Abstract
Genome-wide expression analysis of an industrial strain of Saccharomyces cerevisiae identified the YOR387c and YGL258w homologues as highly inducible in zinc-depleted conditions. Induction was specific for zinc deficiency and was dependent on Zap1p. The results indicate that these sequences may be valuable molecular markers for detecting zinc deficiency in industrial fermentations.
Deficiency of the essential nutrient zinc is a major contributor to retarded yeast fermentation in the brewing process(4). During the processing of wort, zinc ions can form complexes with polyphenols and can also form insoluble complexes of alpha acids (12). In this form the zinc is not accessible to yeast, and direct methods of measuring zinc in wort do not always accurately predict its availability to the yeast. Molecular markers have been used as an alternative method to indirectly monitor fermentation processes through yeast gene expression. Genes encoding heat shock (HSP12) and osmotic shock (SPI1) proteins were used as predictors of stress conditions in industrial fermentations (2, 17) and the maltose genes (MAL) have been used to monitor conditions that affect yeast fermentation activity (19). However, because these genes respond to a broad range of conditions, their usefulness for the identification of the specific cause of a defective fermentation is limited. Here we identify molecular markers useful in monitoring industrial fermentations specifically for conditions of zinc deficiency.
To identify genes useful as molecular markers, genome-wide expression analysis was performed on the Saccharomyces cerevisiae industrial Lager 1 strain (6) growing in zinc-depleted and zinc-replete conditions. The low-zinc medium was essentially LZM (23) except that the carbon source was changed to maltose (LZMM), which is the most abundant sugar available to yeast in beer fermentations (21). Lager 1 seed cultures actively growing in LZMM-40 μM ZnSO4 were harvested and washed three times with sterile distilled water before inoculation into LZMM with and without 40 μM ZnSO4. RNA was isolated from cells at 4 h, when the rates of growth of the two cultures were beginning to diverge (Fig. 1). Radiolabeled (33P) cDNA produced from isolated RNA was hybridized to GeneFilters microarrays (Research Genetics) as outlined by Higgins et al. (10). Genes that were induced or repressed more than fivefold in zinc-depleted conditions are listed in Table 1. The majority of the induced genes have been previously reported to be possible targets for the transcriptional activators Zap1p (14) and Msn2p-Msn4p(8) and were therefore subsequently grouped into these categories. The metalloregulatory protein Zap1p is involved in zinc-responsive transcriptional regulation in Saccharomyces cerevisiae (25, 26). The ZRT1 and ZRT2 genes (induced 8.1- and 7.7-fold, respectively) encode high- and low-affinity zinc permeases, respectively, and both are Zap1p targets (23, 24). Another Zap1p target induced was the ZRT3 gene (induced 8.3-fold), which encodes the yeast vacuolar permease (15). Up-regulation of these genes would enhance the ability of the Lager 1 strain to maintain intracellular zinc levels. The HSP12 and HSP26 genes, encoding heat shock proteins, were also induced by zinc depletion (Table 1). Both of these are targets of Msn2p-Msn4p and, unlike the Zap1p targets, are induced by a broad range of conditions, including general starvation (1, 18). The presence of a general starvation response is further highlighted by the repression of genes involved in sugar utilization (MAL genes), ethanol production (ADH1), and ribosomal functions (Table 1), all of which are vital for yeast growth and metabolic activity.
FIG. 1.
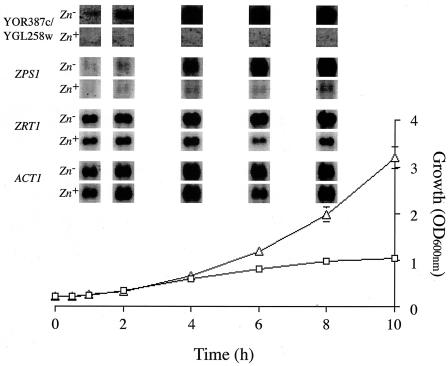
YOR387c and YGL258w differential gene expression is the most rapid and responsive to zinc depletion. Northern blot kinetic analysis of YOR387c and YGL258w, ZPS1, ZRT1, and ACT1 gene expression was performed with total RNA isolated from Lager 1 cells grown in LZMM with (□; Zn+) and without (▵; Zn−) 40 μM ZnSO4 added. Each Northern blot is a representative of a duplicate experiment, and the growth curves are the means of triplicate readings of duplicate experiments with lower than 12% standard errors. OD600nm, optical density at 600 nm.
