IH or RA. One day prior to place learning, mice were habituated to the water maze during a free swim. Place learning was then assessed over six consecutive days using a spaced training regimen that has been demonstrated to produce optimal learning in mice [45]. Each training session consisted of three trials separated by a 10 minute inter-trial interval (ITI). On a given daily session, each mouse was placed into the pool from 1 of 4 quasirandom start points (N, S, E or W) and allowed a maximum of 90 seconds to escape to the platform where the mice were allowed to stay for 15 sec. Mice that failed to escape were led to the platform. The position of the platform remained constant during the trials. 24 h following the final training session, the platform was removed for a probe trial to obtain measures of spatial bias. To assess the performance in the water maze, mean escape latencies and swim distance were analyzed.
Reference memory
Retention tests were carried out 14 days after acquisition of the task. In the retention test, performance in a single session (two trials) was assessed, and the mean average performance of the two trials was calculated.
Elevated plus maze (EPM)
The elevated plus maze (EPM) was used to assess anxiety. The basic measure is the animal preference for dark, enclosed places over bright, exposed places [46]. A 60 w light was placed above the apparatus and the test was video taped by an overhead camera. Mice were placed in the center of the maze facing a closed arm, and allowed to explore for 10 min in isolation. Each mouse received one videotaped trial. Mice prefer to enter into closed arms compared to open arms. Time spent in the dark area is viewed as avoidance or anxiety-like behavior. The following parameters were scored: (a) Percent time spent in open and closed arms; (b) number of entries to closed arms; (c) Time spent in the center. An arm entry was defined as the entry of all four feet into either one of the closed arm. Of note, the maze was cleaned with 30% ethanol between trials to remove any odor cues.
Forced swimming test (FST)
Briefly, mice were individually forced to swim in an open cylindrical container (diameter 14 cm, height 20 cm), with a depth of 15 cm of water at 25±1 °C. The immobility time, defined as the absence of escape-oriented behaviors, was scored during 6 min, as previously described [47], [48], [49]. Each mouse was judged to be immobile when it ceased struggling, and remained floating motionless in the water, making only those movements necessary to keep its head above water. The average percentage immobility was calculated by a blinded experimenter.
NADPH Oxidase Expression
qRT-PCR analysis of p47phox was performed using ABI PRISM 7500 System (Applied Biosystems, Foster City, CA). RNA from frontal cortex in IH and RA exposed mice was prepared with TRIZOL. cDNA synthesis was performed using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Ribosomal 18S rRNA was used as a reference gene to normalize the expression ratios for the gene of interest. Primer sequences were 5′-CAGCCAGCACTATGTGTACA-3′ and 5′-GAACTCGTAGATCTCGGTGAA-3′ for p47phox (91 bp). One microgram of total RNA was used to generate cDNA templates and TaqMan® Master Mix Reagent Kit (Applied Biosystems, Foster City, CA) was used to amplify and quantify the p47phox transcript in 25 µl reactions. Triplicate PCR reactions were performed in 96-well in parallel with the 18S rRNA. The steps involved in the reaction program included: the initial step of 2 minutes at 50°C; denaturation at 95°C for 10 min, followed by 45 thermal cycles of denaturation (15 seconds at 95°C) and elongation (1 min at 60°C). Expression values were obtained from the cycle number (Ct value) using the Biosystems analysis software. P47phox and 18S rRNA were performed in triplicates to determine the Ct-diff. These Ct values were averaged and the difference between the 18S Ct (Avg) and the gene of interest Ct (Avg) was calculated (Ct-diff). The relative expression p47phox was analyzed using the 2-ΔΔCT method. Quantitative results were expressed as the mean ±standard deviation (SD). Statistical significance was evaluated by the Student's t-test.
Measurement of NADPH oxidase activity
NADPH oxidase activity was measured using the standard cytochrome c reduction method, as described previously [50]. Isolated frontal cortices from wild type mice exposed to either IH or RA were homogenized in a RIPA buffer. The homogenate was subjected to centrifugation at 250 g for 10 min to remove cellular debris. Supernatant was then centrifuged at 20,000 g for 20 min at 4 °C to eliminate mitochondria, lysosomes, peroxisomes, Golgi membranes, and rough endoplasmic reticulum. The resulting supernatant was centrifuged at 100,000 g for 60 min at 4°C. The pelleted plasma membrane fraction containing Nox was dissolved in a buffer containing 8 mM piperazine-N,N'-bis 2-ethanesulfonic acid (pH 7.2), 100 mM KCl, 3 mM NaCl, 3.5 mM MgCl2, 1.25 mM EGTA, and proteolytic inhibitors. The solubilized membrane fraction samples were placed in a multiwell plate, and flavin adenine dinucleotide (FAD; 0.01 mM), acetylated cytochrome c (0.1 mM), and GTPγS (0.01 mM, as an activating agent for NADPH oxidase) were added to the samples. Superoxide dismutase (SOD; 100 U/ml) was included to block cytochrome c reduction in half of the samples. After the samples were kept at room temperature for 2 min, sodium dodecyl sulfate (0.1 mM) as an additional activating agent was added. After incubation for 3 min, NADPH (0.2 mM) was added to initiate cytochrome c reduction. Absorbance at 550 nm (A550) in samples incubated and not incubated with SOD was measured for 10 min on a FlexStation III microplate reader (Molecular Devices, Sunnyvale, CA, USA). NOX activity was calculated as the SOD-inhibitable reduction of cytochrome c. All chemicals were from Sigma (St. Louis, MO).
