Abstract
Three novel insertion sequences (ISs) (ISPso1, ISPso2, and ISPso3) of the soil bacterium Paracoccus solventivorans DSM 11592 were identified by transposition into entrapment vector pMEC1. ISPso1 (1,400 bp) carries one large open reading frame (ORF) encoding a putative basic protein (with a DDE motif conserved among transposases [Tnps] of elements belonging to the IS256 family) with the highest levels of similarity with the hypothetical Tnps of Rhodospirillum rubrum and Sphingopyxis macrogoltabida. ISPso2 (832 bp) appeared to be closely related to ISPpa2 of Paracoccus pantotrophus DSM 11072 and IS1248 of Paracoccus denitrificans PdX22, both of which belong to the IS427 group (IS5 family). These elements contain two overlapping ORFs and a putative frameshift motif (AAAAG) responsible for production of a putative transframe Tnp. ISPso3 (1,286 bp) contains a single ORF, whose putative product showed homology with Tnps of ISs classified as members of a distinct subgroup of the IS5 group of the IS5 family. The highest levels of similarity were observed with ISSsp126 of Sphingomonas sp. and IS1169 of Bacteroides fragilis. Analysis of the distribution of ISs of P. solventivorans revealed that ISPso2-like elements are the most widely spread of the elements in nine species of the genus Paracoccus. ISPso1 and ISPso3 are present in only a few paracoccal strains, which suggests that they were acquired by lateral transfer. Phylogenetic analysis of Tnps of the novel ISs and their closest relatives showed their evolutionary relationships and possible directions of lateral transfer between various bacterial hosts.
The classification of the genus Paracoccus (alpha subgroup of the Proteobacteria) has undergone serious changes during the past decade. Several new species have been isolated, and the status of other species has been reevaluated. Currently, the genus consists of 17 species, which are found in different environments. Some of these species, including Paracoccus alcaliphilus (38) P. carotinifaciens (36), P. aminophilus, P. aminovorans (37), and P. kondratievae (11), were isolated from soil. Other species were isolated from environments containing a range of toxic components; e.g., P. alkenifer was isolated from biofilters used in the treatment of waste gases from an animal rendering plant (22), P. methylutens was isolated from groundwater contaminated with dichloromethane (12), P. pantotrophus was isolated from a sulfide-oxidizing, denitrifying fluidized-bed reactor in a plant (30), and P. kocurii was isolated from wastewater from semiconductor manufacturing processes (26). Some strains of P. denitrificans, which was the first Paracoccus species isolated (4), have also been found in a number of different habitats, including sewage, sludge, horse manure, and cow dung (20), as well as in soil. Bacteria belonging to the genus Paracoccus are probably also important components of many wastewater treatment system communities (25). The number of known habitats for bacteria belonging to the genus Paracoccus has expanded. Two new species were isolated recently from the marine environment; P. seriniphilus was isolated from the marine bryozoan Bugula plumosa (27), and P. zeaxanthinifaciens was isolated from seaweed from the coast of the African Red Sea (5). Furthermore, the first paracoccal species associated with human infection (P. yeei) was isolated from the dialysate of a patient with peritonitis (8). These bacteria thus appear to be more widespread than was previously thought.
Paracocci are among the most metabolically versatile bacteria. They are chemoorganoheterotrophs (utilizing a wide variety of organic compounds, including potentially polluting compounds, like acetone) or facultative chemolitoautotrophs (utilizing reduced sulfur compounds, such as sulfides, thiosulfate, thiocyanate, or molecular hydrogen, as energy sources). Methylotrophy is often observed (with such methyl compounds as formate, methanol, trimethylamine, tetramethylammonium), as is growth in anaerobic conditions (nitrate respiration) (1). Because of their versatile metabolism paracocci can play an important role in the cycling of elements in the environment.
It seems that all these physiological properties raise the possibility that Paracoccus species could be used in bioremediation systems, particularly since most species can use nitrate as an alternative electron acceptor. One of these species is P. solventivorans, which was first isolated at the site of a natural gas company as an acetone-degrading, nitrate-reducing bacterium (33). The second isolate of this species, DSM 11592 (used in this study), was found in biofilters used for the treatment of waste gases (22). So far, nothing is known about the genetics of P. solventivorans. It is only known that strain DSM 11592 carries a 5-kb plasmid, pSOV1 (2).
We initiated studies on transposable elements of Paracoccus spp. Such elements significantly enhance the variability and consequently the adaptative capacities of their hosts (7). By using the specific paracoccal entrapment vector pMEC1, we previously identified and characterized several insertion sequences (ISs) and transposons of different strains of P. pantotrophus (3). This analysis allowed us to distinguish transposable elements common in this species (e.g., ISPpa2 of the IS5 family and ISPpa5 of the IS66 family), as well as strain-specific elements (e.g., ISPpa1 of the IS256 family and Tn3434 of the Tn3 family). In order to study the occurrence and frequency of lateral transfer in this group of bacteria, we performed studies to identify and determine the distribution of transposable elements of other paracoccal strains. Here we describe molecular characteristics of ISs caught by pMEC1 in P. solventivorans DSM 11592. These ISs may be used for construction of novel tools for transposon mutagenesis, which may be very useful for genetic analysis of this interesting group of bacteria.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Strains of Paracoccus spp. (Table 1) were grown in Luria-Bertani (LB) medium (31) at 30°C, and Escherichia coli strains were grown at 37°C. When necessary, the medium was supplemented with antibiotics as follows: kanamycin, 50 μg/ml; rifampin, 50 μg/ml; and tetracycline, 2 μg/ml.