TABLE 1.
Highly differentially expressed Lager 1 genes (more than five fold) after growth in zinc-depleted conditions compared to zinc-replete conditions
| Open reading frame | Gene name | Fold induction or repressiona | Gene or protein descriptionb |
|---|---|---|---|
| Induced by zinc depletion | |||
| Putative Zap 1p target genes | |||
| YOR387c | 80 | ||
| YGL258w | 63 | ||
| YOL154w | ZPS1 | 28 | |
| YGL256w | ADH4 | 13 | Alcohol dehydrogenase isoenzyme IV |
| YJL056c | ZAP1 | 10 | Involved in zinc-responsive transcriptional regulation |
| YBL049w | 8.5 | ||
| YKL175w | ZRT3 | 8.3 | Vacuolar Zn efflux |
| YGL255w | ZRT1 | 8.1 | High-affinity zinc transport protein |
| YLR130c | ZRT2 | 7.7 | Low-affinity zinc transport protein |
| YDR284c | DPP1 | 6.8 | Diacylglycerol pyrophosphate phosphatase |
| YGL121c | GPG1 | 6.2 | G protein gamma |
| Putative Msn2p-Msn4p target genes | |||
| YPL223c | GRE1 | 27 | Hydrophilic polypeptide with no known homology |
| YGR088w | CTT1 | 26 | Cytoplasmic catalase T |
| YDR070c | 26 | ||
| YBR072w | HSP26 | 25 | Heat shock protein 26 |
| YMR181c | 23 | ||
| YMR175w | SIP18 | 17 | Salt-induced protein of 18 kDa |
| YOL053c-A | DDR2 | 12 | Induced by heat shock, osmotic shock, and oxidative stress |
| YIR038c | GTT1 | 10 | Glutathione transferase |
| YML128c | GIN3 | 9.5 | Meiotic sister chromatid recombination |
| YFL014w | HSP12 | 9 | 12-kDa heat shock protein |
| YDL124w | 9 | NADPH-dependent reductase | |
| YHR138c | 8.6 | ||
| YBL075c | SSA3 | 7.7 | Cytosolic member of the 70-kDa heat shock protein family |
| YMR107w | 7.5 | ||
| YKL151c | 7.2 | ||
| YHR096c | HXT5 | 6.9 | Hexose transporter |
| YGR256w | GND2 | 6.4 | 6-Phosphogluconate dehydrogenase |
| YDL204w | 6.1 | ||
| YHR104w | GRE3 | 5.8 | α-Keto-aldose reductase |
| YBR116c | 5.7 | ||
| YER150w | SPI1 | 5.6 | Component of cell wall |
| YKL163w | PIR3 | 5.2 | Protein containing tandem internal repeats |
| YKL150w | MCR1 | 5.0 | NADH-cytochrome b5 reductase |
| Other | |||
| YOR374w | ALD4 | 24 | Mitochondrial aldehyde dehydrogenase |
| YER073w | ALD5 | 9.5 | Mitochondrial aldehyde dehydrogenase |
| YNL172w | APC1 | 8.2 | Subunit of ubiquitin-protein ligase |
| YNL056w | 8.2 | ||
| YOL082w | 7.8 | ||
| YMR173w | DDR48 | 7.3 | Flocculent-specific protein |
| YMR118c | 6.9 | Induced by starvation | |
| YLR136c | TIS11 | 6.7 | Homologue of mammalian TIS11 |
| YMR173w-A | 6.4 | Overlaps DDR48 | |
| YNL239w | LAP3 | 6.1 | Aminopeptidase of cysteine protease family |
| YNR077c | 5.8 | ||
| YDR543c | 5.6 | ||
| YKL065c | YET1 | 5.4 | Yeast endoplasmic reticulum 25-kDa transmembrane protein |
| YBR214w | SDS24 | 5.1 | Nuclear protein similar to Schizosaccharomyces pombe sds23 |
| YIR037w | HYR1 | 5.1 | Putative glutathione peroxidase |
| Repressed by zinc depletion | |||
| YOL086c | ADH1 | 15 | Alcohol dehydrogenase |
| YMR116c | ASC1 | 10 | WD repeat protein (G-beta-like protein) |
| YLR150w | MPT4 | 9.2 | Telomeric DNA binding activity |
| YBR299w | MAL32 | 9.1 | α-Glucosidase, hydrolyses maltose |
| YGR292w | MAL12 | 8.5 | α-Glucosidase, hydrolyses maltose |
| YBR298c | MAL31 | 7.2 | High-affinity maltose permease |
| YHR183w | GND1 | 5.3 | 6-Phosphogluconate dehydrogenase/PICK> |
| Ribosomal protein subunits | |||
| YPL131w | RPL1 | ||
| YOR063w | RPL3 | 9.1 | |
| YGL147c | RPL9A | 7.6 | |
| YLR325c | RPL38 | 6.4 | |
| YHR010w | RPL27 | 5.8 | |
| YLR029c | RPL13A | 5.8 | |
| YJL177w | RPL17B | 5.7 | |
