Abstract
We studied a male with acquired prosopagnosia using a battery of implicit association tests (IATs) to investigate whether observing faces varying by social category would activate the patient’s implicit social biases. We also asked him to categorize faces explicitly by race, gender, and political party. The patient, G.B., was marginally slower to categorize black compared to white faces. He showed congruency effects in the race and celebrity IATs, but not in the gender or political IATs. These results indicate that G.B. possesses an implicit social sensitivity to certain facial stimuli despite an inability to overtly recognize familiar faces. The results demonstrate that social biases can be retrieved based on facial stimuli via pathways bypassing the fusiform gyri. Thus the IAT effect can be added to the list of covert recognition effects found in prosopagnosia.
Keywords: prosopagnosia, implicit attitudes, brain lesions, traumatic brain injury, social cognition
1. Introduction
Prosopagnosia, an inability to recognize familiar faces due to neurological damage (Bodamer, 1947; see also Ellis & Florence, 1990), can be observed in brain-damaged patients even when vision, intelligence, and cognitive abilities remain normal (Sorger, Goebel, Schiltz, & Rossion, 2007). The associated brain damage is generally found in inferior temporo-occipital regions (Barton, 2008a; Damasio, Damasio, & van Hoesen, 1982; Haxby, Hoffman, & Gobbini, 2000; Meadows, 1974), especially the fusiform face area (FFA), a region specialized for face perception (Haxby et al., 2000; Kanwisher, McDermott, & Chun, 1997; Kanwisher, Stanley, & Harris, 1999). The FFA is connected to the middle temporal lobe, which represents semantic information about other people (Gorno-Tempini et al., 1998) and to the left anterior temporal pole, which mediates name retrieval (Grabowski et al., 2001). These regions are important in recognition of familiar faces (Schweinberger & Burton, 2003). Prosopagnosia is often associated with other perceptual deficits including object recognition impairments (Barton, Cherkasova, & Hefter, 2004; Clarke, Lindemann, Maeder, Borruat, & Assal, 1997), but in general, prosopagnosic patients do not have difficulty discriminating faces from objects (Damasio et al., 1982; Orban de Xivry, Ramon, Lefevre, & Rossion, 2008; Rossion et al., 2003). For normal perception of faces, the face features are combined into a single configuration, not treated as separate units (Sergent & Signoret, 1992b). This holistic encoding of facial structures is lost in prosopagnosia (Bodamer, 1947; Ellis & Florence, 1990; Van Belle, De Graef, Verfaillie, Busigny, & Rossion, 2010).
The purpose of the current studies was to use IATs to determine whether a patient, despite an inability to overtly recognize familiar faces, could elicit implicit biases about the race, gender, political views, or likability of the person shown, and consequently display an IAT effect due to that bias. To our knowledge, no patient with prosopagnosia has been tested on IATs that use faces. We also tested the patient on his ability to categorize faces explicitly by race and gender, to recognize faces overtly, and to determine if he was faster to respond to faces already seen.
Covert face recognition has been demonstrated in prosopagnosia. As this recognition process is not consciously accessible, investigators must use methods other than verbal report to uncover it. Bruyer et al. (1983) report on a patient who made few errors when learning to associate true first names to famous faces, but made many errors when learning to associate incorrect first names to famous faces, even though the patient could not overtly identify the faces. Bauer (1984) found that during tasks of matching names to known faces, a prosopagnosic patient’s skin conductance responses (SCR) were three times more accurate in matching the correct name to a face than was the patient’s explicit choice. Similarly, Tranel and Damasio (1985; 1988) displayed known and unknown faces to six patients who could not overtly recognize the faces but showed larger amplitude SCRs to the known compared to unknown faces. Jones and Tranel (2001) found normal SCRs to pictures of immediate family and close friends in a child with prosopagnosia. Rizzo et al. (1987) found that prosopagnosic patients scanned photographs of familiar faces in different patterns than unfamiliar faces. Also, reaction times for prosopagnosic patients in a name classification task were longer when a distractor rather than a matching face was shown (de Haan, Bauer, & Greve, 1992; de Haan, Young, & Newcombe, 1987b). Similarly, Young et al. (1988) demonstrated that related face primes shortened patients’ mean RTs when judging familiarity of printed names while unrelated face primes lengthened mean RTs. Also, a patient was systematically faster to react to familiar compared to unfamiliar faces while deciding whether two photographs showed the same or different faces (de Haan, Young, & Newcombe, 1987a). These instances of covert recognition in prosopagnosics have been interpreted as indicating that subcomponents of the physiological process of face categorization remain intact even though they are not available to patients’ consciousness (Sergent & Poncet, 1990; Tranel, 2000).
1.1 Implicit Association Tests
Attitudes have been explored extensively using IATs (Greenwald, McGhee, & Schwartz, 1998; Greenwald & Nosek, 2001). In an IAT, participants are presented two tasks: one requires categorization of images into one of two categories (e.g., white or black faces); the other requires categorization of words into one of two attributes (e.g., pleasant or unpleasant). The two response keys are mapped in either a congruent or incongruent manner according to conventional stereotypes (white=pleasant, black=unpleasant). The typical finding is that participants respond faster to stereotype-congruent than to stereotype-incongruent trials. In this way, reaction times can be used to gauge the strength of association between stimuli and the participant’s implicit attitudes.
1.2 Case Report
The patient, G.B., is a Caucasian male who was born in Egypt and immigrated to the U.S. at age 9 with his family. His first language was French but his primary language is English. He also speaks Spanish. He has 20 years of education, was employed pre-injury as a physician, and since his injury has worked in several medical offices, at both paid and volunteer positions. In 2003, he sustained bilateral temporal lobe lesions, including fusiform gyri, and diffuse axonal injury due to an auto-pedestrian collision. He was diagnosed with prosopagnosia and difficulties with stimulus salience, with some object agnosia and left sensory hemi-inattention. MRI results three years post-injury showed a few nonspecific small patchy foci of hyperintense signal in the supratentorial white matter for the T2 and Flair sequences. In addition, there were two large areas of encephalomalacia with volume loss inferior to the temporal horns (posterior temporal areas extending into parieto-occipital cortex including fusiform gyrus), and a small area of encephalomalacia in the right anterior temporal lobe. His brain injury is consistent with descriptions of brain damage in acquired prosopagnosia (Barton, 2008b; Barton et al., 2004; Damasio et al., 1982; Grafman, Salazar, Weingartner, & Amin, 1986; Takahashi, Kawamura, Hirayama, Shiota, & Isono, 1995). See figure 1.
Figure 1.
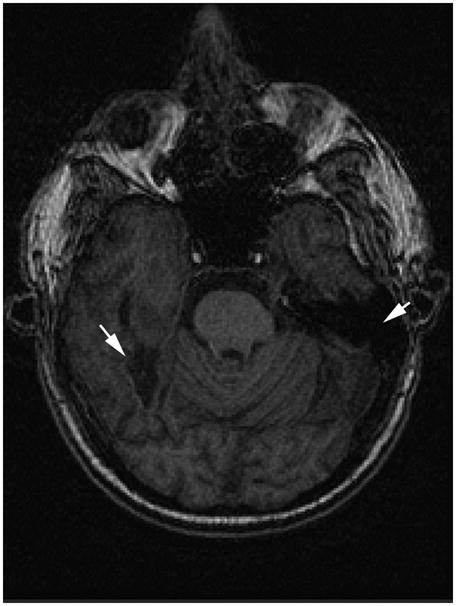
Axial slice showing damage to G.B.’s bilateral fusiform gyri (arrows).
Prior to participation in the studies, G.B. and all control subjects gave informed consent to a protocol that had been approved by the NINDS Institutional Review Board, in accordance with the Declaration of Helsinki (BMJ 1991; 302, 1194). Our testing occurred over a 2.5 year period beginning three years post-injury when G.B. was 53. On standard neuropsychological testing, he showed severe impairment on the Benton Facial Recognition Test (Benton & Van Allen, 1968) with a long form raw score of 35 plus 1 as a correction based on age and education for a corrected long form score of 36. Severe impairment is any score under 37. In the Remote Memory Battery Famous Faces Test (Albert, Butters, & Levin, 1979), he did not recognize any faces without at least one cue. Cuing enabled him to recognize 7 of 48 faces. He failed both the silhouettes and the progressive silhouettes portions of the Visual Object and Space Perception Battery, but passed the other subtests (Warrington & James, 1991). He was severely impaired on the Carey-Diamond Test of Facial Discrimination (Carey & Diamond, 1977; Carey, Diamond, & Woods, 1980).