TABLE 1.
Distribution of ISPso2-related elements in paracoccal strains
| Strain | Reference | Hybridization signal with ISPso2 as a probe | PCR product with primers specific for:
|
||
|---|---|---|---|---|---|
| ISPso2 | ISPpa2 | IS1248 | |||
| P. solventivorans DSM 11592 | 22 | + | + | − | − |
| P. alcaliphilus JCM 7364 | 38 | + | − | − | − |
| P. alkenifer DSM 11593 | 22 | + | − | − | − |
| P. aminophilus JCM 7686 | 37 | + | − | − | − |
| P. aminovorans JCM 7685 | 37 | + | − | − | − |
| P. denitrificans DSM 413 | 28 | + | − | − | + |
| P. denitrificans LMD 22.21 | 28 | + | − | − | + |
| P. pantotrophus DSM 65 | 28 | + | − | + | + |
| P. pantotrophus LMD 82.5 | 28 | + | + | − | + |
| P. pantotrophus DSM 11072 | 18 | + | − | + | + |
| P. pantotrophus DSM 11073 | 18 | + | − | + | + |
| P. methylutens DM12 | 11 | + | − | + | + |
| P. thiocyanatus IAM 12816 | 19 | + | − | − | − |
| P. versutus UW1 | 34 | + | − | − | − |
DNA manipulations.
Plasmid DNA was isolated as described by Birnboim and Doly (6). Cloning experiments, digestion with restriction enzymes, ligation, and agarose gel electrophoresis were performed by using standard procedures, as described previously (31). For Southern hybridization (31) DNA probes were labeled with digoxigenin (Roche). Equal amounts of total genomic DNAs from paracoccal strains were digested to completion with appropriate restriction endonucleases and separated by electrophoresis by using 0.8% agarose gels. DNA was blotted onto BioBond-Plus nylon membranes (Sigma) and hybridized under high-stringency conditions (5× SSC [1× SSC is 150 mM NaCl plus 15 mM sodium citrate, pH 7], 1% blocking reagent [Roche], 0.1% N-lauroylsarcosine, 0.2% sodium dodecyl sulfate [SDS]) at 68°C overnight. The filters were washed twice in 2× SSC-0.1% SDS at room temperature and twice in 0.1× SSC-0.1% SDS at 65°C.
Transformation.
Competent cells of E. coli TG1 were prepared and transformed as described by Kushner (21).
Triparental mating.
Overnight cultures of donor strain E. coli TG1 carrying a mobilizable vector, a P. solventivorans recipient strain, and E. coli DH5α carrying the helper plasmid pRK2013 (10) were spun down at the maximum speed for 2 min at 4°C in a microcentrifuge and washed twice with LB medium to remove the antibiotics. The donor, recipient, and helper were mixed (1:2:1), and a 100-μl sample of the mixture was spread on a plate containing solidified LB medium. After overnight incubation at 30°C, the bacteria were washed off the plate, and suitable dilutions were plated on selective media containing rifampin (selective marker of the recipient strain) and kanamycin to select transconjugants. Spontaneous resistance of the recipient strains to kanamycin was undetectable under these experimental conditions.
Isolation of insertion mutants.
The entrapment vector pMEC1 was introduced into a recipient P. solventivorans strain by triparental mating. An overnight culture of a Kmr transconjugant, carrying pMEC1, was spread on plates containing solidified LB medium supplemented with tetracycline. Appropriate dilutions of the culture were also spread on LB medium lacking tetracycline in order to determine the frequency of transposition. Spontaneous resistance of the strain to tetracycline was undetectable under these experimental conditions.
PCR amplification.
For amplification of transposable elements captured in pMEC1, five nested pairs of cartridge-specific forward and reverse primers were used as described previously (3). For differentiation of ISPso2 and the related element ISPpa2, as well as IS1248, in various strains of Paracoccus the following pairs of forward and reverse primers were used: LPSO2 (5′-AGGATGCATTGATTTCTGTT-3′) and RPSO2 (5′-ATAACCAATAGATGACGAGA-3′); LPPA2 (5′-AGGATGCATTGATTTTCGAC-3′) and RPPA2 (5′-ATAACCAGTAGATGACGACC-3′); and L1248 (5′-CAGGATGCATTGATTTTCAG-3′) and R1248 (5′-ATAACCAATAAATGACGGTT-3′). Amplification was performed with a Mastercycler (Eppendorf) by using the synthetic oligonucleotides described above, Taq polymerase from Qiagen (supplied with buffer), and appropriate template DNAs. Each 50-μl reaction mixture for amplification contained 2 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 200 μM, 50 pmol of each primer, 0.5 U of Taq polymerase, and 1× Q solution (Qiagen). The amplification program was 96°C for 5 min, followed by 35 cycles of 48°C for 30 s, 72°C for 1 min, and 94°C for 1 min; the last cycle was followed by an additional annealing step and a final 10-min extension step.
DNA sequencing and analysis.
The nucleotide sequence was determined by using a terminator sequencing kit and an automatic sequencer (ABI 377; Perkin-Elmer). The transposable elements (present in pMEC1 derivatives) were sequenced first with the appropriate sets of cartridge-specific starters (3) and then with primers complementary to the previously determined sequence. Sequence analysis was done with programs included in the Wisconsin Genetic Computer Group Sequence Analysis Software Package, version 8.1 (9). Comparison searches were performed with IS Finder (http://www-is.biotoul.fr/is.html) and with the BLAST program provided by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST). Phylogenetic analysis was performed by using the parsimony method (DNAPARS in the software package PHYLIP, version 3.57c) (14), as well as the programs SEQBOOT, CONSENSE, and DRAWTREE to perform bootstrap analysis.
