Introduction
The female reproductive axis is unique in that it reaches a senescent state when other organs in the body are generally healthy. The process of oocyte depletion, which begins before birth and ends with menopause, cannot be predicted precisely by chronological age, as its age of onset varies greatly between women. However, a clinical staging system exists, which makes it possible to identify where a woman is in her process of reproductive aging based on her bleeding patterns, which is a better predictor than her age. Staging is useful for several reasons, among them providing a means to attribute women's symptoms during this time to menopausal changes, predicting time to final menstrual period, and identifying health risks. There are now well described symptoms that are linked to specific time points along the menopausal transition, thus validating the concept that menstrual cycle disruption, along with its underlying hormonal changes, are responsible for the common symptoms of the menopausal transition. Moreover, evidence is accruing that at least some menopausal symptoms not previously attributed to estrogen deficiency are successfully treated by exogenous hormones.
In this review, we will describe the known hormonal changes that occur across the menopausal transition and their association with various symptoms and markers of health in women traversing the menopause. We will base our conclusions on the major findings of large chohort studies (N>300) that have been community or population based. Finally, we will present key clinical scenarios in which short term menopausal hormone therapy is likely to be effective.
Staging Reproductive Aging
The STRAW System
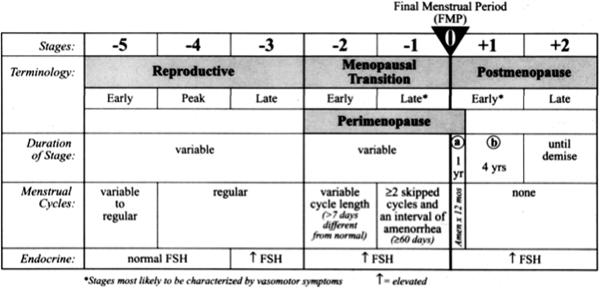
The first nomenclature for the stages of the menopausal transition was developed at the Stages of Reproductive Aging Workshop (STRAW) in Park City Utah in July 2001. Although the Tanner/Marshall staging system is well established for defining the stages of puberty (1), prior to the STRAW workshop there was no similarly accepted system for defining the stages leading to menopause (2). Evidence along with expert opinion was used to develop the staging nomenclature (2).
The STRAW stages are shown in Figure 1. The final menstrual period (FMP), Stage 0, anchors the stages, which are numbered from −5 to +2 (2). The reproductive interval includes stages −5 to −3. In the early reproductive stage, stage −5, menstrual cycles are variable to regular and follicle stimulating hormone (FSH) is well within the normal range. This interval refers to the post-menarcheal period, before menstrual cycles become regular after menarche, which demonstrates considerable inter-individual variation. In the peak reproductive stage, −4, cycles are regular (every 25–35 days; (3)) and FSH remains normal. Again, the duration of this stage is variable (2).
Figure 1.
The late reproductive stage, −3, encompasses a period of regular cycling during which time elevated FSH begins to occur, heralding a biochemically, but not otherwise clinically detectable decline in ovarian reserve (2). Most clinical assays use a 10 mlU/ml FSH level as the cutoff value between normal and diminished ovarian reserve. It is preferable but not always feasible to determine a threshold for the value specific to the laboratory that exceeds two standard deviations of the mean in a young control population at peak reproductive capacity. An elevated estradiol level (>80 pg/ml) in the early follicular phase of the menstrual cycle is of similar significance to an elevated FSH, and can actually suppress FSH, thereby masking the diagnosis of diminished ovarian reserve. An early follicular FSH determination should therefore be interpreted in concert with estradiol. Early follicular phase elevations in FSH are typically intermittent, and therefore difficult to detect with a single sampling. Some women in this late reproductive stage (−3) begin to experience symptoms generally associated with perimenopause, including vasomotor symptoms, breast tenderness, insomnia, migraines, and premenstrual dysphoria (2).
The menopausal transition begins when a woman experiences either: a. a change in her usual intercycle interval of >7days or b. a skipped menstrual period. It is divided into early and late stages, −2 and −1. FSH elevations are greater and more likely to be sustained from cycle to cycle during this time. In the early transition, amenorrhea is intermittent and relatively infrequent. The late transition begins when a woman skips at least two cycles and experiences at least 60 days of amenorrhea.
The postmenopause is divided into two stages in the STRAW nomenclature. It begins with the early stage, +1, defined as the first five years after the final menstrual period. This period of time is further divided into a and b, with a being the first year of amenorrhea and b being the next four years (2). This period is significant for being a time where ovarian hormones undergo further decline, with some intermittent fluctuations. It is known to be a time of accelerated bone loss. Stage +2, the late postmenopausal stage, begins five years after the final menstrual period and continues until demise. FSH remains elevated throughout postmenopause, although over long periods of time FSH eventually declines.
Other Staging Systems
The 60-days amenorrhea definition for the late transition differed from the previously accepted 90-days amenorrhea interval used to define entry into the late menopausal transition (4). In the ReSTAGE study, performed subsequent to STRAW, Harlow, et al, compared four definitions of this stage: the 90 and 60-day intervals, a 42-day running range (using the difference in days between the shortest and longest cycles in a defined time period), and the skipped cycle definition (5). Based on their analysis of prospective menstrual calendar data from the TREMIN Trust, Melbourne Women's Midlife Health Project, Seattle Midlife Women's Health Study, and Study of Women's Health Across the Nation (SWAN), they recommended the use of the 60-day interval (5). This was because, while the latter three criteria occur in more women than the 90-day criterion and are equally predictive of the FMP, the 60-day interval is the most easy to use in clinical practice (5).