G.B.’s index scores on the Wechsler Adult Intelligence Scale-Third Edition (Wechsler, 1981) Verbal, Performance, and Full Scale were in the average range in 2008, which is consistent with his 2006 scores. These scores are lower than expected given his educational and occupational history. His primary index scores on the Wechsler Memory Scale-Third Edition (Wechsler, 1997) ranged from borderline to average in 2006, improving to average to high average in 2008. His scaled scores on the Delis Kaplan executive functions tests (Delis, Kaplan, & Kramer, 2001), where 10 is the mean and 3 is the standard deviation, were as follows: Trailing Making test (number-letter switching), 12; Verbal Fluency test (Letter Fluency total correct), 16; Design Fluency test (Switching total correct), 9; Sorting test (Confirmed Correct Sorts), 12; Twenty Questions test (Initial Abstraction score), 13; Tower Test (Total Achievement score), 18.
2. Study 1 — Overt recognition using objects and unknown faces
2.1 Methods
We tested G.B. and normal controls on numerous visual discrimination tasks, including speeded face versus object, and race and gender categorization tasks.
2.1.1 Participants
In addition to G.B., ten male normal controls aged 48 – 64 years (M = 55.8, SD = 5.3), free of any neurological or psychiatric conditions, with an average of 18.5 years of education (SD = 1.6 years), completed this study.
2.1.2 Stimuli
Categorization ability tests were performed to compare G.B.’s and controls’ ability to distinguish faces from objects and to categorize race and gender. For all categorization tasks, the order of presentation was randomized across participants, as was the mapping of keys to stimuli, unless otherwise mentioned. The face images were obtained from the MacBrain database, the Caltech Frontal face dataset (Faces 1999), and, since only 10 black male faces were included in the MacBrain database and none in the Caltech database, we obtained other faces, including most of the black males, from Google™ and Wikipedia™. Face images were modified using Photoshop to be 3×4 inch black and white images.
Speeded face or object categorization
Participants categorized stimuli as faces or objects. Twenty faces of strangers and 20 objects (e.g., pants, tree) were displayed to measure the participants’ ability to quickly discriminate between the two types. See Figure 2 for examples of objects. Presentation times varied among 33, 50, 67 and 100 ms (see Tanskanen, Nasanen, Ojanpaa, & Hari, 2007). Participants were asked to press the “1” key for a face and the “2” key for an object.
Figure 2.

Examples of stimuli. Top row: pants and tree used in the speeded face or object categorization task. Middle row: examples of white and black faces used in the Race tasks. Used with permission. Bottom row: examples of politicians’ faces used in the Political IAT.
Race categorization
This test required identifying race from images of 20 black and 20 white male faces of strangers, by pressing the “1” (or “2”) key to classify the face as black and the other key for white. See Figure 2 for examples of black and white faces. There were two blocks, one with the black faces mapped to the “1” key and one with the white faces mapped to the “1” key. Faces were displayed until the participant responded or for a maximum of 10 seconds.
Gender categorization
Participants identified gender from images of 10 female and 10 male faces of strangers by pressing the “1” (or “2”) key to classify the face as female and the other key for male. There were two blocks, one with females mapped to the “1” key and one with the males. Faces were displayed until the participant responded or for a maximum of 10 seconds.
2.1.3 Data Analysis
Means of participants’ latencies were computed and compared across conditions and stimulus types using independent samples t-tests in SPSS (Version 11 for the Mac, Cupertino, CA). Single case study t-tests (Crawford, Garthwaite, & Porter, 2010,Crawford, Garthwaite, & Porter, 1998; Sokal & Rohlf, 1995) were used to determine whether the patient’s latencies were significantly different from the mean of the control samples.
2.2 Results
G.B.’s latencies on the speeded tasks were close to the mean for the controls. In the face versus object discrimination task, G.B. was faster to classify faces (M = 1535 ms, SD = 76 ms) than objects (M = 1590 ms, SD = 72 ms) (correct trials only; t(156)=4.69, p<.001), as were controls: faces (M = 1579 ms, SD = 136 ms), objects (M = 1620 ms, SD = 139 ms) (correct trials only; t(1571)=6.01, p<.001). G.B. made 1 error on faces and none on objects, while controls made an average of .7 errors on faces and .7 on objects.
Race categorization task
Controls made few errors (range: 0 – 4). G.B. made 15 errors, 12 on black faces and 3 on white faces (χ2 (1, N=80)= 3.53, p = .06). He made 4 errors on the same black male face, which appeared very white-skinned. Across all participants, 10 of the 29 errors were made on this face. With this face removed from further analysis, G.B. made 8 errors on 4 different black faces, and 1 error each on 3 different white faces, while controls each made up to 1 error on black faces, and up to 2 errors on white faces. Controls classified faces as being Black (M = 1902 ms, SD = 346 ms) or White equally quickly (M = 1938 ms, SD =399 ms) (correct trials only) (t(770)=1.33, ns), while G.B. was, on average, slower to categorize Black faces (M = 2862 ms, SD = 1172 ms) than White faces (M = 2397 ms, SD = 898 ms) (correct trials only; t(65)= 1.84, p = .07). See Figure 3. As shown in Table 2, G.B.’s mean latency difference from black to white faces was significantly different from controls’ using single case study statistics.
Figure 3.
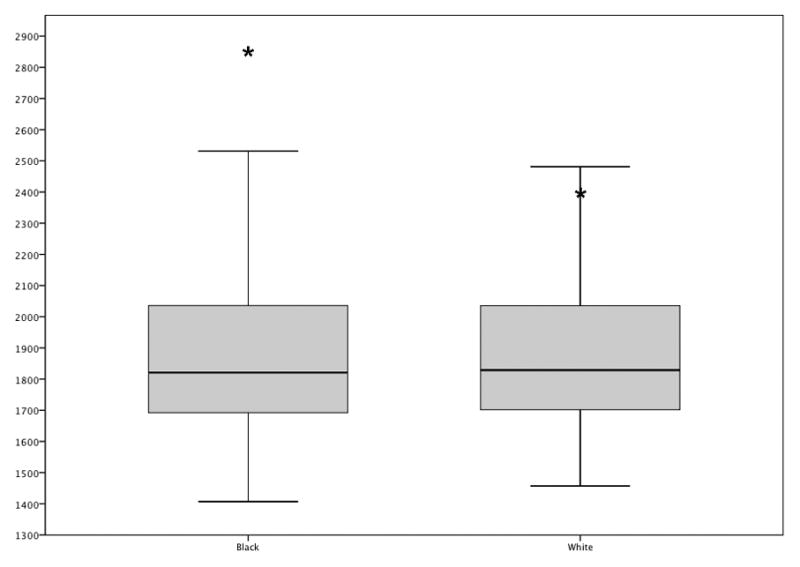
Boxplot of controls’ mean latencies in ms for categorizing African-American and Caucasian faces, correct trials only. Black bars show the median latency for the group of controls, and the bottom and top of the boxes portray the 25th and 75th percentiles. G.B.’s mean latencies are marked with asterisks.
Table 2.
Comparison of mean latency changes across conditions for controls versus patient
| Control sample | Significance Test | Estimated % of the control population obtaining a lower score than patient | Estimated effect size (zcc) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Task | N | Mea n Index as a % | SD | Patient’s Index as a % | t | p | Point | (95% CI) | Point | (95% CI) |
| Race Categorization | 10 | 0.85 | 1.58 | −8.84 | −5.85 | <.001 | 0.01% | 0.00 to 0.05 | −6.13 | −8.98 to − 3.28 |
| Gender Categorization | 10 | −0.30 | 2.89 | −3.34 | −1.00 | .17 | 17.10 % | 3.46 to 40.10 | −1.05 | −1.82 to − 0.25 |
| Race IAT | 10 | 3.36 | 2.63 | 6.85 | 1.27 | .12 | 88.12 % | 67.14 to 98.51 | 1.33 | 0.44 to 2.17 |
| Gender IAT | 10 | 2.12 | 2.24 | −0.70 | −1.20 | .13 | 13.03 % | 1.86 to 34.57 | −1.26 | −2.08 to − 0.397 |
| Political IAT | 24 | 2.36 | 3.67 | 0.91 | −0.39 | .35 | 35.11 % | 20.98 to 50.99 | −0.40 | −0.81 to − 0.03 |
| Celebrity IAT | 9 | 10.26 | 5.26 | 7.98 | −0.41 | .35 | 34.59 % | 13.41 to 60.43 | −0.43 | −1.11 to 0.26 |
| Face Training | 9 | 3.56 | 2.01 | −2.12 | −2.68 | .01 | 1.39% | 0.00 to 9.57 | −2.83 | −4.32 to − 1.31 |
Error trials were removed except for the Celebrity IAT and the associated Face Training task where error trials were included as they were not true errors. Trials with RTs < 10000 were removed. Note that the first trial for each block has been removed for the Gender Categorization task. Also, one face accounting for almost a third of the errors was removed from the Race Categorization task. The Mean Index of the control sample and the Patient’s Index for the Race Categorization task are computed as follows: (Black face mean RT−White face mean RT)/(Black face mean RT + White face mean RT). The Mean Index of the control sample and the Patient’s Index for the Gender Categorization task are computed as follows: (Female face mean RT−Male face mean RT)/(Female face mean RT + Male face mean RT). The Mean Indices of the control samples and the Patient’s Indices for the IATs are computed as follows: (Incongruent mean RT−Congruent mean RT)/(Incongruent mean RT + Congruent mean RT). The Mean Index of the control sample and the Patient’s Index for the Face Training task are computed as follows: (New face mean RT−Trained face mean RT)/(New face mean RT + Trained face mean RT).