Lipid Peroxidation Assay
MDA-586 kits (OxisResearch, Portland OR) were used to measure the relative malondialdehyde (MDA) production, a commonly used indicator of lipid peroxidation (50), in frontal brain cortex according to the manufacturer's instructions. Briefly, after anesthesia with pentobarbital (50 mg/kg intraperitoneally), mice were perfused with 0.9% saline buffer for 5 minutes and the cortex was dissected, snap frozen in liquid nitrogen, and stored at –80°C until assay the following day. Cortical tissues were homogenized in 20 mM phosphate buffer (pH 7.4) containing 0.5 mM butylated hydroxytoluene to prevent sample oxidation. After protein concentration measurements, equal amounts of proteins (2.0–2.5 mg protein from each sample) were used in triplicate to react with chromogenic reagents at 45°C in 500 µL buffer for 1 to 2 hours. The samples were then centrifuged and clear supernatants measured at 586 nm. The level of MDA production was then calculated with the standard curve obtained from the kit according to the manufacturer's instructions.
8-hydroxydeoxyguanosine (8-OHDG)
Levels of 8-OHDG were measured in frontal brain cortex using a commercially available assay (Cell Biolabs, San Diego, CA). Briefly, cortical samples or 8-OHDG standards were first added to an 8-OHDG/BSA conjugate preabsorbed enzyme immunoassay plate. After a brief incubation, an anti–8-OHDG mAb was added, followed by an horseradish peroxidase-conjugated secondary antibody. The 8-OHDG content in the cortical samples was then determined by comparison with the 8-OHDG standard curve.
Data Analysis
To elucidate the nature of interactions between IH and RA conditions, all data were analyzed by one way ANOVA. First, overall statistical significance was determined for the entire training period between the treatment groups. In addition, two-way repeated measures ANOVA were used to analyze each trial blocks, followed by post-hoc Tukey tests. Similar statistical approaches were used to compare probe trial, reference memory, EPM and FST. For all comparisons, a p value <0.05 was considered to achieve statistical significance.
In all the experimental conditions, the data were divided into 6 blocks (containing 3 trials/day). We used a multivariate MANOVA model (SPSS software 11; Chicago) that included latency, pathlength and swim speed and Two between factors: (1) Groups (four levels): RA C57BL6J, IH C57BL6J, RA gp91phox _/Y and IH gp91phox _/Y (2) Condition (two levels): RA or IH. All F statistics are reported using Pillai's Trace. The interaction of three different factors, i.e., time, condition and group were determined using this mixed model repeated measures MANOVA.
Results
Spatial Learning Performance
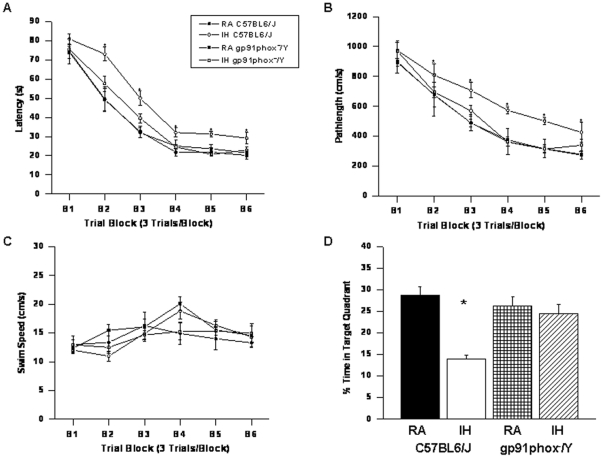
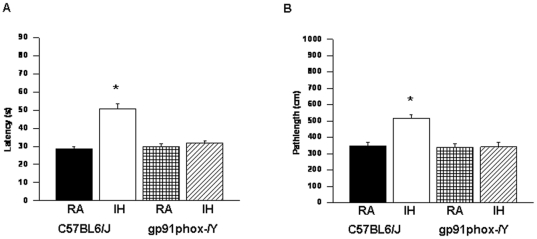
On a standard place discrimination task, wild type mice exposed to 14 days of IH (IH-C57BL6/J) exhibited longer latencies and pathlengths to locate the hidden platform when compared to room air controls RA-C57BL6/J, RA- gp91phox _/Y and gp91phox _/Y mice exposed to 14 days IH (IH- gp91phox _/Y) animals (n = 12 per experimental condition; Figures 2A and B). Overall latency analysis for the entire trial blocks revealed significant changes between the different treatment groups, [F = 41.14; p<0.001] and pathlength, [F = 16.44; p<0.001] indicating that IH adversely affected task performance. Significant differences in latencies were observed during blocks 2 [F = 4.91; p<0.006], 3 [F = 8.38; p<0.001], 4 [F = 3.35; p<0.03], 5 [F = 7.06; p<0.001] and 6 [F = 4.457; p<0.01]. There were no significant differences in Block 1. Repeated measures ANOVA revealed significant differences in pathlengths during blocks 3 [F = 5.25; p<0.004], 4 [F = 4.36; p<0.01], 5 [F = 6.73; p<0.001] and 6 [F = 2.99; p<0.04], with no significant differences in Blocks 1 and 2. There were no significant differences in swim speed in these mice. In the probe-trial test, one-way ANOVA revealed a significant effect of treatment [IH vs. RA: F = 12.87; p<0.001]. The magnitude of impairment was greatest in IH-C57BL6/J mice (Figure 2C). In the reference memory tests, IH-C57BL6/J mice exhibited significant deficits in memory retention in both latency [F = 19.61; p<0.001] and pathlength [F = 12.84; p<0.001]. However, the IH- gp91phox _/Y mice performed similar to normoxic controls (Figure 3).