| YKR094c | RPL40B | 5.7 | |
| YOR293w | RPS10A | 5.6 | |
| YOR096w | RPS30 | 5.5 | |
| YKL180w | RPL17 | 5.5 | |
| YLR048w | RPS0B | 5.5 | |
| YHR203c | RPS7A | 5.4 | |
| YGL123w | RPS2 | 5.3 | |
| YLR062c | 5.3 | ||
| YML073c | RPL6A | 5.2 | |
| YBR191w | RPL21A | 5.1 | |
| YDR450w | RPS18A | 5.1 | |
| YGR027c | RPS31A | 5.0 | |
| YIL148w | RPL40A | 5.0 |
Induction factors were calculated by dividing the expression levels in zinc-depleted conditions by those in zinc-replete conditions. Values are means of duplicate experiments for genes with less than 20% variation between the duplicate experiments.
Gene annotations were obtained with GeneSpring (A. R. Conway, GeneSpring, version 5.0, Silicon Genetics, Redwood City, Calif., 2002).
Further expression analyses were performed on highly induced candidate genes from the listed Zap1p targets in Table 1. These were chosen for their likely specificity for zinc depletion since those activated by the Msn2p-Msn4p complex are subject to a wide range of stresses (16, 20). The Lager 1 genes most highly induced in response to zinc-depleted conditions were YOR387c and YGL258w (Table 1), both of which have no known cell function (11). They are highly homologous genes, with over 92% DNA identity in their coding regions; this homology probably results in cross-hybridization during expression analyses. Since the promoter regions of these genes are almost identical (99.6% over 1 kb of upstream sequence), both transcripts were regarded as one in subsequent tests. The other genes selected for analysis were ZRT1, based on its specificity to zinc-depleted conditions (23), and the highly induced gene ZPS1 (Table 1), whose product has weak homology to zinc metalloproteinases (11). The kinetics and degrees of induction of the selected genes were measured by Northern analyses (10) during a 10-h incubation in zinc-depleted and zinc-replete medium. By the fourth hour all four genes were induced in zinc-depleted conditions (Fig. 1), validating the expression patterns seen in the genome-wide expression analysis (Table 1). YOR387c and YGL258w were rapidly induced, with a very clear increase in transcripts by the second hour of exposure to zinc depletion (Fig. 1). This analysis shows that the highly differential nature of expression of YOR387c and YGL258w and of ZPS1 (Table 1) can be attributed not only to very high levels of expression in zinc-depleted conditions but also to very low expression levels when zinc was present. This is in contrast to what was found for ZRT1, which, although it has increased expression in zinc-depleted conditions, has a relatively high basal level, effectively decreasing its apparent induction. This was surprising since an analysis of ZRT1 expression in a laboratory yeast strain did not show high basal levels (23). This characteristic may be unique to the Lager 1 strain, or other mechanisms may be involved, a possibility supported by observations that changes in nitrogen source affect ZRT1 expression levels (5). Although highly induced by zinc depletion, ZPS1 expression was also found to be affected by changes in pH (13). This is unlikely with YOR387c and YGL258w since an investigation of microarray data using the Yeast Microarray Global Viewer (Laboratoire de Genetique Moleculaire, Ecole Normale Superieure, Paris, France [http://transcriptome.ens.fr/ymgv/who.php]) did not identify any other conditions that significantly affected its expression.