The menopausal transition ends with the FMP (stage 0), which must be determined retrospectively once a woman has reached menopause, defined as amenorrhea for at least 12 months (2). It is in stages −1 and +1 that women tend to experience the most menopausal symptoms (2).
Gracia, et al, compared the STRAW staging definitions with the earlier staging system used by SWAN and their newly developed PENN-5 definition (Table 1). The goal of the study was to determine how well each staging system could be validated by corresponding hormonal changes (6). They followed 427 women ages 35–47 years at baseline for 5 years, including 2263 total observations (6). The women all had regular 22–35 day cycles for 3 months prior to enrollment. Each kept a menstrual calendar and had blood sampled for hormone assays between days 1 to 6 of two consecutive menstrual cycles. Hormones of interest included inhibin B, FSH, LH, Estradiol, DHEAS, and testosterone. They found significant differences in mean inhibin B and FSH levels between the premenopausal and early transition stages of each definition as well as in the extra stage added to the PENN-5 system (6). There were significant differences in LH in the earliest stages of SWAN and STRAW that were not detected in the PENN-5 definition. There were not significant differences in estradiol levels among the premenopausal and early transition stages using any of the staging systems. Similarly, there were no statistically significant differences in testosterone or DHEAS between adjacent stages of any of the systems (6). Adding an additional stage in the early transition period that correlated with statistically significant changes in inhibin B and FSH levels suggests that ovarian reserve can be observed to decline well before overt cycle abnormalities occur. These subtle changes imply that even a single change in cycle length for women in this age group (35–44) should be taken seriously by the physician and considered a reason for counseling patients about the possibility of an early menopausal transition or a reduction in fertility.
Table 1.
Menopausal status definitions
| STRAW definition | PENN-5 definition | ReStage definition | |||
|---|---|---|---|---|---|
| Premenopause (Stages −5 to −3) | Regular cycles, with no change in cycle length | Premenopause | Regular cycles, with no change in cycle length | Premenopause | Regular cycles, with no change in cycle length |
| Early Transition (Stage −2) | 1 cycle length change (≥ 7 days) | Late Premenopause | 1 cycle length change (≥ 7 days) | Early Transition | 1 cycle length change (≥ 7 days) |
| Early Transition | ≥2 cycle length changes (≥ 7 days) | ||||
| Late Transition (Stage −1) | 2–11 months of amenorrhea | Late Transition | 3–11 months of amenorrhea | Late Transition (0.85 probability of FMP within 5 y) | ages 45–49 y: 60 days – 11 months of amenorrhea; ages 40–44 y: at least 60 days of amenorrhea + 60 days of amenorrhea within the next 10 bleeding segments |
| Postmenopause (Stage +1, +2) | ≥ 12 months of amenorrhea | Postmenopause | ≥ 12 months of amenorrhea | Postmenopause | ≥ 12 months of amenorrhea |
Hormonal Correlates of Stages of Reproductive Aging
Melbourne Women's Midlife Health Project
The Melbourne Women's Midlife Health Project (MWMHP) was one of the first major longitudinal studies of the menopausal transition (7). It began with 2,001 women from Melbourne, Australia, aged 45–55 years, who were recruited by random digit telephone dialing (8). The natural history of the menopausal experience was documented in 438 of these women in a population based cohort study starting in 1991 that included annual follicular phase blood samples, menstrual calendars, bi-annual bone mineral density measurements, and home interviews assessing quality of life and physical measures (8). Eligibility included having at least one intact ovary, a menstrual period within the last 3 months, and absence of hormonal therapy (8). The retention rate at the final 9th year of the study was 88% (8).
One major objective of the Melbourne study was to quantify average hormone levels during the menopausal transition and to determine their correlates (9). In 1995, Burger et al. published cross-sectional data from 380 women in the Melbourne Women's Midlife Health Project (MWMHP) concluding that an increase in serum FSH and decreases in estradiol and inhibin were the major endocrine changes associated with the menopause transition (10). This conclusion was based on comparisons of serum hormone levels between women divided into five menstrual cycle-based groups associated with different stages of the menopausal transition (10). In 1998, a longitudinal analysis of the same data (i.e., following each woman over time) indicated that a significant decrease in inhibin B was the first endocrine marker of the early transition (11). These data were based on group comparisons during the 3rd year of the longitudinal study: single serum samples obtained between cycle days 3 and 8 in 110 regularly cycling women (or at random in those with amenorrhea or irregular cycles). FSH was slightly but not significantly raised when comparing premenopausal to early peri-menopausal women (who reported changes in cycle frequency within the past year in addition to a bleed within the past 3 months); inhibin B levels were significantly lower in the latter group (11). Significant falls in E2 and inhibin A as well as elevations in FSH were observed when comparing early perimenopausal to late perimenopausal women (who reported amenorrhea for 3 or more of the preceding months) (11).