Figure 2. gp91phox_/Y mice exposed to IH do not exhibit any deficits in learning and memory functions.
(A and B) Mean latencies (s) and pathlengths (cm) to locate the target platform during place training in C57BL6/J and gp91phox _/Y either exposed to intermittent hypoxia (IH) or maintained in room air (RA) (n = 12 per group. (C) Swim Speed (D) Mean percentage time in the target quadrant during probe trial after completion of water maze testing in either C57BL6/J and gp91phox _/Y exposed to IH or maintained in RA. (n = 12/experimental group; *P<0.05).
Figure 3. gp91phox_/Y mice exposed to IH do not exhibit any deficits in retention.
(A) Mean latencies (s) and (B) pathlengths (cm) to locate the target platform during retention in C57BL6/J and gp91phox _/Y either exposed to intermittent hypoxia (IH) or maintained in room air (RA) during retention of the Morris water maze task. (n = 12/experimental group; *P<0.05).
Repeated measures MANOVA with latency, groups and conditions [F(5,39) = 72.54; P<0.0001]; revealed that RA gp91phox _/Y and RA C57BL6/J mice required significantly less time than their littermates exposed to IH to find the hidden platform in a Morris water maze (Figure 2A); Repeated measures MANOVA with pathlength, groups and conditions [F(5,39) = 21.409; P<0.0001]; indicated that as the training progressed the RA gp91phox _/Y and RA C57BL6/J mice could reach the hidden platform and covered the shortest distance when compared to the distance covered by their littermates exposed to IH in a Morris water maze (Figure 2B). In addition repeated measures MANOVA with swim speed, groups and conditions on the swim speed showed no significant differences between the groups and treatments (Figure 2C).
Elevated Plus Maze
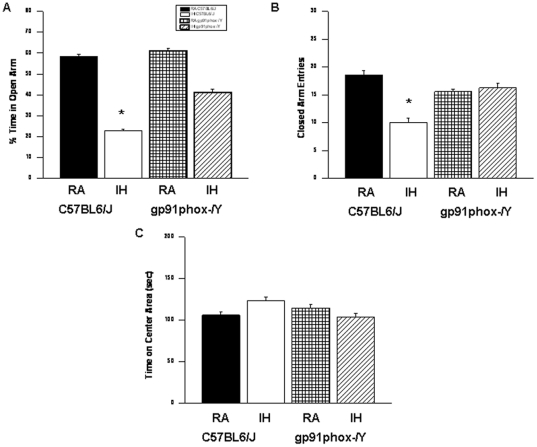
IH-C57BL6/J mice showed significant differences in the percentage of time spent in the open arm [F = 64.13; p<0.001] and in the number of entries into the closed arm [F = 14.74; p<0.001] (Figure 4). The results of the elevated plus maze showed that IH-C57BL6J spent significantly less time in the open arms (Figure 4; group effect, [F = 18.354; p<0.001] and significantly more time in the center area (Figure 4; group effect, [F = 26.945; p<0.001]. The number of entries into the closed arms was significantly increased (Figure 4; condition effect, [F = 11.533; p<0.001].
Figure 4. Exposure to IH induces anxiety in mice.
C57BL6/J mice exposed to IH spend significantly less time in the open arm of the elevated plus maze compared to RA C57BL6/J, or gp91phox _/Y mice exposed to either RA or IH (A). A reduced number of closed-arm entries emerged in wild type mice exposed to IH (B). (C) Time spend in the Center Area was increased in wild type mice exposed to IH (n = 12/experimental group; *P<0.05).
Although, the percentage of time spent in the open arm is commonly used as a measure of anxiety, the time spent on the center platform of the maze and the closed arm entries all reflect anxiety-like behaviors in mice. [79], [87].
Forced Swim Test
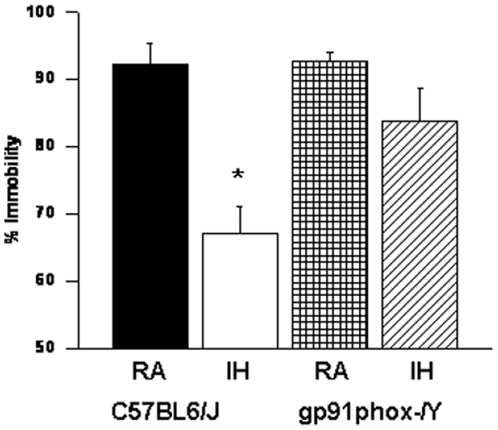
IH-C57BL6/J mice had significantly higher immobility durations during the last 4 min of the FST [F = 25.54; p<0.001] when compared to all other treatment groups, including IH- gp91phox _/Y (Figure 5).