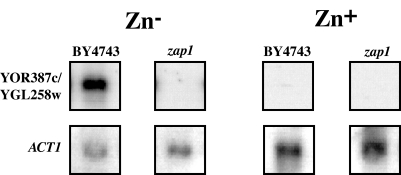
YOR387c and YGL258w were identified in a genome-wide characterization of the zinc-responsive regulon (14) as possible Zap1p targets. To verify this, YOR387c and YGL258w expression in a zap1Δ mutant was measured. The YOR387c and YGL258w transcripts were not evident in the zap1Δ mutant when the mutant was grown in zinc-depleted conditions, whereas they were characteristically present at high levels in the wild type (Fig. 2). This confirmed that YOR387c and YGL258w expression is induced through the Zap1p transcriptional activator in response to zinc deficiency.
FIG. 2.
Induction of YOR387c and YGL258w in zinc-depleted conditions is dependent on Zap1p. YOR387c and YGL258w and ACT1 expression in wild-type (BY4743) and zap1Δ mutant cells grown in Chelex-treated synthetic defined (CSD) medium (14) with (Zn+) and without (Zn−) 10 μM ZnSO4 added was measured.
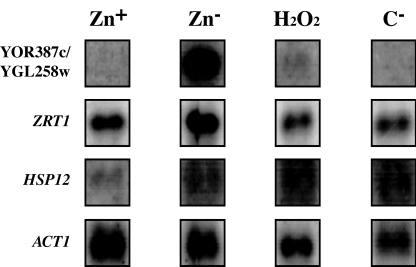
To further analyze the specificity of YOR387c and YGL258w induction, their expression in a Lager 1 strain exposed to oxidative stress and carbon starvation, two conditions known to impact yeast during industrial fermentations (3), was measured. Figure 3 shows that YOR387c and YGL258w expression was not induced in these conditions. A similar pattern of induction was observed with the ZRT1 transcript; however, the basal level of expression in unstressed conditions was higher. The integrity of the stress conditions was confirmed by the induction of the Msn2p-Msn4p-activated gene, HSP12, in all stress conditions compared to induction in the control medium (Fig. 3).
FIG. 3.
YOR387c and YGL258w expression is not induced by oxidative stress or carbon starvation. YOR387c and YGL258w, ZRT1, HSP12, and ACT1 expression was measured in Lager 1 cells grown in modified LZMM with (Zn+) or without (Zn−) 40 μM ZnSO4 added, LZMM (Zn+) with 2 mM H2O2 added (H2O2), and LZMM (Zn+) with no added maltose (C−).