Gender categorization task
Controls made few errors (range: 0 – 2). G.B. made no errors. Controls were equally quick to categorize faces as female (M = 1908, SD = 316 ms) or male (M = 1896, SD = 348 ms) (correct trials only; t(194)=0.24, ns). G.B. was slower to categorize female faces (M = 2190 ms, SD = 464) than male faces (M = 1859 ms, SD = 147) (correct trials only; t(18)=2.15, p=.046; see Figure 4, dark asterisks); however, since G.B. was more than 3 standard deviations slower on the first trial of each block than the mean of the other trials for this task, we removed the first trial of each block and reanalyzed, and the results were not significant (t(16)=1.54, p= .14; see Figure 4, light asterisk). As shown in Table 2, G.B.’s mean latency difference for female compared to male faces was not significantly different from controls’ using single case study statistics once the first trial of each block was removed.
Figure 4.
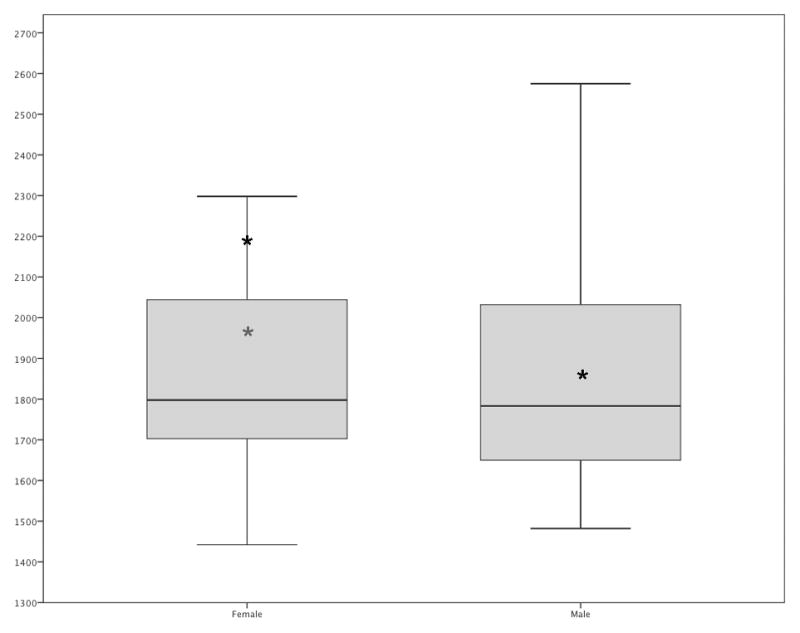
Boxplot of controls’ mean latencies in ms for categorizing female and male faces, correct trials only. Black bars show the median latency for the group of controls, and the bottom and top of the boxes portray the 25th and 75th percentiles. G.B.’s mean latencies are marked with dark asterisks; the light asterisk marks his mean latency for female faces when the first trial of each block was removed. His mean latency for males was not affected.
2.3 Discussion
G.B. had little trouble categorizing an image as a face or an object even with rapid display times, consistent with other prosopagnosic patients (Boucart, Moroni, Despretz, Pasquier, & Fabre-Thorpe, 2010; Damasio et al., 1982; Orban de Xivry et al., 2008; Rossion et al., 2003). He had some difficulty categorizing faces by race, but was easily able to categorize faces by gender. This reflects that he had retained at least some level of perceptual face processing ability (Sergent & Signoret, 1992b). He also had a trend toward being slower to categorize black compared to white faces. While the purpose of these categorization tasks was merely to determine whether the patient could classify faces according to race and gender, the results suggest the need for further testing to explore whether there are differences in task difficulty on race versus gender categorization for people with prosopagnosia. These should use luminance-controlled, standardized, high-resolution images.
3. Study 2 – Overt recognition using known faces
3.1 Methods
Similarly to the race, gender, and political face categorization tasks, we performed this study to determine if G.B. could recognize faces of people personally known to him (family members or coworkers) and faces known but not personally (presidents/candidates, celebrities) among faces of strangers. Only G.B. participated, as the family members’ and coworkers’ images were unique to him. One hundred and thirteen faces were each randomly presented for 2 seconds. After that, the face remained while a question was displayed below it asking, “Do you recognize this person or these people? If yes, type a ‘y’, if no, type an ‘n’”. The face and question remained until the participant responded.
3.2 Results
In all, G.B. recognized 15 of the 98 non-stranger faces. Of these, he recognized only 2/56 celebrity faces, 5/14 coworkers, 6/14 family members (these each included himself or one of his parents, and 2/14 presidents’/candidates’ faces (Barack Obama and John McCain). He did not recognize any of the faces of his children). Of the 15 strangers’ faces, he replied that he recognized only one. See Figure 5.
Figure 5.
Number of non-stranger faces that G.B. correctly recognized.
3.3 Discussion
As expected, G.B.’s overt recognition of faces was poor, especially for those not personally known to him (politicians and celebrities), similar to the results of another prosopagnosic patient (Levine & Calvanio, 1989). After testing, he stated that he had been studying the faces of politicians, but during testing, he recognized only two. He was most successful at recognizing the faces of himself and his parents (consistent with Arsalidou, Barbeau, Bayless, & Taylor, 2010), followed by coworkers. He was, however, not successful at recognizing his children perhaps because children’s facial features change so rapidly as they grow, and because they did not live with him. His pattern of facial recognition was likely due to his high levels of exposure to family members, coworkers, and the top two presidential candidates who were frequently in the news at the time of testing. These results confirm G.B.’s prosopagnosia, in that he demonstrated poor overt recognition of familiar and famous faces.
4. Study 3 – Covert recognition using unknown faces
4.1 Methods
The following questionnaires were given to measure explicit race and gender attitudes: the Should/Would Discrepancy questionnaire (Monteith & Voils, 1998), the Racial Semantic Differential scale (Knutson, Mah, Manly, & Grafman, 2007), the Race Attitudes questionnaire [includes the Discrimination and Diversity scales (Wittenbrink, Judd, & Park, 1997) and the Modern Racism Scale (McConahay, Hardee, & Batts, 1981)], the Gender Semantic Differential scale (Knutson et al., 2007), the Attitudes Toward Women scale (Spence & Helmreich, 1972), and the Gender Attitudes Inventory-ASI (Glick & Fiske, 1996). We also tested G.B. and normal controls on a race and a gender IAT to determine if he had the ability to show an IAT effect, that is, to respond faster due to implicit attitudes based on race and gender stereotypes.
4.1.1 Participants
The same participants as in Study 1 completed this study.
4.1.2 Stimuli
The order of presentation was randomized across participants, as was the mapping of keys to stimuli, unless otherwise mentioned. The face images were obtained from the MacBrain database, the Caltech Frontal face dataset (Faces 1999), and, since few black faces were included in the MacBrain database and none in the Caltech database, we obtained other faces, including most of the black faces, from Google™ and Wikipedia™. Face images were modified using Photoshop to be 3×4 inch black and white images.
In the IATs, participants were first presented with a word training block (pleasant and unpleasant words were used in the race IAT, and strong and weak words were used in the gender IAT), a face training block, and combined words and faces IAT blocks.
Pleasant/unpleasant and strong/weak words were obtained from the ANEW database (Bradley & Lang, 1999). Pleasant/strong words were always mapped to the left key and unpleasant/weak words to the right key. For both IATs, stimuli were displayed until the participant responded or for a maximum of 10 seconds.
Race IAT
Ten faces of strangers were used in each category: black female, black male, white female, and white male, as were 20 unpleasant (M = 2.31, SD = 1.63) and 20 pleasant words (M = 7.46, SD = 1.64) (t (38) = 49.87, p <.001). Half the faces and words were shown in the congruent block and the other half in the incongruent block. Dominance also varied significantly between unpleasant (M = 3.79, SD = 2.48) and pleasant words (M = 6.29, SD = 2.15), (t (38) = 13.23, p < .001), and arousal (unpleasant M = 6.15, SD = 2.45; pleasant M = 5.48, SD = 2.53; t (38) = −2.29, p = .028). The unpleasant and pleasant words did not differ significantly in frequency (t (38) = .006, ns) or number of letters (t (38) = 1.95, ns).