Figure 5. Forced-swim test indicates gp91phox_/Y mice are not depressed following IH.
gp91phox _/Y exposed to IH show less immobility as compared to C57BL6/J mice exposed to IH. *P<0.05. See text for more details.
NADPH Oxidase Expression and Activity
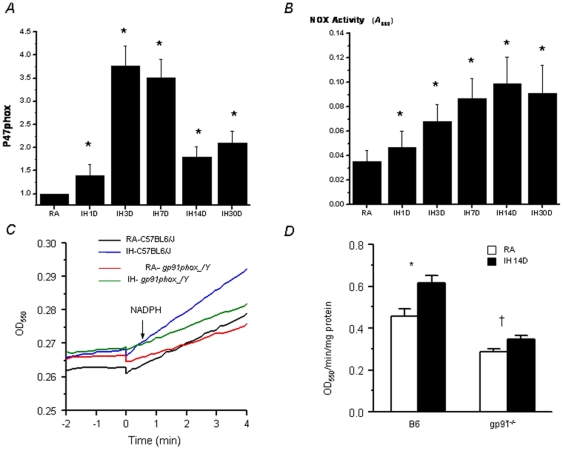
Frontal cortical tissues from IH- and RA-exposed mice were subjected to quantitative RT-PCR. P47phox expression was increased in IH starting at 3 days and sustained thereafter (Figure 6a). Similarly, NADPH oxidase activity was significantly increased in IH-exposed wild type mice (Figure 6b and 6d). Furthermore, such IH-induced increases of NOX activity were attenuated in gp91-/- mice, although the increase was still significant (p = 0.024; n = 6/group; Figure 6c and 6d).
Figure 6. Changes in NADPH oxidase expression and activity.
(A). Changes in P47phox mRNA expression in frontal cerebral cortex in wild type mice exposed to IH (n = 8 per experimental group; *P<0.05). (B). Changes in NADPH oxidase activity in frontal cerebral cortex in wild type mice exposed to IH (n = 8 per experimental group; *P<0.05). (C) Kinetic NADPH oxidase activities in hippocampus measured as NADPH-dependent cytochrome c reduction. Shown are representative tracings from the four experimental groups. (D) Summary of NADPH oxidase activities in hippocampus. IH resulted in a substantial increase in NOX activities in wild type mice (*P = 0.002). Such IH-induced increases of NADPH oxidase activity were much attenuated in gp91phox _/Y mice, although the increase was still significant (†P = 0.024; n = 6 for each group).
Lipid Peroxidation
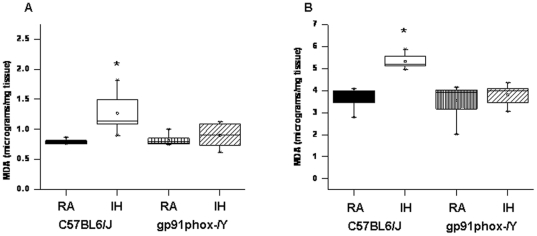
After the behavioral experiments, cortical tissues and hippocampus were harvested and processed for assessment of lipid peroxidation as indicated by MDA levels. Figure 7 shows MDA concentrations in homogenates of cerebral cortex from all treatment groups. A significant increase in MDA levels was observed in IH-C57BL6/J mice [F = 10.38; p<0.001] in the cortex and [F = 35.416; p<0.001] in the hippocampus when compared to all other groups.
Figure 7. Lipid peroxidation was reduced in the frontal cortex of gp91phox_/Y exposed to IH.
MDA tissue levels in cortex and hippocampus of gp91phox _/Y and C57BL6/J mice exposed to either room air (RA) or intermittent hypoxia for 14 days (IH). (n = 6 per experimental group; *P<0.05).
8-OHDG Levels
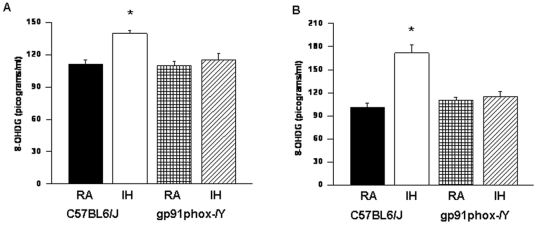
The levels of 8-OHDG in homogenates of cerebral cortex and the hippocampus were significantly higher in IH-C57BL6/J mice [F = 32.50; p<0.001] and [F = 22.214; p<0.001] respectively; when compared to all other groups (Figure 8). However there were no significant differences in the levels of 8-OHDG in cortex of IH- gp91phox _/Y when compared to RA controls.
Figure 8. Oxidative DNA damage was present in the cortex of gp91phox_/Y exposed to IH.
8-OHDG levels in cortex and hippocampus of gp91phox _/Y and C57BL6/J mice exposed to either room air (RA) or intermittent hypoxia for 14 days (IH). (n = 6 per experimental group; *P<0.05).