Nucleotide sequence accession numbers.
Nucleotide sequences determined in this study have been deposited in the GenBank database under accession numbers AY237733 (ISPso1), AY237732 (ISPso2), and AY311510 (ISPso3).
RESULTS AND DISCUSSION
Identification of ISs in P. solventivorans.
To identify the ISs of P. solventivorans, we used the previously described (3) mobilizable entrapment E. coli-Paracoccus shuttle vector pMEC1 (Kmr). This vector carries a selective cartridge (constructed by Schneider et al. [32]), composed of a silent tetA gene under control of the pR promoter of bacteriophage λ and the gene coding for the λ CI repressor. Inactivation of the repressor gene (e.g., through insertion of an IS) results in constitutive expression of tetracycline resistance. The pMEC1 vector was introduced into P. solventivorans DSM 11592, and Tcr clones were selected as described in Materials and Methods. Tcr mutants appeared with a frequency of 2.9 × 10−5. We tested 100 Tcr colonies for plasmid content. Most of the plasmids tested (92%) carried inserts that were smaller than 2 kb, while 8% were the size of pMEC1. Restriction analysis followed by hybridization analysis (with digoxigenin-labeled internal fragments of randomly chosen inserts as probes) revealed the presence of three different classes of elements caught in pMEC1 (0.8, 1.2, and 1.4 kb) (data not shown). To localize the insertion sites of the elements, we used five sets of previously described cartridge-specific primers (3) together with pMEC1-derived plasmids (as template DNAs) in PCRs. We amplified all the inserts, which confirmed that the insertions were within the cI gene (data not shown). The representative elements of each of the classes distinguished were sequenced. A comparison with the nucleotide sequences in databases revealed that these sequences were novel ISs, and they were designated ISPso1, ISPso2, and ISPso3. The G+C contents of the sequences identified were in the range from 60 to 67 mol% (Table 2), while that of P. solventivorans total DNA was 68.5 to 70 mol% (1).
TABLE 2.
Characteristic features of ISs of P. solventivorans
| IS | Length (bp) | G+C content (mol%) | IR length (bp)/no. of mismatchesa | DR length (bp) (sequence) | No. of ORFs | IS family/IS group |
|---|---|---|---|---|---|---|
| ISPso1 | 1,400 | 67 | 28/5 | 8 (ATCACCTT) | 1 | IS256 |
| ISPso2 | 832 | 60 | 14/1 | 2 (TA) | 2 | IS5/IS427 |
| ISPso3 | 1,283 | 62 | 11 | 4 (TAAA) | 1 | IS5/IS5 |
Number of mismatches between two IRs of a given IS.
Structural analysis of ISPso1, ISPso2, and ISPso3.
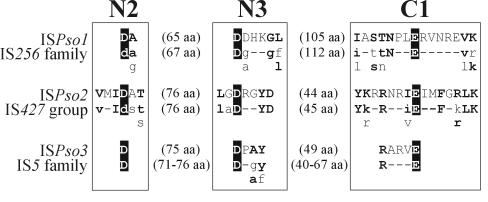
The genetic organization of the three ISs of P. solventivorans DSM 11592 is typical of the genetic organization of the majority of known ISs, since they contain an open reading frame(s) (ORF) for transposase (Tnp) and terminal inverted repeated sequences (IRs). Moreover, they are flanked by direct repeats (DRs) of the target sequence, which are generated upon insertion. The Tnps encoded by all these elements contain three domains (designated N2, N3, and C1), which have three conserved residues (two aspartate [D] residues and one glutamate [E] residue). These residues constitute the DDE motif (so-called catalytic triad) typical of many bacterial Tnps (7). The spacing between these residues, as well as the presence of other conserved residues within the domains, is different in different IS families or groups (7).
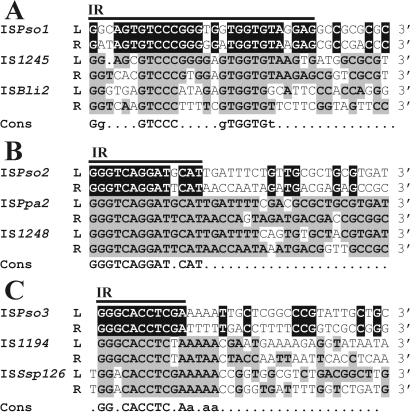
ISPso1 (1,400 bp) carries one large ORF (1,197 bp) (ORF1) encoding a putative basic protein (pI 9.25) consisting of 398 amino acids and having a predicted molecular mass of 44.65 kDa. The ORF1 product contains the DDE motif, in which the acidic residues are separated by 65 and 105 amino acids, respectively (Fig. 1); this motif is highly conserved among elements belonging to the IS256 family (23). Additionally, the nucleotide sequences of the IRs of ISPso1 (Fig. 2A), as well as the size (8 bp) of the DRs resulting from its transposition (Table 2), are also typical of the members of this family (7). Database comparison searches revealed that the predicted translation product of ORF1 exhibited the highest levels of similarity with a hypothetical protein (product of the rrub3476 gene) of Rhodospirillum rubrum (accession no. ZP 00016443) (65% identity and 72% similarity) and with TnpA of Sphingopyxis macrogoltabida (accession no. BAB07803) (57% identity and 67% similarity), both of which exhibit similarity to Tnps encoded by members of the IS256 family. All these data show that ISPso1 can be classified as a new member of the IS256 family.