Gender IAT
Ten white female faces and ten white male faces of strangers were used, as were 20 strong (dominance M = 6.45, SD = 2.23) and 20 weak words (M = 3.12, SD = 2.18) (t(38)= 12.4, p<.001). Faces were repeated in the two blocks, congruent and incongruent. Strong and weak words also varied significantly for valence (weak M = 2.48, SD = 1.57; strong M = 6.71, SD = 1.65; t(38)= 11.86, p< .001) and arousal (strong M = 5.9, SD = 2.46; weak M = 5.17, SD = 2.47; t(38)= 2.7, p=.01). Strong and weak words did not differ significantly in frequency (t(38)= .31, ns) or number of letters (t(38)= .15, ns).
4.1.3 Data Analysis
Means of participants’ latencies were computed and compared across conditions and stimulus types using independent samples t-tests in SPSS (Version 11 for the Mac, Cupertino, CA). Computation of the IAT effect for Greenwald’s D scores was performed according to an improved algorithm (Greenwald, Nosek, & Banaji, 2003). The D score divides the difference between the incongruent and congruent RTs by the standard deviation of the individual’s RTs. This removes the effect of an individual’s latency variability from the measure. To determine if the patient had a significantly different profile from controls, the following ratio: (Incongruent − Congruent)/(Incongruent + Congruent) was calculated for each participant. It results in the percentage of performance decrease between congruent and incongruent conditions. Then, the patient’s index was compared to the mean for the controls using a t-test modified for single-case studies (Crawford et al., 2010; Crawford & Howell, 1998; Sokal & Rohlf, 1995).
4.2 Results
On the race questionnaires, G.B. and the controls scored nearly identically and showed little explicit racism. On the gender questionnaires, the controls’ results indicated low sexism, while G.B.’s results reflected a slightly more sexist attitude.
Race IAT Results
As shown in Table 1, the controls’ mean latency difference between congruent and incongruent conditions indicates a significant increase (correct trials only, all stimulus types; t(790) = 4.3, p<.001). G.B.’s mean latency difference indicated a trend toward significance, (t(72)=1.7, p=.085). See Figure 6. As shown in Table 1, the mean Greenwald’s D score (which removes the effects of the individual’s standard deviations in response times) showed both controls and G.B. had moderate effects of congruency. Table 2 shows that G.B.’s performance change was not significantly different than controls’.
Table 1.
IAT Results
| IAT | Race | Gender | Political | Celebrity | ||||
|---|---|---|---|---|---|---|---|---|
| Mean Latency Differences between Congruent and Incongruent trials-- | ||||||||
| Controls: | +144 | +92 | +53 | +514 | ||||
| G.B.: | +375 | −32 | +19 | +433 | ||||
| Mean RTs-- | ||||||||
| Con | Inc | Con | Inc | Con | Inc | Con | Inc | |
| Controls: | 2077 (SD 438) | 2221* (SD 495) | 2065 (SD 367) | 2157* (SD 470) | 1446 (SD 649) | 1499* (SD 670) | 2207 (SD 628) | 2721* (SD 907) |
| G.B.: | 2549 (SD 799) | 2923 (SD 1032) | 2303 (SD 500) | 2271 (SD 446) | 4091 (SD 1864) | 4166 (SD 1740) | 2498 (SD 738) | 2932* (SD 976) |
| D Scores-- | ||||||||
| Controls: | .41 | .24 | .13 for faces | .70 | ||||
| G.B.: | .36 | −.03 | .06 | .35 | ||||
RTs are in ms.
Significant difference between congruent and incongruent condition with p < .05.
Figure 6.
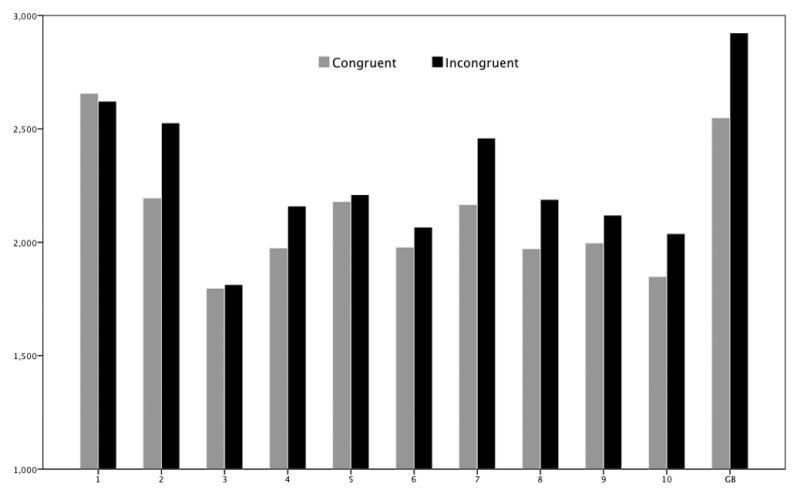
Mean latencies in ms for congruent and incongruent trials per subject for the race IAT. G.B. showed the same pattern as most control participants of a longer mean RT in incongruent trials compared to congruent trials.
Gender IAT Results
As shown in Table 1, the controls’ mean latency difference between congruent and incongruent conditions indicates a significant increase (correct trials only, all stimulus types; t(805) = 3.08, p=.002). G.B.’s mean latency difference indicated no significant difference (t(77) = −0.30, ns). See Figure 7. As shown in Table 1, the mean Greenwald’s D score for controls indicated a small effect, while G.B.’s score was close to zero. As shown in Table 2, G.B.’s performance change was not shown to be significantly different than the controls’. Despite G.B.’s gender bias on the questionnaires, he did not take significantly longer to respond to female faces, 2087 ms (SD = 298), than to male faces, 2086 ms (SD = 443) (t(40) = 0.01, ns).
Figure 7.
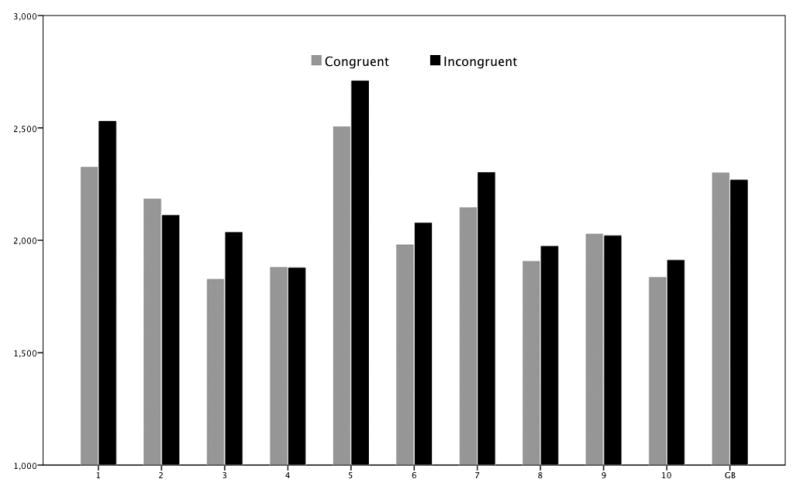
Mean latencies in ms for congruent and incongruent trials per subject for the gender IAT.
4.3 Discussion
G.B. s IATs results also showed a mixed effect of congruency. In the race IAT, he showed an IAT effect as measured by the D score measure, which removes the effects of standard deviations in response latencies. His latencies in the incongruent compared to the congruent condition indicated a trend toward significance. In addition, the difference in the RT ratio between incongruent and congruent conditions for G.B. was not significantly different from the controls’, and the controls showed a significant effect of congruency. For the gender IAT, G.B. showed little difference in latencies between congruent and incongruent conditions, while most controls showed significant effects of congruency; however the differences in percentages of performance change between incongruent and congruent conditions did not meet a significance level of .05 between G.B. and the controls with this small control sample size. G.B. did hold some views corresponding to gender stereotypes, more so than the controls, and would normally be expected to have a greater gender IAT effect, but that was not the case. However, a meta-analysis of the correlations between explicit measures such as self-report and implicit measures as measured by IATs found that the average correlation was only .24 (Hofmann, Gawronski, Gschwendner, Le, & Schmitt, 2005), so it is not unexpected that the explicit and implicit measures do not always correspond in individual subjects. This demonstrates that humans are capable of overcoming their entrenched biases based on the surface properties of people (in this case, gender) by allowing higher brain regions and explicit thought processes to control their behavior.
The faces used in these two IATs were of unknown people and the results indicate that G.B. was able to show a stereotypical bias (for race) as long as he could detect the key physical features defining race. We also wanted to use images of known people in an IAT, so that it would more critically depend on the function of the fusiform gyrus and face recognition systems. We investigated this in Study 4, along with the effects of training.