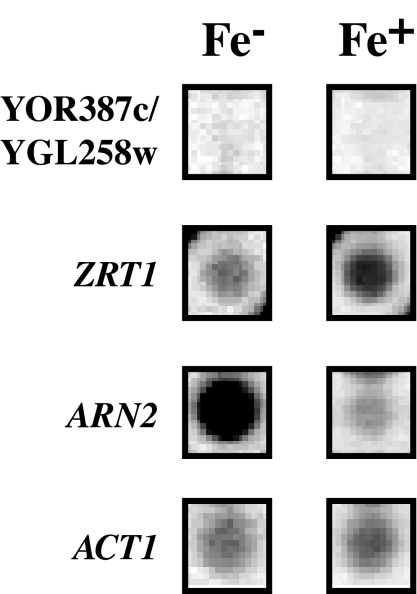
Iron, another trace element in yeast, is, like zinc, essential for yeast growth and metabolic activity (22). To determine the effect of iron depletion on YOR387c and YGL258w expression, transcript levels in the Lager 1 strain growing in low-iron medium (7) were measured by genome-wide expression analysis. From Fig. 4 it is clear that YOR387c and YGL258w were not induced in response to iron-depleted conditions and therefore are not part of a general response to depletion of divalent cations. ZRT1 on the other hand showed some induction. The induction of the ARN2 gene, which encodes a siderochrome iron transporter (9), confirmed the iron-depleted condition (Fig. 4).
FIG. 4.
YOR387c and YGL258w expression is not induced by iron depletion. Shown are YOR387c and YGL258w, ZRT1, ARN2, and ACT1 expression patterns from GeneFilters microarray analysis of Lager 1 cells grown for 4 h in low-iron medium with (Fe+) and without (Fe−) 25 μM FeCl3 added.
Genome-wide expression analysis was useful in identifying YOR387c and YGL258w as molecular markers of zinc-deficient conditions. The expression of these genes was highly inducible from a virtually undetectable basal level, making them easily discernible targets for detection of differential expression by rapid methods of gene transcript analysis such as real-time PCR. These experiments and publicly available microarray information confirmed the specificity of YOR387c and YGL258w induction for zinc-depleted conditions and show that they are induced 2 h before zinc depletion is seen to affect growth of the culture to a significant level. They are therefore not only effective predictors of defective fermentation but also identifiers of the actual cause so that subsequent preventative measures can be carried out.
Acknowledgments
This work was supported by Linkage Project grant LP0210873 from the Australian Research Council and Carlton United Breweries.
We thank Geoff Kornfeld and Anthony Beckhouse for support and assistance.
REFERENCES
- 1.Amoros, M., and F. Estruch. 2001. Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae genes HSP26 and HSP104 in a gene- and stress type-dependent manner. Mol. Microbiol. 39:1523-1532. [DOI] [PubMed] [Google Scholar]
- 2.Aranda, A., A. Querol, and M. L. del Olmo. 2002. Correlation between acetaldehyde and ethanol resistance and expression of HSP genes in yeast strains isolated during the biological aging of sherry wines. Arch. Microbiol. 177:304-312. [DOI] [PubMed] [Google Scholar]
- 3.Attfield, P. V. 1997. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat. Biotechnol. 15:1351-1357. [DOI] [PubMed] [Google Scholar]
- 4.Bromberg, S. K., P. A. Bower, G. R. Duncombe, J. Fehring, L. Gerber, V. K. Lau, and M. Tata. 1997. Requirements for zinc, manganese, calcium, and magnesium in wort. J. Am. Soc. Brew. Chem. 55:123-128. [Google Scholar]
- 5.Cox, K. H., A. B. Pinchak, and T. G. Cooper. 1999. Genome-wide transcriptional analysis in S. cerevisiae by mini-array membrane hybridisation. Yeast 15:703-713. [DOI] [PubMed] [Google Scholar]
- 6.Day, R. E., P. J. Rogers, I. W. Dawes, and V. J. Higgins. 2002. Molecular analysis of maltotriose transport and utilization by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 68:5326-5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eide, D., and L. Guarente. 1992. Increased dosage of a transcriptional activator gene enhances iron-limited growth of Saccharomyces cerevisiae. J. Gen. Microbiol. 138:347-354. [DOI] [PubMed] [Google Scholar]
- 8.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heymann, P., J. F. Ernst, and G. Winkelmann. 1999. Identification of a fungal triacetylfusarinine C siderophore transport gene (TAF1) in Saccharomyces cerevisiae as a member of the major facilitator superfamily. Biometals 12:301-306. [DOI] [PubMed] [Google Scholar]