FIG. 1.
Comparison of the amino acid sequences of the predicted DDE motifs of the putative Tnps encoded by ISPso1, ISPso2, and ISPso3 with the appropriate family- and group-specific consensus sequences (23). In the consensus sequences uppercase letters indicate conservation within the family, lowercase letters indicate predominant amino acids, and dashes indicate nonconserved residues. Residues forming the DDE motif are indicated by a black background. The N2, N3, and C1 domains are enclosed in boxes and labeled. The residues conserved in the domains of the Tnps analyzed and the consensus sequences are indicated by boldface type. The numbers in parentheses are the distances (in amino acids [aa]) between the residues forming the DDE motif.
FIG. 2.
Alignment of the terminal nucleotide sequences of ISPso1 (A), ISPso2 (B), and ISPso3 (C) and their relatives. The identical residues of the termini of an IS of P. solventivorans are indicated by a black background. The putative IRs are indicated by bars. The nucleotides of other ISs identical to those of ISPso1 (IS1194 [accession no. Y13626] and ISBli2 [accession no. AF195203]), ISPso2 (ISPpa2 [accession no. AY179508] and IS1248 [accession no. PDU08856]), or ISPso3 (IS1246 [accession no. NC 003350] and ISSsp126 [accession no. SSP277295]) are indicated by boldface type and a gray background. The consensus sequence (Cons) of the IRs compared is included in each panel. L, sequences at the 5′ (left) end; R, complementary sequences at the 3′ (right) end of the elements.
Another element, ISPso2 (832 bp), appeared to be highly homologous (87% identity at the nucleotide sequence level) to the previously described ISPpa2 of P. pantotrophus DSM 11072 (3) and IS1248 of P. denitrificans PdX22 (39), both of which are members of the IS427 group (IS5 family). Moreover, all these elements have the same structure, since they contain two overlapping ORFs (ORF1 [347 bp] and ORF2 [569 bp]) and nearly identical IRs (Fig. 2B). Within the ORF1-ORF2 overlap (159 bp) there is a putative frameshift motif (AAAAG), which was shown to promote generation of fusion Tnps in some IS1 and IS3 family members (13, 16, 41). The putative fusion protein encoded by ISPso2, whose predicted molecular mass is 28.8 kDa and whose pI is 10.74, has a DDE motif (Fig. 1) that matches the consensus sequence determined for the IS427 group products (23). The levels of identity and similarity between the corresponding ORFs of ISPso1 and ORFs of ISPpa2 or IS1248 were approximately 90 and 94%, respectively, for ORF1 and 85 and 88%, respectively, for ORF2. However, on the basis of the classification guidelines, the levels of identity below 95% for amino acid sequences and below 90% for DNA sequences allowed classification of these ISs as different, closely related elements (24).
Analysis of the nucleotide sequence of ISPso3 (1,286 bp) revealed the presence of one major ORF (ORF1), spanning 84% of the element. ORF1 encodes a putative peptide consisting of 374 amino acids with predicted molecular mass of 39.4 kDa and a pI of 9.91. The amino acid sequence of the ISPso3-encoded putative Tnp contains the N3 and C1 domains of the invariant DDE motif (Fig. 1) conserved in the IS4 and IS5 families (29) with a distance of 49 bp, which is characteristic of the IS5 family (23). In fact, comparison searches with the ORF1 product in databases revealed homology with Tnps encoded by ISs classified as members of a distinct subgroup of the IS5 group of the IS5 family (http://www-is.biotoul.fr/is.html). The highest levels of similarity were observed with Tnps encoded by ISSsp126 of Sphingomonas sp. strain LB126 (51% identity and 60% similarity) (40), IS1169 residing in plasmid pIP421 of Bacteroides fragilis BF-F238 (approximately 43% identity and 52% similarity) (35), and IS1168 present in pIP417 of Bacteroides vulgatus BV17 (approximately 43% identity and 52% similarity) (17). ISPso3 is flanked by identical 11-bp terminal IRs, which exhibit similarity to the IRs of the ISs mentioned above (Fig. 2C). Transposition of this IS resulted in generation of 4-bp DRs (Table 2), a size typical of other elements of the IS5 group (23).
Occurrence of ISPso1, ISPso2, and ISPso3 in different strains of Paracoccus spp.
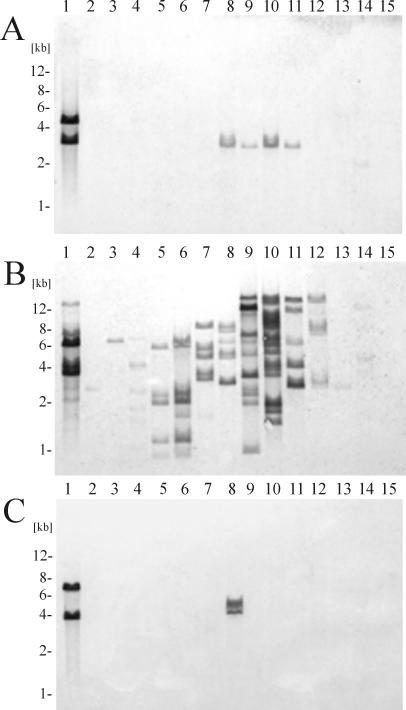
Hybridization analysis was performed to study the distribution of the ISs studied in different strains of Paracoccus spp. To do this, ISs, PCR performed with cartridge-specific primers (3) were probed with total DNAs of paracocci (listed in Table 1) digested with EcoRI and PstI. These restriction enzymes did not cut the ISs analyzed, and therefore the number of hybridized DNA fragments was thought to be equivalent to the minimum number of copies of a given element within the genome.