Discussion
OSA is a clinical condition that is now recognized as having important neurobehavioral consequences that stem, at least in part, from pathological inflammatory processes that are associated with the disease [51]–[53]. During sleep, patients with OSA undergo repeated periods of IH, which essentially correspond to recurring episodes of hypoxia and reoxygenation, leading to increased levels of ROS that are considered as contributing to end-organ injury, including the CNS [54]–[57]. In the present study, we provide conclusive results suggesting that increased activity of NADPH oxidase-mediated oxidative stress pathways mediates, at least in part the effects of IH on cognitive and behavioral functions. Reactive oxygen species (ROS) can be generated from various subcellular compartments, including mitochondria, the cellular membrane, lysosomes, peroxisomes, and the endoplasmic reticulum [24], [58]–[61]. For example, NADPH oxidase [24], [62], [63], xanthine oxidase [63], phospholipase A2 [64], lipoxygenases and cyclooxygenase [58], and cytochrome P450 [65] have all been identified as sources of ROS in various subcellular compartments under both physiological and pathological conditions. Although NADPH oxidase is primarily expressed in phagocytic cells, increasing evidence suggests that various subunits of NADPH oxidase are also expressed in nonphagocytic cells such as sympathetic ganglion neurons and cortical neurons [66]–[68], thereby supporting the conceptual framework that neurons in general express NADPH oxidase [69]. In this context, NADPH oxidase has been implicated in conditions that remotely resemble hypoxia-reoxygenation [70]–[76]. Activation of NADPH oxidase leads to generation of the superoxide ion (O− 2), a ROS which can be converted to the highly reactive hydroxyl radical and to peroxynitrite, a highly damaging RNS [77], [78]. To date, five NADPH oxidase enzyme (NOX) isoforms have been identified (NOX 1–5), and localization studies have shown that of the five NOX enzymes, the NOX2 and NOX4 isoforms are highly localized in the hippocampus CA1 and cerebral cortex [79], [80]. Our study provides insight into the functional role of NADPH oxidase activation and its contribution to intermittent hypoxia induced cognitive deficits. Our studies in the Morris Water Maze revealed preservation of spatial learning and memory in gp91phox _/Y mice as compared to the wild type mice after exposures to intermittent hypoxia. Furthermore, swim speed was not different between the groups, demonstrating that the differences were not due to differences in locomotor ability or coordination. The Morris Water Maze is a hippocampal-dependent test of learning and memory, and thus the enhanced performance on the Morris Water Maze of the gp91phox _/Y mice after IH, but not in normoxic conditions, is likely reflective of the attenuation of oxidative stress and enhanced neuronal survival in the hippocampus.
As would be anticipated from such considerations, we indeed found that IH exposures markedly increased the expression and activity of NADPH oxidase in wild type mice, thereby confirming the assumption that conditions mimicking the oxygenation patterns of sleep apnea induce activation of NADPH oxidase in cortical brain regions. While we can not infer from our current findings on the cellular source of NADPH oxidase contribution to IH-induced cognitive and behavioral deficits, the near complete abrogation of such deficits in the gp91phox _/Y mice clearly and conclusively assigns a critical role for NADPH oxidase in this context. Moreover, NADPH oxidase inhibition is neuroprotective of hippocampus CA1 pyramidal cells 7 days after ischemia [81]–[83]. Of note, we have previously shown that the IH-induced cognitive and behavioral deficits are attenuated by reductions in oxidative stress and inflammatory signaling cascades through pharmacological interventions [9], [10], as well as through attenuation of oxidative stress via targeted genetic manipulations of manganese superoxide dismutase, platelet-activating factor receptor, or nitric oxide synthase [16], [18], [19].
Depressive and anxiety symptoms are frequent in OSA patients [40], [84], [85]. Moreover, depression may account for the fatigue seen in OSA patients, even after OSA severity has been controlled [38]. The prefrontal cortex is particularly vulnerable to sleep disruption, and hypoxemia further creates an unfavorable cellular environment for the restorative processes to occur [2]. The elevated plus-maze is the most frequently utilized animal model for assessing anxiety-like behaviors [86], [87] since it enables researchers to observe the conflict between two innate rodent behaviors, namely the avoidance of open space exposure as countering the tendency to explore novel environments [87]. Our results show that intermittent hypoxia modified anxiety-like behavior in wild type mice. In contrast, gp91phox _/Y exposed to intermittent hypoxia showed preserved performances in this test, suggesting that regions underlying these behavioral patterns are susceptible to IH, most likely via the oxidant stress mediated by activation of NADPH oxidase. Our findings are not surprising considering the previous reports on the palliative effect of apocynin, a putative NADPH oxidase antagonist, on neuronal viability in the context of intermittent hypoxia [88]. Furthermore, Veasey and collaborators further expanded on the critical role played by NADPH oxidase in the injury to locus coeruleus neurons and the excessive sleepiness that developed as a consequence of IH during sleep [89], [90].