5. Study 4-- Covert recognition using known faces
5.1 Methods
We asked G.B. to categorize politicians by political party by pressing the “1” (or “2”) key to classify the face or name as Democrat and the “2” (or “1”) key for Republican. We also measured his political attitudes explicitly with a political questionnaire (Wyer et al., 1991b), and implicitly using a political IAT similar to one used in a previous study (Knutson, Wood, Spampinato, & Grafman, 2006). The political IAT versions differed in that each used politicians popular at the time. Also, in the earlier Political IAT, congruency was based on the political party containing more candidates for whom each participant was likely to vote; while in this version, congruency was based on G.B.’s self-rating of preferred political party on the political questionnaire (Wyer et al., 1991a).
In addition, in order to investigate whether G.B. could access his stored social attitudes about a person (how likable he found famous people) from viewing their faces, we created the celebrity IAT. Other studies report that prosopagnosic patients can make normal judgments of another trait, trustworthiness, by viewing faces, since encoding of facial identity and trait judgment from faces may be functionally independent (Todorov & Duchaine, 2008). First, we constructed a survey listing the names of well-known celebrities to determine normal controls’ attitudes toward them. Using the results, we created an IAT using images of the faces of the most liked and disliked celebrities to investigate differences between G.B. and a second group of normal controls in judging attitudes toward celebrities. We also wanted to determine if G.B. had a training effect of shorter latencies to stimuli to which he had been recently exposed, as has been shown in normal controls (Yonelinas, Otten, Shaw, & Rugg, 2005). For the training effect measure, liked and disliked celebrity faces were shown in a random order. Immediately afterward, we tested subjects on their ability to remember the faces (similar to Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, 2000; Yonelinas et al., 2005) by showing them the recently shown faces randomly mixed with 28 new celebrity faces. For each, subjects performed a two-step rating. The first was to indicate whether or not they had seen the face during training by pressing the “1” or “2” key, respectively. The second step, presented only if they pressed the “1” key, was to press the “r” key if they remembered something specific about seeing the face (e.g., what they thought about when the face was shown, or what the face looked like on the screen). Otherwise, if they could not recollect anything specific but knew they saw it, they were to press the “k” key. The new faces used in this training were not used afterward.
Following this, participants completed the celebrity IAT using both the trained and untrained faces, along with pleasant/unpleasant words. The congruent condition involved having the participant press the “1” key if the face shown was likable and the “2” key if the face was not likable, while mapping the pleasant words to the “1” key, and the unpleasant words to the “2” key. The incongruent condition involved having the participant press the “2” key if the face was likable and the “1” key if the face was not likable. As the order of administration has been shown to affect latency results (Greenwald et al., 1998; Nosek, Banaji, & Greenwald, 2002), subjects received the tasks in the same order: congruent, incongruent, congruent, incongruent.
5.1.1 Participants
Twenty-four healthy participants (13 male) aged 22 – 46 years (M = 28.6, SD = 6.3) with an average of 17 years of education (SD = 2.4 years) completed an earlier version of the Political IAT with politicians popular at the time (Knutson et al., 2006). These participants had been screened to assure they held strong political views. G.B. performed the political IAT using more contemporary politicians.
Eighteen normal controls (17 male) aged 45 – 65 years (M = 55.7, SD = 7.2) with an average of 19.1 years of education (SD = 3.3 years) completed the celebrity likability survey. G.B. and a different group of nine male normal controls aged 45–64 years (M = 54.1, SD = 6.9) with an average of 17.7 years of education (SD = 3.6 years) performed the celebrity IAT and training effect measure. All controls were free of any neurological or psychiatric conditions. An additional control was excluded since he did not recognize more than half of the celebrities.
5.1.2 Stimuli
Politician face categorization
Faces and names were of well-known U.S. politicians (6 Democrats and 6 Republicans). There were two blocks, one with the Democrats mapped to the “1” key and one with the Republicans mapped to the “1” key. Faces were displayed until the participant responded or for a maximum of 10 seconds.
Political IAT
See Knutson et al. (2006) for details on the earlier version of this IAT. The Political IAT that G.B. completed included thirty-six names and faces mapped to the same or different keys as 36 pleasant and 36 unpleasant words. Half of each of the names and faces were well-known Democratic politicians and half were Republican. See Figure 2 for examples of faces of politicians. Politicians whose names were used in the first group had their faces used in the second group and vice-versa. There were three training blocks and four congruent and four incongruent blocks. Half of the pleasant and half of the unpleasant words were randomly assigned to the face conditions and the other half of each type to the name conditions. Pleasant words were rated with a valence > 6.55 (M = 7.67, SD= 0.60), and unpleasant words were rated with a valence < 4.13 (M = 2.55, SD= 0.58; t(80)= 39.35, p<.001). Pleasant and unpleasant words also varied significantly for dominance (pleasant M= 6.13, SD= 0.70; unpleasant M= 3.96, SD= 0.80; t(80)= 13.14, p<.001), but not for arousal (pleasant M= 5.55, SD= 1.27; unpleasant M= 5.68, SD= 0.95; t(80)= −.52, ns). The pleasant and unpleasant words did not differ significantly in frequency (t(80)= −.07, ns) or number of letters (t(80)= .39, ns). Stimuli were displayed until the participant responded or for a maximum of 10 seconds.
Likability Survey
The likability survey used the names of 121 celebrities including TV or movie stars, sports figures, and singers from several decades ago to the present (e.g., Doris Day, Miley Cyrus). The extended time range of celebrities included people made famous prior to and after G.B.’s injury. The survey used a 7-point Likert scale (1=Like a Lot, 7= Dislike a Lot) to determine attitudes toward the celebrities, or a 0 if the name was not recognized. If more than two of the survey participants did not recognize a name, we did not include that celebrity in the IAT.
Celebrity IAT
28 well-liked and 28 disliked celebrity faces were mapped to the same or different keys as 20 pleasant and 20 unpleasant words. Two congruent and two incongruent blocks were run; faces were shown once in a congruent block and once in an incongruent block. We obtained the face images from Google. The pleasant and unpleasant words were obtained from the ANEW database (Bradley & Lang, 1999). The unpleasant and pleasant words varied significantly in valence (unpleasant M = 2.11, SD = 1.45; pleasant M = 7.71, SD = 1.62; t(38)=34.10, p<.001, where 1=very unpleasant to 10= very pleasant). The words also varied significantly in dominance (unpleasant M = 3.84; SD = 2.51; pleasant M = 6.1, SD = 2.23; t(38)=11.25, p<.001), and arousal (unpleasant M = 6.23, SD = 2.52; pleasant M = 5.61, SD = 2.56; t(38)=2.20, p=.03). Stimuli were displayed until the participant responded or for a maximum of 10 seconds. For the training effect measure, 28 celebrity faces were shown in a random order for 5.25 seconds each (based on Hicks & Marsh, 1999). Half of those faces were chosen randomly from the liked and half from the disliked celebrities. Superlab 4.0.7 was used to create the experiments.
5.1.3 Data Analysis
The same analyses were performed for the political and celebrity IATs as for the race and gender IATs. In addition, since the RT results for the celebrity IAT were positively skewed, mean RTs were log10-transformed. The results better approximated a normal distribution.
5.2 Results
Politician categorization task
G.B. made 10 errors, 7 for Democrats and 3 for Republicans (χ2 (1, N=24)= 2.74, ns). His mean latencies to categorize faces as Democrats (M = 5691, SD = 3211 ms) or Republicans (M = 4187, SD = 1826 ms) were not significantly different (correct trials only; t(34)= 1.73, ns).
On the political orientation questionnaire, G.B. rated himself as neutral. For political party preference, G.B. rated himself as a 2 (Democratic).
Political IAT Results
As shown in Table 1, the healthy participants’ mean latency difference between congruent and incongruent conditions was significant (correct face and name trials only; t(6136)= 3.14, p=.002), and their Greenwald’s D score for faces indicated a small effect. G.B.’s mean latency difference was not significant (correct face and name trials only; t(485)=.13, ns), nor was his Greenwald’s D score. Note that although the healthy participants as a whole showed an IAT effect, four of them individually did not. As shown in Table 2, G.B.’s performance change was not significantly different than controls’.
Celebrity IAT Results
As shown in Table 1, the controls’ mean latency difference for congruent versus incongruent conditions was significant (all trials, all stimulus types; t(1725)=13.69, p<.001). Note that since the task of categorizing the faces as to their likability is highly subjective, all trials (whether considered “correct” or “incorrect” according to the celebrity likability survey results) were included in the results. Results did not change when incorrect trials were excluded however. G.B.’s mean latency difference also was significant (t(190)=3.47, p=.001). See Figure 8. Both the controls and G.B. had mean RT log10 ratios of incongruent over congruent trials significantly greater than zero; that is, they both showed an IAT effect (controls: t(862)=18.98, p<.001. G.B.: t(95)=5.71, p<.001). Table 1 shows the mean D score for controls and G.B.. As shown in Table 2, G.B.’s performance change was not significantly different from controls’.