We detected two copies of ISPso1 in parental strain DSM 11592 (Fig. 3A, lane 1) and a single copy of homologous sequences in all of the P. pantotrophus strains tested (DSM 65, LMD 82.5, DSM 11072, DSM 11073) (Fig. 3A, lanes 8 to 11). The presence of an ISPso1-like sequence (on restriction fragments that were the same size) in all strains of P. pantotrophus strongly suggests that this element was acquired by the common ancestor before branching of these strains. A weak hybridization signal was also observed with a single band of total DNA of P. versutus UW1 (Fig. 3A, lane 14), which is phylogenetically closely related to P. pantotrophus (1). On the other hand, ISPso3 (a member of the IS5 family) was present only in one strain of P. pantotrophus (LMD 82.5) (Fig. 3C, lane 8) in addition to the parental strain (two copies in DSM 11592) (Fig. 3C, lane 1), which suggests that it was recently acquired by a lateral transfer event.
FIG. 3.
Analysis of the distribution of ISPso1-like (A), ISPso2-like (B), and ISPso3-like (C) elements by DNA hybridization. The lanes contained EcoRI-PstI-digested DNAs of P. solventivorans DSM 11592 (lane 1), P. alkenifer DSM 11593 (lane 2), P. aminophilus JMC 7686 (lane 3), P. aminovorans JCM 7685 (lane 4), P. denitrificans DSM 413 (lane 5), P. denitrificans LMD 22.21 (lane 6), P. methylutens DM12 (lane 7), P. pantotrophus DSM 65 (lane 8), P. pantotrophus LMD 82.5 (lane 9), P. pantotrophus DSM 11072 (lane 10), P. pantotrophus DSM 11073 (lane 11), P. alcaliphilus JCM 7364 (lane 12), P. thiocyanatus IAM 12816 (lane 13), P. versutus UW1 (lane 14), and pSOV1 of P. solventivorans DSM 11592 (lane 15). The positions of the markers are indicated on the left.
Sequences homologous to ISPso2 were present in all of the strains of Paracoccus spp. tested. The copy number of ISPso2 varied from 1 to approximately 14. We observed hybridization with different signal intensities, which reflected the fact that the sequences detected were not identical. As mentioned above, ISPso2 and its two closest relatives (ISPpa2 and IS1248) are highly homologous. These elements do cross-hybridize with each other (3; data not shown), and therefore we were not able to distinguish them by hybridization. In order to study the distribution and diversity of ISPso2-like elements in paracocci, we designed specific primers for each of the three related elements (the nucleotide sequences of the primers are given in Materials and Methods) and used them (together with total DNAs of the strains analyzed) in a PCR analysis. The results obtained showed that these elements are highly divergent. We did not observe PCR amplification in the majority of the paracoccal species tested (Table 1). We were able to detect ISPso2 only in parental strain DSM 11592 and in P. pantotrophus LMD 82.5. IS1248 was detected in all strains of P. pantotrophus and P. denitrificans, as well as in P. methylutens DM12, while ISPpa2 was present in all but one strain (LMD 82.5) of P. pantotrophus and in P. methylutens DM12. The simultaneous presence of ISPpa2 and IS1248 in some strains (Table 1) is not surprising since it was previously shown that ISPpa2-like elements are harbored by related plasmids of two strains of P. pantotrophus (DSM 11073 and DSM 65) (3), which suggests the possibility that they were disseminated by lateral transfer. However, the possibility that different copies of the ancestor element evolved divergently in the same host cannot be eliminated.
None of the ISs identified hybridized with the only natural plasmid (2) harbored by P. solventivorans DSM 11592, plasmid pSOV1 (Fig. 3, lane 15); therefore, all of them reside in the chromosome of this strain.
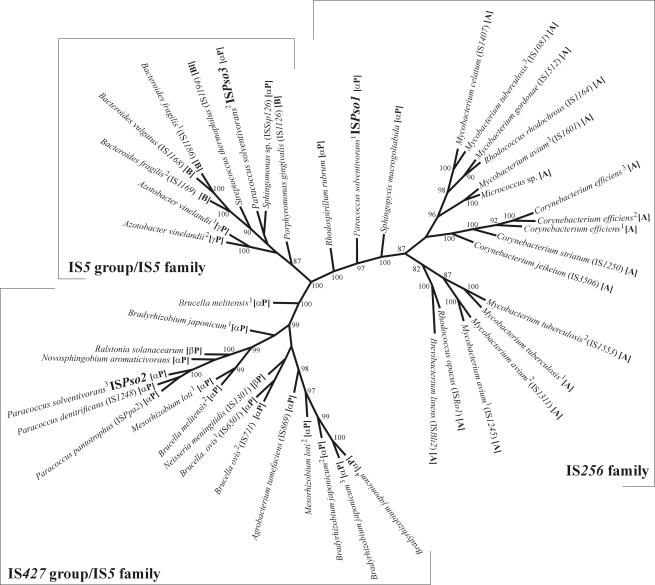
Phylogenetic analysis.