In 1999, the MWMHP group published conclusions based on 6 years of longitudinal data analysis of serum FSH, circulating estradiol (E2), and inhibin A and B in 150 women who experienced their FMP during follow-up; a total of 795 blood samples were taken (average, 5.3 per woman; Figure 2) (9). The 18 months on either side of the FMP correlated with the greatest change in FSH; levels increased 5 fold during this time from an average of 17.5 to 100.5 (9). Significantly, the association of FSH levels with time to FMP was found to be stronger than the association of age with time to FMP (9). The modifying effects of age and BMI were both statistically significant: between ages 46–54, log(FSH) increased linearly then flattened by age 56; BMI was inversely related to FSH (9). Mean estradiol levels were largely variable during the premenopausal years, then began to decrease about 2 years before the FMP(9). The maximum rate of change was at the time of the FMP, followed by a plateau within 2 years (9). Estradiol was affected by age, as log(E2) decreased almost linearly between ages 48 and 56 years, but the magnitude of change was less than for FSH; BMI was not observed to be related to E2 (9). Inhibin A and B levels also decreased prior to the FMP: inhibin A decreased by 50% in the 2.5 years before the FMP, inhibin B by 40% (9). This was the first longitudinal study to report changes in inhibin in relation to menopause (9). Although this study identified hormonal trends in the few years on either side of the LMP, it did not identify a reliable hormonal marker of menopausal status for an individual woman. Nonetheless, the Melbourne investigators helped to clarify the time line of hormone changes in a longitudinal cohort, and identified the loss of inhibin B, likely associated with a critical reduction in the size of the follicle cohort, that heralds the transition. This observation helped explain the clinical finding that ovarian reserve and fertility potential declines long before any observable, major disruption in menstrual cyclicity.
The MWMHP also measured testosterone and its binding protein, sex hormone binding globulin (SHBG) in a total of 1046 blood samples taken from 172 women ages 46–62 years (12). Mean SHBG was found to decrease during this time, most abruptly between −4 and −2 years from the FMP; however, after adjusting for other factors about one third of this decline was found to be due to decreases in estradiol and increases in BMI (12). The free androgen index (FAI) increased with time relative to the FMP, consistent with the decline in SHBG, which is the primary carrier for circulating testosterone. FAI was positively associated with BMI but not associated with age or estradiol (12). Total serum testosterone was not found to change during the menopausal transition. DHEAS did not change relative to FMP but did decrease with age (12). Taken together, these findings do not support the notion that women become suddenly or dramatically deficient in androgens with passage through the menopausal transition.
Study of Women's Health Across the Nation
The Study of Women's Health Across the Nation (SWAN) is a prospective cohort study of the natural menopausal transition that began in 1995. (13) Participants include a representative, community-based sample of African-American, non-Hispanic Caucasian, Chinese, Hispanic, and Japanese women from multiple sites across the country (13). The initial cross-sectional study to identify women for the longitudinal study included 16,065 randomly selected women aged 40–55 years (13). Eligibility criteria included age 42–52 years, an intact uterus and at least one ovary, no current exogenous hormone use, at least one menstrual period in the previous 3 months, and self-identification with one of the designated race/ethnic groups (13). From the cross-sectional study, 6557 women were found to be eligible for the longitudinal study; 3306 women participated (13). SWAN has a number of substudies that assess bone density, psychiatric morbidity, sleep and cardiovascular markers such as carotid intimal medial thickness and coronary calcium accrual in relation to menopause. Some of the major contributions of SWAN have served to clarify the relationships between menopausal stages, hormones and intermediate disease outcomes. Key findings include:
A strong association with progression from the early to late menopausal transition and increased hot flashes, depressive symptoms and major depression(14, 15).
Serial study of bone mineral density reflects a similar trajectory to symptoms in that the late menopausal transition is the stage at which bone demineralization becomes detectable(16).
Transient short term memory deficits associated with the late transition and amenable to hormone treatment, but only if it is given prior to the FMP (17).
A general trend towards increased total cholesterol, LDL, and apolipoprotein B associated with progress through the transition(18) and, a loss of the protective effect of HDL as women become postmenopausal(19).
The Daily Hormone Study of SWAN will be presented in detail in the next section.
The Penn Ovarian Aging Study
The longitudinal Penn Ovarian Aging Study (POAS) that took place from 1995 to 2007 included a cohort of 436 women identified by random-digit dialing in Philadelphia County, Pennsylvania (20). The group included equal numbers of African American and white women (n=218) ages 35 to 47, all of whom had regular menstrual cycles for the previous three cycles, intact uterus, and at least one ovary (20). Women using psychotropic or hormonal medications, were pregnant or breast-feeding, had serious health problems known to compromise ovarian function, or had a history of alcohol or drug abuse within the past year were excluded (20). The data were collected during twelve assessment periods, the first 6 at approximately 8- to 9-month intervals, and the remaining conducted annually with a 2-year gap between periods 10 and 11 (20). Blood was drawn for hormone level assessment during days 1–6 of the cycle in two consecutive menstrual cycles or 1 month apart in noncycling women, therefore a maximum of 24 hormone samples per participant (20). Additional information collected included anthropometric measures, an interview questionnaire about overall health, and a self-assessment concerning perception of overall health (20).