Figure 8.

Mean latencies in ms for congruent and incongruent trials per subject for the celebrity IAT. G.B. showed the same pattern as control participants of a longer mean RT in incongruent trials compared to congruent trials.
G.B. performed poorly in recognizing whether a celebrity’s face was previously shown, making 21 errors on 56 faces, while controls averaged less than 2 errors (range 0 to 5; SD = 1.8).
The controls showed a training effect; that is, they were faster to response to faces they had seen in the training. Their mean latency for the untrained faces was 2626 ms (SD = 911) (all face trials), and for the trained faces, 2478 ms (SD = 821), for a significant decrease of 179 ms (t(1006)=3.28, p=.001). G.B. did not show a training effect. His mean latency for the untrained faces was 3061 ms (SD = 808), and for the trained faces, 3194 ms (SD = 1023), for an increase of 132 ms (t(110)=0.76, ns). See Figure 9. As shown in Table 2, G.B.’s pattern of performance change from trained to new faces was significantly different from controls’.
Figure 9.

Mean latencies in ms for trained and untrained faces per subject in the celebrity IAT. G.B. is the only participant who had a longer mean RT for trained faces than for untrained faces.
5.3 Discussion
In the political IAT, G.B. showed little difference in latencies between congruent and incongruent conditions. Twenty of 24 controls completing a previous version of the political IAT (see Knutson et al., 2006) showed significant effects of congruency. But note that control participants had been screened so that only those with strong political views were included and their IAT effect was small, whereas G.B. rated himself as neutral on the political orientation questionnaire. In addition, G.B.’s results were not significantly different from the controls according to Crawford et al.’s t-tests modified for single case studies (Crawford et al., 2010; Crawford & Howell, 1998; Sokal & Rohlf, 1995). It is possible that G.B. was unable to encode the faces of politicians since this requires going beyond mere surface characteristics (e.g., skin color) into face identification, which requires assembling several features into a unified face and is difficult for someone with prosopagnosia (Boutsen & Humphreys, 2002; Busigny & Rossion, 2010; Levine & Calvanio, 1989; Saumier, Arguin, & Lassonde, 2001; Van Belle et al., 2010). This may have prevented him from retrieving semantic knowledge regarding the politicians and their political parties and/or his implicit attitudes toward them. This is consistent with his difficulty in categorizing faces by political party. However, G.B. did show an IAT effect in the celebrity IAT, demonstrating his ability to retrieve implicit attitudes toward the celebrities even without overt face recognition. Thus we feel the lack of an IAT effect in the political IAT is more likely due to G.B.’s neutral political orientation, for even though he identified himself as a Democrat, he rated his political orientation as neutral.
The results on the recall task confirm G.B.’s difficulties in learning and recalling faces. In the training task, for controls, prior exposure to the faces shortened their later latencies on the celebrity IAT to those faces. G.B. did not show this training effect.
6. General Discussion
These studies examined whether a person who was unable to overtly recognize faces would show a social IAT effect (based on attitudes) when faces are used as stimuli; that is, would he be able to extract enough information from the faces, so that he could access stored social knowledge of the person enabling his social biases to emerge? The biases could be based on facial features (race or gender stereotypes) or face recognition (political beliefs, familiarity, or likability). We also investigated whether training on the faces would decrease his latency to those faces.
Overt face recognition
G.B., consistent with other prosopagnosics, was severely impaired on the Benton Facial Recognition Test (Benton & Van Allen, 1968) and on the Remote Memory Battery Famous Faces Test (Albert et al., 1979), but showed little difficulty distinguishing between objects and faces. He had extreme difficulty recognizing familiar faces, especially those not personally known to him (politicians and celebrities). He performed poorly when asked to indicate whether a face had been shown to him in a training session, and he did not show the normal training effect of a shorter RT to previously seen faces.
Covert face recognition
Overall, controls showed significant effects of attitude for each IAT they performed (race, gender, political and celebrity). Seeing the face photographs activated stored attitudes about blacks vs. whites, men vs. women, preferred vs. non-preferred politicians, and liked vs. disliked celebrities. G.B. showed an IAT effect in the race and celebrity IATs, even with poor overt recognition of known faces from the latter. The lack of IAT effect for the political IAT may be due to his neutral political attitude, for on the political orientation questionnaire, he rated himself as neutral. It is not clear why he did not show an IAT effect in the gender IAT, since his results on the questionnaires showed a slight amount of gender stereotyping, but while the control group as a whole showed an IAT effect, each individual control subject did not (2 of 10 controls did not) and he was not shown to be significantly different from the control sample. The control group’s mean D score was only .24, considered a small effect. It may be that this test needs more power in order to better detect an effect, as only 10 female and 10 male faces were used and only two blocks were run.
Single case study statistics (Crawford et al., 2010; Crawford & Howell, 1998; Sokal & Rohlf, 1995) reveal that the patient’s results were significantly different from controls’ in differential speed of classifying black and white faces by race (see Table 2). The patient also was different from controls in that only he did not show a training effect for celebrity faces. However, none of the IAT’s showed a significant pattern of difference between the patient’s mean latency differences and the controls’, giving some evidence that while not always showing an IAT effect, his results could not be distinguished from the normal control sample’s, even though he had difficulty recognizing familiar faces.
How does this social covert recognition without awareness occur? Increasing evidence shows that parallel pathways in the brain are able to process some amount of face identification based on surface features (Sergent’s “physiognomic invariants”) to elicit social biases, even though the identity of the face is not available to patients’ consciousness (Sergent & Poncet, 1990; Sergent & Signoret, 1992a; Tranel, 2000). Covert face recognition requires at least some degree of encoding of facial representation (Schweinberger & Burton, 2003). Face processing is mediated by a distributed neural system including the occipital face area (OFA) and the fusiform face area (FFA) (Schiltz et al., 2006; Schweinberger & Burton, 2003). The OFA is involved in the early perception of facial features, while the FFA represents a unified face configuration (Gauthier et al., 2000; Haxby et al., 2000; Liu, Harris, & Kanwisher, 2009; Nichols, Betts, & Wilson, 2010) and mediates the recognition of identity (Haxby et al., 2000; Schiltz et al., 2006). In addition to its connection to the fusiform gyrus for face processing (Freeman, Ambady, & Holcomb, 2010; A. Harris & G. K. Aguirre, 2008; A. M. Harris & G. K. Aguirre, 2008; Steeves et al., 2009), the OFA connects via alternative pathways to the prefrontal cortex (PFC) and anterior temporal lobes (Philippi, Mehta, Grabowski, Adolphs, & Rudrauf, 2009). Specifically, the inferior fronto-occipital fasciculus connects the occipital cortex with prefrontal areas, while the inferior longitudinal fasciculus connects the visual areas with the anterior temporal lobe and amygdala (Catani, Howard, Pajevic, & Jones, 2002; Catani, Jones, Donato, & Ffytche, 2003). These alternative pathways may allow retrieval of social biases even when the fusiform gyrus is lesioned (see also Fox, Iaria, & Barton, 2008) as they connect the OFA to ventral and medial PFC, which mediate social attitudes related to stereotypes (Gozzi, Raymont, Solomon, Koenigs, & Grafman, 2009; Knutson et al., 2007; Milne & Grafman, 2001; Quadflieg et al., 2009) and which mediate valence processing (Dolcos, LaBar, & Cabeza, 2004). The ventromedial PFC projects to and from the amygdala and hippocampus, allowing formation of somatic markers which may trigger a covert response (Damasio, Tranel, & Damasio, 1990). The anterior temporal lobes represent conceptual social knowledge (Zahn et al., 2007; Zahn et al., 2009) and the amygdala is well-known for its emotion-related responses to faces (Adolphs, 2002; Rolls, 2008). These connections might allow for non-conscious preferences or intuitions (see Lieberman, 2000) about faces to be retained. Taken together, these alternative pathways bypass the conscious recognition and face holistic processing that comes from fusiform gyrus activation, and can therefore allow the retrieval of social biases and valence information after viewing a face even when there is fusiform gyri damage and no overt recognition (Levine & Calvanio, 1989).
7. Conclusions
We have shown for the first time that the IAT effect can be added to the list of covert recognition effects found in prosopagnosia (Bobes et al., 2003; Schweinberger & Burton, 2003; Tranel, 2000; Tranel, Damasio, & Damasio, 1995). In some of the IATs, G.B. demonstrated the ability to perform covert recognition of faces without overt awareness of explicit information such as the name of the person or the category to which he or she belongs. Some of the attitudes went above and beyond stereotypes based on surface feature extraction such as skin color in the race IAT, but were based on knowledge of the person such as likability in the celebrity IAT. While G.B. did not have an IAT effect in each IAT, he did in two of the four, demonstrating that it is possible for a patient with prosopagnosia to show covert recognition of faces. We encourage further study of prosopagnosia using IATs on other patients.