Comparisons of the Tnps encoded by ISPso1, ISPso2, and ISPso3 in databases allowed identification of their closest homologues. These sequences were used for construction of a phylogenetic tree, which showed the evolutionary relationships (Fig. 4) and allowed estimation of possible directions of lateral transfer of transposable elements between various bacterial hosts. As expected, the phylogenetic analysis separated the Tnps into three subgroups (comprising sequences with similarity to IS256 products and products of two groups belonging to the IS5 family), which were well supported by bootstrap values (Fig. 4).
FIG. 4.
Unrooted bootstrap tree (1,000 replicates) for putative Tnps encoded by ISPso1, ISPso2, and ISPso3 and their closest relatives (identified in available databases), constructed by the parsimony method. The tree was constructed by using SEQBOOT, DNAPARS, CONSENSUS, and DRAWTREE in the software package PHYLIP (14). The numbers at the nodes indicate bootstrap values for the nodes based on 1,000 bootstrap resamplings (values less than 80 are not shown). The taxonomic groups (as described by Garrity and Holt [15]) are indicated in brackets, as follows: A, Actinobacteria; B, Bacteroidetes; αP, alpha subgroup of the Proteobacteria; βP, beta subgroup of the Proteobacteria; γP, gamma subgroup of the Proteobacteria; Bl, bacilli. The sequences used for this analysis were sequences of Agrobacterium tumefaciens (accession no. X53945), Azotobacter vinelandii (ZP 00089333 [superscript 1] and ZP 00088382[superscript 2]), Bacteroides fragilis (S44507 [superscript 1] and I40184[superscript 2]), Bacteroides vulgatus (I40597), Bradyrhizobium japonicum (NP 769117 [superscript 1], NP 769117 [superscript 2], NP 771828 [superscript 3], and NP 774822 [superscript 4]), Brevibacterium linens (AAF09242), Brucella melitensis (NP 540341 [superscript 1] and NP 540319 plus NP 540320 [superscript 2]), Brucella ovis (Q08082 [superscript 1] and M94960 [superscript 2]), Corynebacterium efficiens (NP 737799 [superscript 1], NP 737110 [superscript 2], and NP 702969 [superscript 3]), Corynebacterium jeikeium (NP 848205), Corynebacterium striatum (AAG03374), Mesorhizobium loti (NP 109452 plus NP 109453 [superscript 1] and NP 108276 [superscript 2]), Micrococcus sp. (AAK62483), Mycobacterium avium (AAA69904 [superscript 1], CAA11709 [superscript 2], and AAC71696 [superscript 3]), Mycobacterium celatum (CAA65977), Mycobacterium gordonae (AAB54010), Mycobacterium tuberculosis (NP 218157 [superscript 1], NP 338287 [superscript 2], and NP 337078 [superscript 3]), Neisseria meningitidis (Z49092), Novosphingobium aromaticivorans (ZP 00093225 plus ZP 00093229), P. denitrificans (AAC43507 plus AAC43509), P. pantotrophus (AAO21198 plus AAO21199), P. solventivorans (AAO84921 [superscript 1], AAP76386 [superscript 2], and AAO48787 plus AAO48788 [superscript 3]), Porphyromonas gingivalis (CAA10225), Ralstonia solanacearum (BAA97979 plus BAA97980), Rhodococcus opacus (AAB57888), Rhodococcus rhodochrous (BAA11042), Rhodospirillum rubrum (ZP 00016443), Sphingomonas sp. (CAB87573), Sphingopyxis macrogoltabida (BAB07803), and Streptococcus thermophilus (AF454495). Some of the Tnps of the IS427 group are encoded by two overlapping ORFs; therefore, two accession numbers are given above. In these cases the sequences of the transframe fusion Tnps (generated in silico within the putative frameshift motif) were used for the analysis. The designations of the defined ISs are the GenBank and ISDatabase designations.
As Fig. 4 shows, the Tnp encoded by ISPso2 is closely related to a number of Tnps produced by bacteria belonging to the Proteobacteria, the majority of which (all but two) are classified (like paracocci) in the alpha subgroup of the Proteobacteria. This suggests that a unique transfer event might have occurred in a putative progenitor of bacteria belonging to this class. The widespread distribution of these elements in paracocci (as shown by hybridization analysis [Fig. 3B]) seems to support this hypothesis. The presence within the paracoccal cluster of a closely related Tnp of Ralstonia solanacearum (a member of the beta subgroup of the Proteobacteria) (Fig. 4) suggests, however, that both common ancestry and lateral transfer are part of the evolutionary history of these ISs.
Interestingly, Tnps of the ISPso1 type were found exclusively in bacteria belonging to two phylogenetically unrelated taxa, the alpha subgroup of the Proteobacteria and the Actinobacteria. The ISPso1 sequence has a high G+C content (Table 1), which is typical of both groups of bacteria. The Tnps of members of the alpha subgroup of the Proteobacteria (P. solventivorans, Rhodospirillum rubrum, and Sphingopyxix macrogoltabida, which are classified in different orders) do not form a separate cluster, which might suggest that they were acquired by different transfer events.
The ISPso3-like Tnps, located on the same branch of the phylogenetic tree (Fig. 4), are produced by members of different groups of bacteria (including the gram-negative alpha and gamma subgroups of the Proteobacteria and Bacteroidetes, as well as gram-positive bacilli), indicating their broad host range and frequent transfer between various bacterial genera.
The ISs described in this paper are the first transposable elements identified in P. solventivorans. Some of these elements might be useful for strain or species identification. These elements are members of two different families, the IS256 family (ISPso1) and the IS5 family (ISPso2 and ISPso3). Several elements classified in the IS5 family (which is relatively heterogeneous and comprises several groups) were also identified in P. pantotrophus (3), which suggests that ISs belonging to this family predominate in paracocci.