As mentioned above, Gracia, et al, devised a staging system that captured women at the earliest stages of the menopausal transition. The POAS focused on predicting entry into the earliest stages of the menopausal transition. They further investigated this staging system in 2009, at which time the authors' objective was to estimate the probability and identify risk factors for entering the transition to menopause as well as moving into each stage (21). They used the PENN-5 staging definitions as described above. Data from 9 years of the PENN ovarian aging study was used, including 10 assessment periods of 436 women (out of which 125 discontinued before the 9 year completion) (21). The likelihood of entering the menopausal transition and moving into each subsequent stage was increased for each unit increase in FSH (P<0.001) and with each unit decrease in inhibin B (P<0.001) in the adjusted multivariable model (21). The women in the late transition (stage 4) had the largest change in FSH, compared to women in the early transition (stage 3; odds ratio= 1.9 [95%CI=]; (21). Decreases in inhibin B resulted in odds ratios similar to FSH (21). Current smoking increased the odds of transition into each stage (odds ratio 1.3, CI 95%)(21). BMI, alcohol use, and age at menarche were not significant predictors of the transition; age and race predicted transitions in some but not all stages. Estradiol levels did not change as significantly between stages but higher levels increased the odds of entering the transition (P=0.013) (21).
The POAS was the first study to determine associations between depression and hormonal changes during the transition to menopause. Using the cohort described above with 6 assessment periods over a 4 year interval and adjusting for known predictors of depression, they found an increased likelihood of depressive symptoms during the transition to menopause that decreased after menopause (22). These symptoms were almost twice as likely in the early transition phase and decreased with age (22). This was consistent with earlier SWAN findings that early perimenopausal women had higher rates of psychologic distress (23). This likelihood was lower for those with a rapidly increasing FSH but higher for those with increasing estradiol (22), which occurs in the early transition (24). They later had similar findings with a 9 year longitudinal assessment of the same cohort: depressed mood was again found to be increased in the early menopausal transition and then significantly associated with progression through stages of the transition after adjusting for other risk factors (25). Again, incidence of depressed mood significantly decreased postmenopause (25). Subsequent to these findings, others have demonstrated the effectiveness of exogenous estradiol (50–100mcg transdermally) in treating menopausal transition-related depression and depressive symptoms (LEE COHEN, CLAUDO SOARES).
This group also made important contributions in terms of showing associations between obesity and hormonal markers of ovarian reserve. They showed that antimullerian hormone (AMH) and inhibin B were both negatively associated with body size (26, 27). They further examined these associations in 2008 in a cross-sectional study measuring the AMH and inhibin B levels along with antral follicle counts of 36 women 40–52 years, with half of the women with a BMI<25 and half with a BMI>25 (without PCOS) (28). Adding antral follicle count as a measurement of reserve helped to distinguish if the previous associations between AMH, inhibin B, and obesity were due to decreased ovarian reserve in obese women or the effects of obesity on ovarian hormones, whether it be in production, sequestration, or clearance(28). They found significanly lower levels of AMH in obese vs. normal weight women. Inhibin B was also lower in obese women compared to normal weight women, but the group difference was not statistically significant (P=0.08). An ultrasound assessment of antral follicle count (AFC) was also lower in obese women compared to those of normal weight, but statistically significantly so (28). These findings are consistent with the idea that altered AMH levels in obese women are affected by reasons other, or in addition to, decreased ovarian reserve.
They later studied the relationship between obesity and reproductive hormone levels in the menopausal transition period using linear regression models with data from a larger group of women (20). They compared hormone levels between obese and nonobese women at different stages of the transition. The study included all 436 participants for 12 years; 137 women discontinued the study before the 12 years were up (20). Premenopausal obese and overweight women had significantly lower estradiol levels compared with nonobese women; this was independent of age, race, and smoking (20). In the postmenopausal period, however, obese women had relatively higher estradiol levels (20). Their inhibin B findings suggested an earlier decline in ovarian reserve in obese women: premenopausal obese women had significantly lower levels than premenopausal nonobese women but this actually reversed in the late transition stage (20). Somewhat counterintuitively, FSH was found to be lower in postmenopausal obese compared with nonobese women and not significantly different early in the menopause transition (20). In addition to BMI, waist circumference and waist-to-hip ratio were examined; these correlations were similar to the BMI findings (20). Taken together, the data suggest that obesity adversely affects markers of ovarian aging that come from the ovary (i.e., estradiol, AMH, inhibin), such that it appears obese women have less ovarian reserve, but pituitary hormones (FSH, and in some studies, LH) are lower in obese women relative to non-obese women, suggesting that both hypothalamic-pituitary suppression and reduced ovarian function characterize obesity. Despite these differences in pituitary and ovarian hormones between obese and non-obese women, there is no observed difference in age at menopause between the two groups.
Seattle Midlife Women's Health Study
The Seattle Women's Health Study (SMWHS) studied the natural menopausal transition from 1990 to 2006 in a population-based cohort. The focus of these studies was symptoms, hormones, stress, and stages of the menopausal transition. Participants provided annual data by questionnaires, menstrual calendars, and health diaries; in 1996 a subset began providing 3-day monthly diary data as well as first morning voided urine specimens 8–12 times per year for endocrine assays (29). This went through 2000 and then was quarterly from 2001 to 2005 (29). In 2000, based on data from 184 midlife women in their study, they classified women into three stages of the transition: early (flow and/or cycle length changes by at least 7 days), middle (irregularity without skipping), and late (skipped periods, with the cycle length exceeding 60 days) (30).