Acknowledgments
We thank G.B. for his participation, Joshua Poore for his comments on an earlier manuscript, Tianxia Wu for her statistical guidance, and the anonymous reviewers for their insightful comments. This study was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health. The authors declare no conflict of interest. Some of the faces were obtained from the MacBrain Face Stimulus Set. Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set. The Caltech Frontal face dataset (Faces 1999) was collected by Markus Weber at the California Institute of Technology and can be obtained at http://www.vision.caltech.edu/html-files/archive.html.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kristine M. Knutson, Email: knutsonk@ninds.nih.gov.
Karen A. DeTucci, Email: kdetucci@yahoo.com.
References
- Adolphs R. Recognizing emotion from facial expressions: Psychological and neurologival mechanisms. Behavioral and Cognitive Neuroscience Reviews. 2002;1(1):21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Albert M, Butters N, Levin J. Temporal gradients in the retrograde amnesia of patients with alcoholic Korsakoff’s Disease. Archives of Neurology. 1979;36:211–216. doi: 10.1001/archneur.1979.00500400065010. [DOI] [PubMed] [Google Scholar]
- Arsalidou M, Barbeau EJ, Bayless SJ, Taylor MJ. Brain responses differ to faces of mothers and fathers. Brain and Cognition. 2010;74:47–51. doi: 10.1016/j.bandc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Barton JJS. Prosopagnosia associated with a left occipitotemporal lesion. Neuropsychologia. 2008a;46:2214–2224. doi: 10.1016/j.neuropsychologia.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Barton JJS. Structure and function in acquired prosopagnosia: Lessons from a series of 10 patients with brain damage. Journal of Neuropsychology. 2008b;2:197–225. doi: 10.1348/174866407x214172. [DOI] [PubMed] [Google Scholar]
- Barton JJS, Cherkasova MV, Hefter R. The covert priming effect of faces in prosopagnosia. Neurology. 2004;63(11):2062–2068. doi: 10.1212/01.wnl.0000145772.77040.24. [DOI] [PubMed] [Google Scholar]
- Bauer BM. Autonomic recognition of names and faces in prosopagnosia: A neuropsychological application of the Guilty Knowledge Test. Neuropsychologia. 1984;22(4):457–469. doi: 10.1016/0028-3932(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Bobes MA, Lopera F, Garcia M, Diaz-Comas L, Galan L, Valdes-Sosa M. Covert matching of unfamiliar faces in a case of prosopagnosia: An ERP study. Cortex. 2003;39:41–56. doi: 10.1016/s0010-9452(08)70073-x. [DOI] [PubMed] [Google Scholar]
- Bodamer J. Die Prosop-Agnosie. Archiv fur Psychiatrie und Nervenkrankheiten. 1947;179:6–53. doi: 10.1007/BF00352849. [DOI] [PubMed] [Google Scholar]
- Boucart M, Moroni C, Despretz P, Pasquier F, Fabre-Thorpe M. Rapid categorization of faces and objects in a patient with impaired object recognition. Neurocase. 2010;16(2):157–168. doi: 10.1080/13554790903339637. [DOI] [PubMed] [Google Scholar]
- Boutsen L, Humphreys GW. Face context interferes with local part processing in a prosopagnosic patient. Neuropsychologia. 2002;40:2305–2313. doi: 10.1016/s0028-3932(02)00088-x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Technical report C-1. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. Affective norms for English words (ANEW): Stimuli, instruction manual and affective ratings. [Google Scholar]
- Bruyer R, Laterre C, Seron X, Feyereisen P, Strypstein E, Pierrard E, Rectem D. A case of prosopagnosia with some preserved covert remembrance of familiar faces. Brain Cognition. 1983;2:257–284. doi: 10.1016/0278-2626(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Busigny T, Rossion B. Acquired prosopagnosia abolishes the face inversion effect. Cortex. 2010;46:965–981. doi: 10.1016/j.cortex.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Carey S, Diamond R. From piecemeal to configurational representation of faces. Science. 1977;195:312–314. doi: 10.1126/science.831281. [DOI] [PubMed] [Google Scholar]
- Carey S, Diamond R, Woods B. Development of face recognition --A maturational component. Developmental Psychology. 1980;16:257–269. [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Clarke S, Lindemann A, Maeder P, Borruat FX, Assal G. Face recognition and postero-inferior hemispheric lesions. Neuropsychologia. 1997;35(12):1555–1563. doi: 10.1016/s0028-3932(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH, Porter S. Point and interval estimates of effect sizes for the case-controls design in neuropsychology: Rationale, methods, implementations, and proposed reporting standards. Cognitive Neuropsychology. 2010;27(3):245–260. doi: 10.1080/02643294.2010.513967. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. The Clinical Neuropsychologist. 1998;12:482–486. [Google Scholar]
- Damasio AR, Damasio H, van Hoesen GW. Prosopagnosia: Anatomic basis and behavioral mechanisms. Neurology. 1982;32:331–341. doi: 10.1212/wnl.32.4.331. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- de Haan EHF, Bauer RM, Greve KW. Behavioural and physiological evidence for covert face recognition in a prosopagnosic patient. Cortex. 1992;28(1):77–95. doi: 10.1016/s0010-9452(13)80167-0. [DOI] [PubMed] [Google Scholar]
- de Haan EHF, Young A, Newcombe F. Face recognition without awareness. Cognitive Neuropsychology. 1987a;4(4):385–415. [Google Scholar]
- de Haan EHF, Young A, Newcombe F. Faces interfere with name classification in a prosopagnosia patient. Cortex. 1987b;23:309–316. doi: 10.1016/s0010-9452(87)80041-2. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. NeuroImage. 2004;23(1):64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3(11):1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Ellis HD, Florence M. Bodamer’s (1947) paper on prosopagnosia. Cognitive Neuropsychology. 1990;7(2):81–105. [Google Scholar]
- Fox CJ, Iaria G, Barton JJS. Disconnection in prosopagnosia and face processing. Cortex. 2008;44:996–1009. doi: 10.1016/j.cortex.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Ambady N, Holcomb PJ. The face-sensitive N170 encodes social category information. Neuroreport. 2010;21:24–28. doi: 10.1097/WNR.0b013e3283320d54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. Journal of Cognitive Neuroscience. 2000;12(3):495. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Glick P, Fiske ST. The Ambivalent Sexism Inventory: Differentiating hostile and benevolent sexism. Journal of Personality and Social Psychology. 1996;70(3):491–512. [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Tempini ML. The neural systems sustaining face and proper-name processing. Brain. 1998;121(11):2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Gozzi M, Raymont V, Solomon J, Koenigs M, Grafman J. Dissociable effects of prefrontal and anterior temporal cortical lesions on stereotypical gender attitudes. Neuropsychologia. 2009;47:2125–2132. doi: 10.1016/j.neuropsychologia.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Ponto LLB, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Human Brain Mapping. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J, Salazar AM, Weingartner H, Amin D. Face memory and discrimination: An analysis of the persistent effects of penetrating brain wounds. International Journal of Neuroscience. 1986;29(1):125–139. doi: 10.3109/00207458608985643. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: The Implicit Association Test. Journal of Personality and Social Psychology. 1998;74:1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA. Health of the Implicit Association Test at age 3. Zeitschrift fur Experimentelle Psychologie. 2001;48(2):85–93. doi: 10.1026//0949-3946.48.2.85. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and Using the Implicit Association Test: I. An Improved Scoring Algorithm. Journal of Personality and Social Psychology. 2003;85(2):197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Harris A, Aguirre GK. The representation of parts and wholes in face-selective cortex. Journal of Cognitive Neuroscience. 2008;20(5):863–878. doi: 10.1162/jocn.2008.20509. [DOI] [PubMed] [Google Scholar]
- Harris AM, Aguirre GK. The effects of parts, wholes, and familiarity on face-selective responses in MEG. Journal of Vision. 2008;8(10):1–12. doi: 10.1167/8.10.4. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hicks JL, Marsh RL. Remember-know judgments can depend on how memory is tested. Psychonomic Bulletin & Review. 1999;6(1):117–122. doi: 10.3758/bf03210818. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Gawronski B, Gschwendner T, Le H, Schmitt M. A meta-analysis on the correlation between the Implicit Association Test and explicit self-report measures. Personality and Social Psychology Bulletin. 2005;31(10):1369–1385. doi: 10.1177/0146167205275613. [DOI] [PubMed] [Google Scholar]