Acknowledgments
This work was supported by the State Committee for Scientific Research, Poland (grant 6 P04A 048 21).
REFERENCES
- 1.Baj, J. 2000. Taxonomy of the genus Paracoccus. Acta Microbiol. Pol. 49:185-200. [PubMed] [Google Scholar]
- 2.Baj, J., E. Piechucka, D. Bartosik, and M. Wlodarczyk. 2000. Plasmid occurrence and diversity in the genus Paracoccus. Acta Microbiol. Pol. 49:265-270. [PubMed] [Google Scholar]
- 3.Bartosik, D., M. Sochacka, and J. Baj. 2003. Identification and characterization of transposable elements of Paracoccus pantotrophus. J. Bacteriol. 185:3753-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beijerinck, M., and D. C. J. Minkman. 1910. Bildung und Verbrauch von Stickoxydul durch Bakterien. Zentralbl. Bakteriol. Parasitenkd. Infektionsk. 25:30-63. [Google Scholar]
- 5.Berry, A., D. Janssens, M. Humbelin, J. P. Jore, B. Hoste, I. Cleenwerck, M. Vancanneyt, W. Bretzel, A. F. Mayer, R. Lopez-Ulibarri, B. Shanmugam, J. Swings, and L. Pasamontes. 2003. Paracoccus zeaxanthinifaciens sp. nov., a zeaxanthin-producing bacterium. Int. J. Syst. Evol. Microbiol. 53:231-238. [DOI] [PubMed] [Google Scholar]
- 6.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandler, M., and J. Mahillon. 2002. Insertion sequences revised, p. 305-366. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. American Society for Microbiology, Washington, D.C.
- 8.Daneshvar, M. I., D. G. Hollis, R. S. Weyant, A. G. Steigerwalt, A. M. Whitney, M. P. Douglas, J. P. Macgregor, J. G. Jordan, L. W. Mayer, S. M. Rassouli, W. Barchet, C. Munro, L. Shuttleworth, and K. Bernard. 2003. Paracoccus yeeii sp. nov. (formerly CDC group EO-2), a novel bacterial species associated with human infection. J. Clin. Microbiol. 41:1289-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 11:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host-range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doronina, N. V., Y. A. Trotsenko, B. B. Kuznetzov, and T. P. Tourova. 2002. Emended description of Paracoccus kondratievae. Int. J. Syst. Evol. Microbiol. 52:679-682. [DOI] [PubMed] [Google Scholar]
- 12.Doronina, N. V., Y. A. Trotsenko, V. I. Krausowa, and N. E. Suzina. 1998. Paracoccus methylutens sp. nov.—a new aerobic facultatively methylotrophic bacterium utilizing dichloromethane. Syst. Appl. Microbiol. 21:230-236. [DOI] [PubMed] [Google Scholar]
- 13.Escoubas, J. M., M. F. Prere, O. Fayet, I. Salvignol, D. Galas, D. Zerbib, and M. Chandler. 1991. Translational control of transposition activity of the bacterial insertion sequence IS1. EMBO J. 10:705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felsenstein, J. 1989. PHYLIP, phylogenetic inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 15.Garrity, G. M., and J. G. Holt. 2001. The road map to the manual, p. 119-166. In G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, vol. 1. Springer-Verlag, New York, N.Y.
- 16.Haas, M., and B. Rak. 2002. Escherichia coli insertion sequence IS150: transposition via circular and linear intermediates. J. Bacteriol. 184:5833-5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haggoud, A., G. Reysset, H. Azeddoug, and M. Sebald. 1994. Nucleotide sequence analysis of two 5-nitroimidazole resistance determinants from Bacteroides strains and of a new insertion sequence upstream of the two genes. Antimicrob. Agents Chemother. 38:1047-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan, S. L., I. R. McDonald, A. J. Kraczkiewicz-Dowjat, D. P. Kelly, F. A. Rainey, J. C. Murrell, and A. P. Wood. 1997. Autotrophic growth on carbon disulfide is a property of novel strains of Paracoccus denitrificans. Arch. Microbiol. 168:225-236. [DOI] [PubMed] [Google Scholar]
- 19.Katayama, Y., A. Hirashi, and H. Kuraishi. 1995. Paracoccus thiocyanatus sp. nov., a new species of thiocyanate-utilizing facultative chemolithotroph, and transfer of Thiobacillus versutus to the genus Paracoccus as Paracoccus versutus comb. nov. with emendation of the genus. Microbiology 141:1469-1477. [DOI] [PubMed] [Google Scholar]
- 20.Kelly, D. P., F. A. Rainey, and A. P. Wood. December2000, posting date. The genus Paracoccus. In M. Dworkin, S. Falkow, E. Rosenberg, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, 3rd ed. [Online.] Springer Verlag, New York, N.Y. http://link.springer-ny.com/link/service/books/10125/.
- 21.Kushner, S. R. 1978. An improved method for transformation of E. coli with ColE1-derived plasmids, p. 17-23. In H. B. Boyer and S. Nicosia (ed.), Genetic engineering. Elsevier/North-Holland, Amsterdam, The Netherlands.