The cumulative evidence supports the assumption that the structural and neurobehavioral consequences of IH exposures in adult rodents involve a number of interrelated pathways, namely glutamate excitoxicity, oxidative stress, mitochondrial dysfunction, up-regulation of pro-inflammatory mediators, and altered regulation of pro- and anti-apoptotic gene cascades [91]–[93]. However, the explicit mechanistic involvement of NADPH oxidase in the CNS end-organ injury was not thoroughly explored. We propose that activation of NADPH oxidase by IH would be lead to the observed increases in oxidative stress markers. Earlier findings from our laboratory showed that intermittent hypoxia increases NADPH oxidase subunit protein expression in hypoxia sensitive brain regions involved in learning and memory in rats, and that administration of green tea polyphenols in drinking water attenuated the increase in NADPH oxidase gene expression under IH conditions [94].
In summary, we have shown that excessive NADPH oxidase-mediated superoxide release induced by IH contributes to the cellular damage and consequent behavioral impairments associated with severe forms of OSA. This study suggests that NADPH oxidase may be a promising target for OSA treatment, especially in halting the progression of OSA-associated cognitive and behavioral morbidities.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by National Institutes of Health grant HL-086662. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Montplaisir J, Bédard MA, Richer F, Rouleau I. Neurobehavioral manifestations in obstructive sleep apnea syndrome before and after treatment with continuous positive airway pressure. Sleep. 1992;15:S17–9. doi: 10.1093/sleep/15.suppl_6.s17. [DOI] [PubMed] [Google Scholar]
- 2.Beebe DW, Gozal D. Obstructive sleep apnea and prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 3.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–7. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 4.Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–7. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 5.Gozal D, McLaughlin Crabtree V, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188–193. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001a;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gozal E, Row BW, Schurr A, Gozal D. Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat. Neurosci Lett. 2001b;305:197–201. doi: 10.1016/s0304-3940(01)01853-5. [DOI] [PubMed] [Google Scholar]
- 8.Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res. 2002;52:449–53. doi: 10.1203/00006450-200209000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, et al. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med. 2003;168:469–75. doi: 10.1164/rccm.200211-1264OC. [DOI] [PubMed] [Google Scholar]
- 10.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 2003;167:1548–53. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- 11.Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D. Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res. 2007;177:308–314. doi: 10.1016/j.bbr.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldbart AD, Row BW, Kheirandish L, Cheng Y, Brittian KR, et al. High fat/refined carbohydrate diet enhances the susceptibility to spatial learning deficits in rats exposed to intermittent hypoxia. Brain Res. 2006;1090:190–196. doi: 10.1016/j.brainres.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 13.Kheirandish L, Row BW, Li RC, Brittian KR, Gozal D. Apolipoprotein E deficient mice exhibit increased vulnerability to intermittent hypoxia-induced spatial learning deficits. Sleep. 2005;28:1412–1417. doi: 10.1093/sleep/28.11.1412. [DOI] [PubMed] [Google Scholar]
- 14.Ramanathan L, Gozal D, Siegel JM. Chronic hypoxia leads to oxidative stress in the rat cerebellum and pons. J Neurochem. 2005;93:47–52. doi: 10.1111/j.1471-4159.2004.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Chi L, Row BW, Xu R, Ke Y, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 16.Row BW, Kheirandish L, Li RC, Hardie M, Bazan NG, et al. Platelet-activating factor receptor deficient mice are protected from experimental sleep apnea-induced spatial learning deficits. J Neurochem. 2004;89:189–196. doi: 10.1111/j.1471-4159.2004.02352.x. [DOI] [PubMed] [Google Scholar]
- 17.Gozal D, Nair D, Goldbart AD. Physical activity attenuates intermittent hypoxia-induced spatial learning deficits and oxidative stress. Am J Respir Crit Care Med. 2010;182:104–12. doi: 10.1164/rccm.201001-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, et al. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis. 2004;17(1):44–53. doi: 10.1016/j.nbd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Shan X, Chi L, Ke Y, Luo C, Qian SY, et al. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol Dis. 2007;28(2):206–215. doi: 10.1016/j.nbd.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 21.Dringen, R Oxidative and antioxidative potential of brain microglial cells. Antioxid. Redox Signal. 2005;7:1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- 22.Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–8. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 23.Vallet, P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, et al. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 24.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 25.Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, et al. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tejada-Simon M, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, et al. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumimoto H, Ueno N, Yamasaki T, Taura M, Takeya R. Molecular mechanism underlying activation of superoxide-producing NADPH oxidases: roles for their regulatory proteins. Jpn J Infect Dis. 2004;57:S24–S25. [PubMed] [Google Scholar]