- Jones RD, Tranel D. Severe developmental prosopagnosia in a child with superior intellect. Journal of Clinical and Experimental Neuropsychology. 2001;23(3):265–273. doi: 10.1076/jcen.23.3.265.1183. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Stanley D, Harris A. The fusiform face area is selective for faces not animals. Neuroreport. 1999;10(1):183. doi: 10.1097/00001756-199901180-00035. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Human Brain Mapping. 2007;28:915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KM, Wood JN, Spampinato MV, Grafman J. Politics on the brain: an fMRI investigation. Social Neuroscience. 2006;1:25–40. doi: 10.1080/17470910600670603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DN, Calvanio R. Prosopagnosia: A defect in visual configural processing. Brain and Cognition. 1989;10:149–170. doi: 10.1016/0278-2626(89)90051-1. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Intuition: A social cognitive neuroscience approach. Psychological Bulletin. 2000;126:109–137. doi: 10.1037/0033-2909.126.1.109. [DOI] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N. Perception of face parts and face configurations: An fMRI study. Journal of Cognitive Neuroscience. 2009;22(1):203–211. doi: 10.1162/jocn.2009.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahay JB, Hardee BB, Batts V. Has racism declined in America? It depends on who is asking and what is asked. Journal of Conflict Resolution. 1981;25(4):563–579. [Google Scholar]
- Meadows JC. The anatomical basis of prosopagnosia. Journal of Neurology and Neurosurgical Psychiatry. 1974;37:489–501. doi: 10.1136/jnnp.37.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne E, Grafman J. Ventromedial prefrontal cortex lesions in humans eliminate implicit gender stereotyping. Journal of Neuroscience. 2001;21:RC150, 151–156. doi: 10.1523/JNEUROSCI.21-12-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith MJ, Voils CI. Proneness to prejudiced responses: Toward understanding the authenticity of self-reported discrepancies. Journal of Personality & Social Psychology. 1998;75(4):901–916. doi: 10.1037//0022-3514.75.4.901. [DOI] [PubMed] [Google Scholar]
- Nichols DF, Betts LR, Wilson HR. Decoding of faces and face components in face-sensitive human visual cortex. Frontiers in Psychology. 2010;1(28):1–13. doi: 10.3389/fpsyg.2010.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosek BA, Banaji MR, Greenwald AG. Harvesting implicit group attitudes and beliefs from a demonstration web site. Group Dynamics: Theory, Research, and Practice. 2002;6(1):101–115. [Google Scholar]
- Orban de Xivry JJ, Ramon M, Lefevre P, Rossion B. Reduced fixation on the upper area of personally familiar faces following acquired prosopagnosia. Journal of Neuropsychology. 2008;2:245–268. doi: 10.1348/174866407x260199. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. The Journal of Neuroscience. 2009;29(48):15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadflieg S, Turk DJ, Waiter GD, Mitchell JP, Jenkins AC, Macrae CN. Exploring the neural correlates of social stereotyping. Journal of Cognitive Neuroscience. 2009;21(8):1560–1570. doi: 10.1162/jocn.2009.21091. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Hurtig R, Damasio AR. The role of scanpaths in facial recognition and learning. Annals of Neurology. 1987;22(1):41–45. doi: 10.1002/ana.410220111. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Face processing in different brain areas, and critical band masking. Journal of Neuropsychology. 2008;2:325–360. doi: 10.1348/174866407x258903. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- Saumier D, Arguin M, Lassonde M. Prosopagnosia: A case study involving problems in processing configural information. Brain and Cognition. 2001;46:255–316. doi: 10.1006/brcg.2000.1271. [DOI] [PubMed] [Google Scholar]
- Schiltz C, Sorger B, Caldara R, Ahmed F, Mayer E, Goebel R, Rossion B. Impaired face discrimination in acquired prosopagnosia is associated with abnormal response to individual faces in the right middle fusiform gyrus. Cerebral Cortex. 2006;16:574–586. doi: 10.1093/cercor/bhj005. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Burton AM. Covert recognition and the neural system for face processing. Cortex. 2003;39:9–30. doi: 10.1016/s0010-9452(08)70071-6. [DOI] [PubMed] [Google Scholar]
- Sergent J, Poncet M. From covert to overt recognition of faces in a prosopagnosic patient. Brain. 1990;113:989–1004. doi: 10.1093/brain/113.4.989. [DOI] [PubMed] [Google Scholar]
- Sergent J, Signoret JL. Implicit access to knowledge derived from unrecognized faces in prosopagnosia. Cerebral Cortex. 1992a;2:389–400. doi: 10.1093/cercor/2.5.389. [DOI] [PubMed] [Google Scholar]
- Sergent J, Signoret JL. Varieties of functional deficits in prosopagnosia. Cerebral Cortex. 1992b;2:375–388. doi: 10.1093/cercor/2.5.375. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf JF. Biometry. San Francisco, CA: Freeman; 1995. [Google Scholar]
- Sorger B, Goebel R, Schiltz C, Rossion B. Understanding the functional neuroanatomy of acquired prosopagnosia. Neuroimage. 2007;35(2):836–852. doi: 10.1016/j.neuroimage.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Spence JT, Helmreich R. The attitudes toward women scale: An objective instrument to measure attitudes toward the rights and roles of women in contemporary society. JSAS Catalog of Selected Documents in Psychology. 1972;67:66–67. [Google Scholar]
- Steeves J, Dricot L, Goltz HC, Sorger B, Peters J, Milner AD, Rossion B. Abnormal face identity coding in the middle fusiform gyrus of two brain-damaged prosopagnosic patients. Neuropsychologia. 2009;47(12):2584–2592. doi: 10.1016/j.neuropsychologia.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M, Hirayama K, Shiota J, Isono O. Prospagnosia: A clinical and anatomical study of four patients. Cortex. 1995;31:317–329. doi: 10.1016/s0010-9452(13)80365-6. [DOI] [PubMed] [Google Scholar]
- Tanskanen T, Nasanen R, Ojanpaa H, Hari R. Face recognition and cortical responses: Effect of stimulus duration. Neuroimage. 2007;35(4):1636–1644. doi: 10.1016/j.neuroimage.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Todorov A, Duchaine B. Reading trustworthiness in faces without recognizing faces. Cognitive Neuropsychology. 2008;25(3):395–410. doi: 10.1080/02643290802044996. [DOI] [PubMed] [Google Scholar]
- Tranel D. Non-conscious brain processing indexed by psychophysiological measures. In: Mayer EA, Saper CB, editors. The Biological Basis for Mind Body Interactions. Vol. 122. Elsevier Science BV; 2000. pp. 317–332. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR. Knowledge without awareness: An automomic index of facial recognition by prosopagnosics. Science. 1985;228:1453–1454. doi: 10.1126/science.4012303. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR. Non-conscious face recognition in patients with face agnosia. Behavioral Brain Research. 1988;30(3):235–249. doi: 10.1016/0166-4328(88)90166-0. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio A. Double dissociation between overt and covert face recognition. Journal of Cognitive Neuroscience. 1995;7(4):425–432. doi: 10.1162/jocn.1995.7.4.425. [DOI] [PubMed] [Google Scholar]
- Van Belle G, De Graef P, Verfaillie K, Busigny T, Rossion B. Whole not hole: Expert face recognition requires holistic perception. Neuropsychologia. 2010 doi: 10.1016/j.neuropsychologia.2010.04.034. epub. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. A new test of object decision: 2D silhouettes featuring a minimal view. Cortex. 1991;27(3):370–383. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale: Revised. New York: Psychological Corporation, Harcourt Brace Jovanovich; 1981. [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wittenbrink B, Judd CM, Park B. Evidence for racial prejudice at the implicit level and its relationship with questionnaire measures. Journal of Personality & Social Psychology. 1997;72(2):262–274. doi: 10.1037//0022-3514.72.2.262. [DOI] [PubMed] [Google Scholar]
- Wyer RSJ, Budesheim TL, Shavitt S, Riggle ED, Melton RJ, Kuklinski JH. Image, issues, and idealogy: The processing of information about political candidates. Journal of Personality and Social Psychology. 1991a;61(4):533–545. doi: 10.1037//0022-3514.61.4.533. [DOI] [PubMed] [Google Scholar]
- Wyer RSJ, Budesheim TL, Shavitt S, Riggle ED, Melton RJ, Kuklinski JH. Image, issues, and ideology: The processing of information about political candidates. Journal of Personality and Social Psychology. 1991b;61:533–545. doi: 10.1037//0022-3514.61.4.533. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience. 2005;25(11):3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AW, Hellawell D, De Haan EH. Cross-domain semantic priming in normal subjects and a prosopagnosic patient. The Quarterly Journal of Experimental Psychology, A. 1988;40:561–580. doi: 10.1080/02724988843000087. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(15):6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, Grafman J. The neural basis of human social values: Evidence from functional MRI. Cerebral Cortex. 2009;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]