In 2009 they published data about cortisol levels during the menopausal transition (using the stages they defined in 2000) from 132 women in their cohort (31). Their hormonal levels were calculated from urinary assays from the first-voided morning urine on day 6 of the menstrual cycle. Overnight cortisol levels were associated significantly with estrone, testosterone, FSH, epinephrine, and norepinephrine levels (31). However, they did not find a correlation between these levels and perceived stress or to severity of perimenopausal symptoms, suggesting the levels were more related to biological factors rather than a response to stressors (31). In terms of the stages, cortisol progressively increased from the late reproductive period to the late transition, then decreased by early postmenopause (31). Furthermore, they later looked at 418 women in their cohort who had at least one elevated perceived stress from health reports and found that none of the hormonal or menstrual cycle factors related to the menopausal transition were significantly associated with perceived stress (29). This included stage in transition, hot flashes, estrogen levels, FSH levels, and hormone therapy use; however, depressed mood, employment (positive association), and perceived health were all associated with perceived stress (29).
Menstrual Cycle Hormone Changes Across the Transition
Annual or semi-annual hormone measurements have typically been confined to the follicular phase of the menstrual cycle or taken at random. This sampling paradigm, while practical, leads to incomplete information about the luteal phase of the menstrual cycle and does not allow investigation of the physiology of the process of folliculogenesis. The first investigation of the daily hormone patterns of older reproductive women was carried out by Sherman, et al (32). Three cardinal features were described for the menopausal transition cycles studied: a foreshortened follicular phase, a monotropic elevation of FSH, and decreased luteal progesterone production. These authors concluded that a relative lack of inhibin was responsible for the FSH rise in the absence of follicle failure. Most of these observations, based upon a cohort of only 8 women, have held up over time.
In the early 1980's, Metcalf, et al, performed weekly urinary sampling on relatively small cohorts of perimenopausal women. In these studies, intermittent excretion of very variable amounts of estrogen with similarly variable gonadotropin output were first described (33, 34). Although women no longer appeared to excrete progesterone after the FMP, intermittent production of estrogen was observed (35). In a subsequent study of 100 women who underwent weekly urinary sampling, no decrease in luteal phase progesterone metabolite excretion was observed, arguing against luteal dysfunction in association with the menopausal transition (36). This latter point remains somewhat controversial and will be addressed in detail in subsequent paragraphs.
A few investigators have examined follicle and oocyte dynamics in reproductive aging, which provide further information. Klein, et al, examined ovarian follicle development and sampled oocytes in reproductively aged women 40–45, all of whom reported regular menstrual cycles (37). These studies showed an accelerated follicular phase, with normal indices of follicle growth and a monotropic rise of FSH. The early follicular phase was marked by early estradiol elevation. Santoro, et al, examined the follicle dynamics of older women, who had already evidenced menstrual cycle abnormalities, and observed accelerated folliculogenesis with ovulation at a smaller follicle size than midreproductive aged control women (38). The accelerated follicular phase of reproductive aging women that had been attributed to a decrease in inhibin B—even before it could be measured—has been confirmed by multiple investigators (39–42). There may be further detrimental consequences to this monotropic rise in FSH on oocytes themselves, as the oocytes of reproductively aged women appear to have a disorganized meiotic spindle assembly (43). Moreover, this loss of inhibin restraint may lead to `overshoot' of subsequent estrogen production, as large excursions of E1c have been observed in conjunction with decreased pregnanediol glucuronide (Pdg) in some studies of perimenopausal women (24).
The SWAN Daily Hormone Study (DHS) is an ancillary study to SWAN in which a subcohort of 990 women collected daily, first-morning voided urine for an entire menstrual cycle or for up to 50 days, whichever came first (44). This is the largest cohort study of perimenopausal hormonal excretion patterns ever assembled. Women in the SWAN DHS collected daily urine specimens (method described in Santoro et al (44)) for one complete menstrual cycle or up to 50 days, whichever came first, each year. These urine samples were assayed for excretion products of the pituitary and ovary. Women also kept a daily diary with information about symptoms and social dimensions of each day during the frequent urine collection periods (13).
Cross-sectional data analysis from the 848 women who comprised the first DHS collection revealed correlations between hormonal patterns and age, ethnicity, smoking status, and BMI (45). BMI was found to be strongly related to virtually all menstrual cycle characteristics except E1c excretion. Women with a BMI greater than 25 were less likely to have cycles with evidence of luteal activity, measured by a 3-fold increase in Pdg above the nadir identified during the follicular phase (45), a modification of the method described by Kassam et al (46). In addition to differences in cycle characteristics, they were found to have lower overall excretion of gonadotropins and luteal phase progesterone metabolites, indicating that body size may negatively affect corpus luteum function (45).
When comparing ethnic groups, Japanese- and Chinese-American women were found to have lower levels of E1c excretion; the other hormones of interest were not associated with ethnicity (45). Older age was associated with greater cycle variability, including longer and more irregular cycles (45). Smokers were found to have reduced luteal progesterone metabolite excretion but no significant differences in cycle length or other hormones (45). Peak Pdg was also higher in a small comparison group of midreproductive-aged women (45). No difference was noted in E1c between the the SWAN participants and the midreproductive-aged women (45).