- 10.Higgins, V. J., A. G. Beckhouse, A. D. Oliver, P. J. Rogers, and I. W. Dawes. 2003. Yeast genome-wide expression analysis identifies a strong ergosterol and oxidative stress response during the initial stages of an industrial lager fermentation. Appl. Environ. Microbiol. 69:4777-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges, P. E., A. H. Z. McKee, B. P. Davis, W. E. Payne, and J. I. Garrels. 1999. Yeast Protein Database (YPD): a model for the organization and presentation of genome-wide functional data. Nucleic Acids Res. 27:69-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreder, G. C. 1999. Yeast assimilation of trub-bound zinc. J. Am. Soc. Brew. Chem. 57:129-132. [Google Scholar]
- 13.Lamb, T. M., W. Xu, A. Diamond, and A. P. Mitchell. 2001. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276:1850-1856. [DOI] [PubMed] [Google Scholar]
- 14.Lyons, T. J., A. P. Gasch, L. A. Gaither, D. Botstein, P. O. Brown, and D. J. Eide. 2000. Genome-wide characterization of the Zap1p zinc-responsive regulon in yeast. Proc. Natl. Acad. Sci. USA 97:7957-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDiarmid, C. W., L. A. Gaither, and D. J. Eide. 2000. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 19:2845-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Pastor, M. T., G. Marchler, C. Schuller, A. Marchler-Bauer, H. Ruis, and F. Estruch. 1996. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress-response element (STRE). EMBO J. 15:2227-2235. [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Torrado, R., P. Carrasco, A. Aranda, J. Gimeno-Alcaniz, J. E. Perez-Ortin, E. Matallana, and M. L. del Olmo. 2002. Study of the first hours of microvinification by the use of osmotic stress-response genes as probes. Syst. Appl. Microbiol. 25:153-161. [DOI] [PubMed] [Google Scholar]
- 18.Praekelt, U. M., and P. A. Meacock. 1990. HSP12, a new small heat-shock gene of Saccharomyces cerevisiae—analysis of structure, regulation and function. Mol. Gen. Genet. 223:97-106. [DOI] [PubMed] [Google Scholar]
- 19.Rogers, P. J., I. W. Dawes, A. D. Oliver, R. E. Day, and V. J. Higgins. 2002. Effective communication with yeast through gene expression using genome-wide transcriptional analysis, paper 18, p. 1-6. In Proceedings of the 27th Convention of the Institute and Guild of Brewing—Asia Pacific Section, Adelaide, Australia. The Institute and Guild of Brewing, London, United Kingdom.
- 20.Schmitt, A. P., and K. McEntee. 1996. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93:5777-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stambuk, B. U., and P. S. de Araujo. 2001. Kinetics of active α-glucoside transport in Saccharomyces cerevisiae. FEMS Yeast Res. 1:73-78. [DOI] [PubMed] [Google Scholar]
- 22.Van Ho, A., D. McVey Ward, and J. Raplan. 2002. Transition metal transport in yeast. Annu. Rev. Microbiol. 56:237-261. [DOI] [PubMed] [Google Scholar]
- 23.Zhao, H., and D. Eide. 1996. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc. Natl. Acad. Sci. USA 93:2454-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao, H., and D. Eide. 1996. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J. Biol. Chem. 271:23203-23210. [DOI] [PubMed] [Google Scholar]
- 25.Zhao, H., and D. J. Eide. 1997. Zap1p, a metalloregulatory protein involved in zinc-responsive transcriptional regulation in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:5044-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao, H., E. Butler, J. Rodgers, T. Spizzo, S. Duesterhoeft, and D. Eide. 1998. Regulation of zinc homeostasis in yeast by binding of the ZAP1 transcriptional activator to zinc-responsive promoter elements. J. Biol. Chem. 273:28713-28720. [DOI] [PubMed] [Google Scholar]