- 28.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 29.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79(3):155–69. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Lomax KJ, Leto TL, Nunoi H, Gallin JI, Malech HL. Recombinant 47-kilodalton cytosol factor restores NADPH oxidase in chronic granulomatous disease. Science. 1989;245:409–412. doi: 10.1126/science.2547247. [DOI] [PubMed] [Google Scholar]
- 31.Royer-Pokora B. Novel approaches for cloning human genes: the chronic granulomatous disease (CGD). Dis. Markers. 1987;5:57–58. [PubMed] [Google Scholar]
- 32.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 33.Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 35.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan R. Obstructive sleep apnoea and depression – diagnostic and treatment implications. Aust N Z J Psychiatry. 1992;26:586–91. doi: 10.3109/00048679209072093. [DOI] [PubMed] [Google Scholar]
- 37.Borak J, Cieslicki JK, Koziej M, Matuszewski A, Zielinski J. Effects of CPAP treatment on psychological status in patients with severe obstructive sleep apnoea. J Sleep Res. 1996;5:123–7. doi: 10.1046/j.1365-2869.1996.d01-60.x. [DOI] [PubMed] [Google Scholar]
- 38.Bardwell WA, Ancoli-Israel S, Dismdale JE. Types of coping strategies are associated with increased depressive symptoms in patients with obstructive sleep apnea. Sleep. 2001;24:905–9. doi: 10.1093/sleep/24.8.905. [DOI] [PubMed] [Google Scholar]
- 39.Sánchez AI, Buela-Casal G, Bermúdez MP, Casa-Maldonado F. The effects of continuous positive air pressure treatment on anxiety and depression levels in apnea patients. Psychiatry Clin Neurosci. 2001;55:641–6. doi: 10.1046/j.1440-1819.2001.00918.x. [DOI] [PubMed] [Google Scholar]
- 40.Akashiba T, Kawahara S, Akahoshi T. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest. 2002;122:861–5. doi: 10.1378/chest.122.3.861. [DOI] [PubMed] [Google Scholar]
- 41.El-Sheikh M, Kelly RJ, Buckhalt JA, Hinnant JB. Children's sleep and adjustment over time: the role of socioeconomic context. Child Development. 2010;81:870–883. doi: 10.1111/j.1467-8624.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- 42.Row BW, Kheirandish L, Li RC, Hardie M, Bazan NG, et al. Platelet-activating factor receptor deficient mice are protected from experimental sleep apnea-induced spatial learning deficits. J Neurochem. 2004;89:189–196. doi: 10.1111/j.1471-4159.2004.02352.x. [DOI] [PubMed] [Google Scholar]
- 43.Kheirandish L, Row BW, Li RC, Brittian KR, Gozal D. Apolipoprotein E deficient mice exhibit increased vulnerability to intermittent hypoxia-induced spatial learning deficits. Sleep. 2005;28:1412–1417. doi: 10.1093/sleep/28.11.1412. [DOI] [PubMed] [Google Scholar]
- 44.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 45.Gerlai R, Clayton NS. Analysing hippocampal function in transgenic mice: an ethological perspective. Trends Neurosci. 1999;22:47–51. doi: 10.1016/s0166-2236(98)01346-0. Review. [DOI] [PubMed] [Google Scholar]
- 46.Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48:254–60. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- 47.Eckeli AL, Dach F, Rodrigues ALS. Acute treatment with GMP produces antidepressant-like effects in mice, Neuro Rep. 2000;11:1839–1843. doi: 10.1097/00001756-200006260-00008. [DOI] [PubMed] [Google Scholar]
- 48.Zomkowsi ADE, Rosa AO, Lin J, Santos ARS, Calixto JB, et al. Evidence for serotonin receptor subtypes involvement in agmatine antidepressant like-effect in the mouse forced swimming test, Brain Res. . 2004;1023:256–263. doi: 10.1016/j.brainres.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 49.Kaster MP, Raupp I, Binfaré RW, Andreatini R, Rodrigues SAC. Antidepressant-like effect of lamotrigine in the mouse forced swimming test: evidence for the involvement of noradrenergic system. Euro J Pharmacol. 2007;565:119–124. doi: 10.1016/j.ejphar.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Miller FJ, Jr, Griendling KK. Functional evaluation of nonphagocytic NAD(P)H oxidases, Methods Enzymol. 2002;353:220–233. doi: 10.1016/s0076-6879(02)53050-0. [DOI] [PubMed] [Google Scholar]
- 51.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. . Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 52.Day R, Gerhardstein R, Lumley A, Roth T, Rosenthal L. The behavioral morbidity of obstructive sleep apnea. Prog Cardiovasc Dis. 1999;41:341–354. doi: 10.1053/pcad.1999.0410341. [DOI] [PubMed] [Google Scholar]
- 53.Bass JL, Corwin M, Gozal D, Moore C, Nishida H, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–816. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- 54.Gozal D, Kheirandish-Gozal L. The multiple challenges of obstructive sleep apnea in children: morbidity and treatment. Curr Opin Pediatr. 2008;20:654–658. doi: 10.1097/MOP.0b013e328316ec2d. [DOI] [PubMed] [Google Scholar]
- 55.Jun J, Savransky V, Nanayakkara A, Bevans S, Li J, et al. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1274–R1281. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, et al. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol. 2007;102:557–56. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 57.Angermüller S, Islinger M, Völkl A. Peroxisomes and reactive oxygen species, a lasting challenge. Histochem Cell Biol. 2009;131:459–63. doi: 10.1007/s00418-009-0563-7. [DOI] [PubMed] [Google Scholar]
- 58.Dröge W. The plasma redox state and ageing. Ageing Res Rev. 2002;1(2):257–78. doi: 10.1016/s1568-1637(01)00008-3. Review. [DOI] [PubMed] [Google Scholar]