- 22.Lipski, A., K. Reichert, B. Reuter, C. Spröer, and K. Altendorf. 1998. Identification of bacterial isolates from biofilters as Paracoccus alkenifer sp. nov. and Paracoccus solventivorans with emended description of Paracoccus solventivorans. Int. J. Syst. Bacteriol. 48:529-536. [DOI] [PubMed] [Google Scholar]
- 23.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merlin, C., J. Mahillon, J. Nesvera, and A. Toussaint. 2000. Gene recruiters and transporters: the modular structure of bacterial mobile elements, p. 363-409. In C. M. Thomas (ed.), Horizontal gene pool. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 25.Neef, A., A. Zaglauer, H. Meier, R. Amann, H. Lemmer, and K. H. Schleifer. 1996. Population analysis in a denitrifying sand filter: conventional and in situ identification of Paracoccus spp. in methanol-fed biofilms. Appl. Environ. Microbiol. 62:4329-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohara, M., Y. Katayama, M. Tsuzaki, S. Nakamoto, and H. Kuraishi. 1990. Paracoccus kocurii sp. nov., a tetramethylammonium-assimilating bacterium. Int. J. Syst. Bacteriol. 40:292-296. [DOI] [PubMed] [Google Scholar]
- 27.Pukall, R., M. Laroche, R. M. Kroppenstedt, P. Schumann, E. Stackebrandt, and R. Ulber. 2003. Paracoccus seriniphilus sp. nov., an l-serine-dehydratase-producing coccus isolated from the marine bryozoan Bugula plumosa. Int. J. Syst. E vol. Microbiol. 53:443-447. [DOI] [PubMed] [Google Scholar]
- 28.Rainey, F. A., D. P. Kelly, E. Stackebrandt, J. Burghardt, A. Hiraishi, Y. Katayama, and A. P. Wood. 1999. A re-evaluation of the taxonomy of Paracoccus denitrificans and a proposal for the combination Paracoccus pantotrophus comb. nov. Int. J. Syst. Bacteriol. 49:645-651. [DOI] [PubMed] [Google Scholar]
- 29.Rezsohazy, R., B. Hallet, J. Delcour, and J. Mahillon. 1993. The IS4 family of insertion sequences: evidence for a conserved transposase motif. Mol. Microbiol. 9:1283-1295. [DOI] [PubMed] [Google Scholar]
- 30.Roberston, L. A., and J. G. Kuenen. 1983. Thiosphaera pantotropha gen. nov. sp. nov., a facultative anaerobic, facultatively autotrophic sulphur bacterium. J. Gen. Microbiol. 129:2847-2855. [Google Scholar]
- 31.Sambrook, J., and D. W. Russel. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Schneider, D., D. Faure, M. Noirclerc-Savoye, A. C. Barrière, E. Coursange, and M. Blot. 2000. A broad-host-range plasmid for isolating mobile genetic elements in gram-negative bacteria. Plasmid 44:201-207. [DOI] [PubMed] [Google Scholar]
- 33.Siller, H., F. A. Rainey, E. Stackebrandt, and J. Winter. 1996. Isolation and characterization of a new gram-negative, acetone-degrading, nitrate-reducing bacterium from soil, Paracoccus solventivorans sp. nov. Int. J. Syst. Bacteriol. 46:1125-1130. [DOI] [PubMed] [Google Scholar]
- 34.Taylor, B., and D. S. Hoare. 1969. New facultative Thiobacillus and a reevaluation of the heterotrophic potential of Thiobacillus novellus. J. Bacteriol. 100:487-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinh, S., A. Haggoud, G. Reysset, and M. Sebald. 1995. Plasmids pIP419 and pIP421 from Bacteroides: 5-nitroimidazole resistance genes and their upstream insertion sequence elements. Microbiology 141:927-935. [DOI] [PubMed] [Google Scholar]
- 36.Tsubokura, A., H. Yoneda, and H. Mizuta. 1999. Paracoccus carotinifaciens sp. nov., a new aerobic gram-negative astaxanthin-producing bacterium. Int. J. Syst. Bacteriol. 49:277-282. [DOI] [PubMed] [Google Scholar]
- 37.Urakami, T., H. Araki, H. Oyanagi, K. Suzuki, and K. Komagata. 1990. Paracoccus aminophilus sp. nov. and Paracoccus aminovorans sp. nov., which utilize N,N-dimethylformamide. Int. J. Syst. Bacteriol. 40:287-291. [DOI] [PubMed] [Google Scholar]
- 38.Urakami, T., J. Tamaoka, K. Suzuki, and K. Komagata. 1989. Paracoccus alcaliphilus sp. nov., an alkaliphilic and facultatively methylotrophic bacterium. Int. J. Syst. Bacteriol. 39:116-121. [Google Scholar]
- 39.Van Spanning, R. J. M., A. P. De Boer, D. J. Slotboom, W. N. Reijnders, and A. H. Stouthamer. 1995. Isolation and characterization of a novel insertion sequence element, IS1248, in Paracoccus denitrificans. Plasmid 34:11-21. [DOI] [PubMed] [Google Scholar]
- 40.Wattiau, P., L. Bastiaens, R. van Herwijnen, L. Daal, J. R. Parsons, M. E. Renard, D. Springael, and G. R. Cornelis. 2001. Fluorene degradation by Sphingomonas sp. LB126 proceeds through protocatechuic acid: a genetic analysis. Res. Microbiol. 152:861-872. [DOI] [PubMed] [Google Scholar]
- 41.Zheng, J., and M. A. McIntosh. 1995. Characterization of IS1221 from Mycoplasma hyorhinis: expression of its putative transposase in Escherichia coli incorporates a ribosomal frameshift mechanism. Mol. Microbiol. 16:669-685. [DOI] [PubMed] [Google Scholar]