Later in 2004, SWAN investigators analyzed the hypothalamic-pituitary response to estrogen in their participants to determine if modifications in this feedback loop occur at the onset of menopause (47). They focused on the 160 women in the DHS who did not have luteal activity (19% of DHS participants) (47). These anovulatory women were classified into three groups based on their hormonal patterns: group 1 had both an estrogen increase and LH surge within 2 days (indicating normal ovarian function and a normal hypothalamic-pituitary response to estrogen), group 2 had an estrogen increase only (this surge of estrogen was adequate to elicit a surge in younger ovulating women, indicating appropriate ovarian function but an inadequate hypothalamic-pituitary response to estrogen), and group 3 had neither (these women had similar estrogen levels as the other groups throughout the cycle but lacked the estrogen peak) (47). The percentages of days with hot flashes or night sweats were significantly higher for group 3 women than for either group 1 or group 2 women (47). The failure of estrogen to elicit an LH surge in Group 2 suggests that a decrease in estrogen sensitivity accompanies the menopausal transition. This decreased sensitivity to estrogen is consistent with the fact that exogenous estrogen is therapeutic for perimenopausal symptoms (48) even though they may have equivalent or higher circulating estrogen levels than younger women (24).
Among women with ovulatory cycles, body mass index is a major predictor of hormonal patterns, with higher BMI tertile being strongly associated with decreases in all 4 menstrual cycle hormones: LH, FSH, E1c and Pdg (45). As women completed consecutive years of study, a small but significant decrease in luteal progesterone has also been observed, lending credence to the concept that the menopausal transition is characterized by progressive luteal dysfunction (49).
Anovulatory cycles become more common during the menopausal transition. In an initial analysis of cycles in which Pdg excretion was not shown to rise, Weiss, et al, identified evidence for abnormalities of hypothalamic-pituitary function in perimenopausal women. These investigators described their findings as `estrogen insensitivity' inasmuch as failure of an LH surge was observed in a proportion of these cycles in which a completely normal duration and amount of E1c excretion was observed(47). In the 3-year longitudinal follow-up study of the anovulatory cycles, it was observed that initially anovulatory cycles did not necessarily represent a progression through the transition(50). In other words, women do not appear to go from ovulatory to anovulatory cycles on a consistent basis as they traverse the menopause. In summary, menstrual cycle studies of the transition have confirmed that the early menopausal transition, when cyclicity is for the most part preserved, is characterized by highly variable patterns of gonadotropin and sex steroid output. As women progress through the transition, follicle failure appears to occur, and sex steroid production wanes dramatically but intermittently. Eventually, menstrual cycles cease, but evidence of estrogen production occurs for a period of 6 months to 2 years, after which time women achieve a steady state of hypergonadotropic hypogonadism. These findings lend further strength to the inhibin hypothesis, and indicate that the initial loss of restraint on FSH secretion leads to the early transition patterns. Once follicle numbers become insufficient to sustain folliculogenesis, uncompensated ovarian failure ensues and eventually becomes permanent. Until that time, ovarian function can be highly erratic and may well account for the symptomatology that so often accompanies the menopausal transition.
Predicting the FMP
Although much progress has been made in the past several years in terms of better understanding hormonal correlates of reproductive aging and the menopausal transition, a hormonal marker that predicts the timing of the FMP has not yet been clearly defined. This would be clinically useful for several reasons, as it would justify measuring hormone levels in the clinical setting for prognostic or diagnostic purposes. Both the North American Menopause Society (51) and the American College of Obstetrics and Gynecology (52) recommend hormone use for the shortest possible duration; having an idea of when perimenopausal symptoms could be expected to end would aid in decision making about the duration of hormone therapy use. It could also aid in decision making about family planning, whether it be method of contraception or timing of childbearing.
In 1982, Metcalf, et al, sought to find an ovarian marker of the FMP and came up with the conclusion that “an endometrial rather than a hormonal event might determine the time at which menstruation stops during the menopausal transition” (34). This was based on hormonal data from weekly urine samples of eight women near the time of FMP (34). Postmenopausal FSH and LH levels were similar to the elevated levels during the transition when women were having long anovulatory cycles (34). However, and somewhat surprisingly, estrogen levels were not consistently different when comparing the postmenopausal and late transition periods. Both were lower than earlier time points along the transition, but not as low as the subsequent postmenopausal levels (i.e., those found more than 2 years after the FMP; (34). As mentioned above, the MWMHP also studied hormonal trends near the time of the FMP without identifying a reliable hormonal marker of menopausal status (9).
The Michigan Bone Health and Metabolism study group sought to find an endocrine biomarker for predicting the FMP by studying hormone assays of AMH, inhibin B, and FSH in 300 follicular phase specimens from 50 Caucasian women in pre- and perimenopause (53). The data was obtained annually for 6 years and fitted to individual profiles with the outcomes of interest being time to FMP and age at FMP (53). They concluded that out of the 3 hormones of interest, AMH was the best marker; however their assay was not sensitive enough to follow levels sufficiently close to the FMP (53). AMH levels declined to low and nondetectable levels 5 years before the FMP (53). Baseline AMH level was associated with age at FMP (53). Similarly, inhibin B declined to nondetectable levels 4 years prior to the FMP; however, baseline inhibin was not associated with age at FMP (53). FSH was not as strongly correlated to time to FMP nor was it related to age at FMP (53). This was consistent with previous findings by Burger, et al, that both FSH and E2 were not reliable markers of menopause due to within- and between-subject variability (54). While their findings suggest that low AMH may be a signal of the late stage of the menopausal transition, the clinical usefulness of this association remains unrealized, likely due to technical limitations of assay sensitivity.