- 59.Kubota H, Fujioka Y, Yoshino H, Koji H, Yoshihara K. Cardiac swinging calcified amorphous tumors in end-stage renal failure patients. Ann Thorac Surg. 2010;90:1692–4. doi: 10.1016/j.athoracsur.2010.04.097. [DOI] [PubMed] [Google Scholar]
- 60.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–27. doi: 10.1089/ars.2009.2625. Review. [DOI] [PubMed] [Google Scholar]
- 61.Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muralikrishna Adibhatla R, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med; 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 64.Yasui H, Hayashi S, Sakurai H. Possible involvement of singlet oxygen species as multiple oxidants in p450 catalytic reactions. Drug Metab Pharmacokinet. 2005;20:1–13. doi: 10.2133/dmpk.20.1. [DOI] [PubMed] [Google Scholar]
- 65.Fukui T, Lassegue B, Kai H, Alexander RW, Griendling KK. Cytochrome b-558 alpha-subunit cloning and expression in rat aortic smooth muscle cells. Biochim Biophys Acta. 1995;1231:215–219. doi: 10.1016/0005-2728(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 66.Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:RC111(1–5). doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000;20:RC53(1–5). doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 69.Jang HJ, Hwang S, Cho KY, Kim do K, Chay KO, et al. Taxol induces oxidative neuronal cell death by enhancing the activity of NADPH oxidase in mouse cortical cultures. Neurosci Lett. 2008;443:17–22. doi: 10.1016/j.neulet.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 70.Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–63. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, et al. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci. 2009;29:2545–52. doi: 10.1523/JNEUROSCI.0133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Groeger G, Mackey AM, Pettigrew CA, Bhatt L, Cotter TG. Stress-induced activation of Nox contributes to cell survival signalling via production of hydrogen peroxide. J Neurochem. 2009;109:1544–54. doi: 10.1111/j.1471-4159.2009.06081.x. [DOI] [PubMed] [Google Scholar]
- 73.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–63. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, et al. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12:1371–82. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ha JS, Lee JE, Lee JR, Lee CS, Maeng JS, et al. Nox4-dependent H2O2 production contributes to chronic glutamate toxicity in primary cortical neurons. Exp Cell Res. 2010;316:1651–61. doi: 10.1016/j.yexcr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 76.Baran AS, Richert AC. Obstructive sleep apnea and depression. CNS Spectr. 2003;8(2):128–34. doi: 10.1017/s1092852900018356. [DOI] [PubMed] [Google Scholar]
- 77.Maneen MJ, Cipolla MJ. Peroxynitrite diminishes myogenic tone in cerebral arteries: role of nitrotyrosine and F-actin. Am J Physiol Heart Circ Physiol. 2007;292:H1042–1050. doi: 10.1152/ajpheart.00800.2006. [DOI] [PubMed] [Google Scholar]
- 78.Nanetti L, Taffi R, Vignini A, Moroni C, Raffaelli F, et al. Reactive oxygen species plasmatic levels in ischemic stroke. Mol Cell Biochem. 2007;303:19–25. doi: 10.1007/s11010-007-9451-4. [DOI] [PubMed] [Google Scholar]
- 79.Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 80.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, et al. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 81.Raz L, Zhang QG, Zhou CF, Han D, Gulati P, et al. Role of Rac1 GTPase in NADPH oxidase activation and cognitive impairment following cerebral ischemia in the rat. PLoS One. 2001;2010 5:e12606. doi: 10.1371/journal.pone.0012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen H, Song YS, Chan PH. J Cereb Blood Flow Metab; 2009. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang QG, Raz L, Wang R, Han D, De Sevilla L, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64:1195–2000; quiz, 1274-6. doi: 10.4088/jcp.v64n1009. [DOI] [PubMed] [Google Scholar]
- 85.File SE. Behavioural detection of anxiolytic action. In: Elliot JM, Heal DJ, Marsden CA, editors. Experimental Approaches to Anxiety and Depression. New York: John Wiley & Sons;; 1992. pp. 25–44. [Google Scholar]
- 86.Pellow S, Chopin P, File SE, Briley M. Validation of open-closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 87.Hui-guo L, Kui L, Yan-ning Z, Yong-jian X. Apocynin attenuate spatial learning deficits and oxidative responses to intermittent hypoxia. Sleep Med. 2010;11:205–212. doi: 10.1016/j.sleep.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 88.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 89.Zhan G, Serrano F, Fenik P, Hsu R, Kong L, et al. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 2005;172:921–929. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goldbart A, Row BW, Kheirandish L, Schurr A, Gozal E, et al. Intermittent hypoxic exposure during light phase induces changes in cAMP response element binding protein activity in the rat CA1 hippocampal region: water maze performance correlates. Neuroscience. 2003;122:585–90. doi: 10.1016/j.neuroscience.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 91.Gozal D, Row BW, Kheirandish L, Liu R, Guo SZ, et al. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. J Neurochem. 2003;86:1545–52. doi: 10.1046/j.1471-4159.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- 92.Gozal E, SachlebenJr LR, Guo SZ, Gozal D. Differential NMDA glutamate receptor changes following sustained or intermittent hypoxia in the rat brain. Sleep. 2002;25:A333. [Google Scholar]
- 93.Burckhardt IC, Gozal D, Dayyat E, Cheng Y, Li RC, et al. Green tea catechin polyphenols attenuate behavioral and oxidative responses to intermittent hypoxia. Am J Respir Crit Care Med. 2008;177:1135–41. doi: 10.1164/rccm.200701-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]