Tehrani, et al, concluded that AMH was a good predictor of menopause status in late reproductive-aged women (55). They studied 147 women ages 40–50 with regular cycles as part of the Tehran Lipid and Glucose Study cohort with assessments three times at 3-year intervals (55). 60 of the 147 women reached menopause during this time; they had an 88% probability of predicting which women would not reach menopause within the next 6 years based on an AMH threshold of 0.39 ng/mL (55). They found that over time women “maintained the same relative position with respect to the age-adjusted mean leavel of AMH in the cohort” (55). Previous studies have found minimal variability in AMH levels from one cycle to another in individual women (56, 57). This lack of within-individual variation supports the potential for clinical use of a single AMH measurement in late reproductive women for predicting the FMP.
The ReSTAGE investigators sought to further refine the criteria for the transition to be more predictive of the FMP occurring within 5 years by refining the use of markers of menstrual cyclicity. They concluded that persistence, or recurrence of certain markers (in this case, 60 days of amenorrhea), within the next 10 bleeding segments after their initial occurrence, increased the reliability of entry into the late menopausal transition for women in the 40–44 year age group (58). They found this persistence to be a better predictor of the FMP occurring within 5 years for women in this younger age group; for women between ages 45–49, they concluded that a single 60-day interval remained a useful marker (58). Both repeat bouts of prolonged amenorrhea in 40–44 year olds and a single bout of prolonged amenorrhea in a 45–49 year old have a 0.85 probability of the FMP taking place within 5 years (58).
SWAN marshaled its longitudinal data to determine a set of variables for predicting timing of the FMP. This study included 2,662 women, 706 of whom had their FMP (defined retrospectively, after 12 months of amenorrhea) during the 6-year follow-up period (59). They found several factors to be associated with a shorter time to FMP: more advanced baseline age, greater number of vasomotor symptoms, and more variable or less frequent menses (59). Baseline age was the strongest predictor; the effect was greater for non-white than for white ethnic groups (59). Current smokers were 68% more likely to have an earlier FMP than nonsmokers, including never or past smokers (59). Regular exercise and having a higher educational level were both associated with a longer time to FMP (59). E2 levels proved to have a complex relationship to the measured outcome: both low E2 and high E2 (>100 pg/mL) were associated with a shorter time to FMP (59). Women with a higher follicular phase FSH levels at baseline had a shorter time to FMP, as expected (59). They concluded that “in the most extreme cases, i.e., age 54, high estradiol level, current smoking, and high follicle-stimulating hormone level, the FMP can be estimated to within 1 year” (59).
Summary and Conclusions
The menopausal transition is an `irregularly irregular' period of midlife. It involves the acquisition of irreversible ovarian infertility in a woman, along with permanent cessation of menses. Changes in key ovarian markers that predict fertility are found well before any menstrual cycle irregularity is observed. This `pre-clinical' period of ovarian senescence is poorly defined and remains to be better characterized in non-infertile women. In between the first onset of cycle irregularity and the final menstrual period, a series of hormonal changes occur. The weight of the evidence suggests that these changes are saccadic and thus regular menstrual cycles may disappear, only to resume again for a period of months to years. The clinically observable transition from the early to the late menopausal transition seems to be associated with the biggest increase in symptoms associated with menopause: hot flashes, adverse mood, poor sleep and vaginal symptoms. Although most of these symptoms will subside over time in a majority of affected women, short term treatment with hormones may well be indicated to alleviate distress.
Many health and lifestyle related factors are associated with progress through the transition and menstrual cyclicity. Of these, BMI is one of the most overweening covariates. Women with higher BMI, although they do not have an earlier age at FMP, are much more likely to report perimenopausal hot flashes, to have lower—not higher—reproductive hormone secretion/excretion, to have heavier bleeding and to have more menstrual irregularity preceding their FMP. Interestingly, the relationship between BMI and hot flashes changes after menopause. After the FMP, the larger the BMI the lless likely a woman is to have hot flashes. It is likely that adipose-derived estrogenic steroids protect the postmenopausal obese woman, while other mechanisms subserve hot flashes when the same woman is younger and still producing endogenous estrogen. Current research has been able to describe health processes up to the FMP for many women; however, the question of whether or not long-term health outcomes can be predicted by a woman's traversal of the menopause awaits further follow-up.
Although there is not a current test or battery of tests that can predict the FMP for any one woman, some demographic and biochemical variables are becoming more prominent and likely to help develop a model. Of all the hormones studied to date, AMH appears to be the most promising marker. Further research is critical to characterize the hormonal patterns that lead up to the FMP and how the hormonal environment of the postmenopause influences later-life health outcomes.
References
- 1.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969 Jun;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Climacteric. 2001 Dec;4(4):267–72. [PubMed] [Google Scholar]
- 3.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967 Jan-Mar;12(1 Pt 2):77–126. [PubMed] [Google Scholar]
- 4.Brambilia DJ, McKinlay JB, Johannes CB. Defining the perimenopause for application in epidemiologic investigations. American Journal of Epidemiology. 1994;140:1091–5. doi: 10.1093/oxfordjournals.aje.a117209. [DOI] [PubMed] [Google Scholar]
- 5.Harlow SD, Cain K, Crawford S, Dennerstein L, Little R, Mitchell ES, Nan B, Randolph JF, Jr., Taffe J, Yosef M. Evaluation of four proposed bleeding criteria for the onset of late menopausal transition. J Clin Endocrinol Metab. 2006 Sep;91(9):3432–8. doi: 10.1210/jc.2005-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gracia CR, Sammel MD, Freeman EW, Lin H, Langan E, Kapoor S, Nelso DB. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12(2):128–35. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- 7.Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women's Midlife Health Project. Hum Reprod Update. 2007 Nov-Dec;13(6):559–65. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- 8.Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: a 9-year prospective population-based study. The Melbourne Women's Midlife Health Project. Climacteric. 2004 Dec;7(4):375–89. doi: 10.1080/13697130400012163. [DOI] [PubMed] [Google Scholar]
- 9.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999 Nov;84(11):4025–30. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 10.Burger HG, Dudley EC, Hopper JL, Shelley JM, Green A, Smith A, Dennerstein L, Morse C. The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab. 1995 Dec;80(12):3537–45. doi: 10.1210/jcem.80.12.8530596. [DOI] [PubMed] [Google Scholar]
- 11.Burger HG, Cahir N, Robertson DM, Groome NP, Dudley E, Green A, Dennerstein L. Serum inhibins A and B fall differentially as FSH rises in perimenopausal women. Clin Endocrinol (Oxf) 1998 Jun;48(6):809–13. doi: 10.1046/j.1365-2265.1998.00482.x. [DOI] [PubMed] [Google Scholar]
- 12.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000 Aug;85(8):2832–8. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- 13.Sowers M. Menopause: Biology and Pathology. Academic Press; San Diego: 2000. SWAN: a multicenter, multiethnic community-based cohort study of women and the menopausal transition. [Google Scholar]
- 14.Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, Sternfeld B, Matthews K. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health. 2006 Jul;96(7):1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bromberger JT, Schott LL, Kravitz HM, Sowers M, Avis NE, Gold EB, Randolph JF, Jr., Matthews KA. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women's Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010 Jun;67(6):598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008 Mar;93(3):861–8. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009 May 26;72(21):1850–7. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009 Dec 15;54(25):2366–73. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodard GA, Brooks MM, Barinas-Mitchell E, Mackey RH, Matthews KA, Sutton-Tyrrell K. Lipids, menopause, and early atherosclerosis in Study of Women's Health Across the Nation Heart women. Menopause. 2010 Nov 19; doi: 10.1097/gme.0b013e3181f6480e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010 Jul;17(4):718–26. doi: 10.1097/gme.0b013e3181cec85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sammel MD, Freeman EW, Liu Z, Lin H, Guo W. Factors that influence entry into stages of the menopausal transition. Menopause. 2009 Nov-Dec;16(6):1218–27. doi: 10.1097/gme.0b013e3181a8f62b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004 Jan;61(1):62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 23.Bromberger J, Kravitz H, Wej H, Brown C, Youk A, Cordal A, Powell L, Matthews K. History of depression and women's current health and functioning during midlife. Gen Hosp Psychiatry. 2005;27:200–8. doi: 10.1016/j.genhosppsych.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996 Apr;81(4):1495–501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 25.Freeman EW, Sammel MD, Lin H, Gracia CR, Pien GW, Nelson DB, Sheng L. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007 Aug;110(2 Pt 1):230–40. doi: 10.1097/01.AOG.0000270153.59102.40. [DOI] [PubMed] [Google Scholar]
- 26.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF., 3rd Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007 Jan;87(1):101–6. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 27.Gracia CR, Freeman EW, Sammel MD, Lin H, Nelson DB. The relationship between obesity and race on inhibin B during the menopause transition. Menopause. 2005 Sep-Oct;12(5):559–66. doi: 10.1097/01.gme.0000172268.24949.94. [DOI] [PubMed] [Google Scholar]
- 28.Su HI, Sammel MD, Freeman EW, Lin H, DeBlasis T, Gracia CR. Body size affects measures of ovarian reserve in late reproductive age women. Menopause. 2008 Sep-Oct;15(5):857–61. doi: 10.1097/gme.0b013e318165981e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods NF, Mitchell ES, Percival DB, Smith-DiJulio K. Is the menopausal transition stressful? Observations of perceived stress from the Seattle Midlife Women's Health Study. Menopause. 2009 Jan-Feb;16(l):90–7. doi: 10.1097/gme.0b013e31817ed261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell ES, Woods NF, Mariella A. Three stages of the menopausal transition from the Seattle Midlife Women's Health Study: toward a more precise definition. Menopause. 2000 Sep-Oct;7(5):334–49. doi: 10.1097/00042192-200007050-00008. [DOI] [PubMed] [Google Scholar]
- 31.Woods NF, Mitchell ES, Smith-Dijulio K. Cortisol levels during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Menopause. 2009 Jul-Aug;16(4):708–18. doi: 10.1097/gme.0b013e318198d6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman BM, West JH, Korenman SG. The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab. 1976 Apr;42(4):629–36. doi: 10.1210/jcem-42-4-629. [DOI] [PubMed] [Google Scholar]
- 33.Metcalf MG, Livesey JH. Gonadotrophin excretion in fertile women: effect of age and the onset of the menopausal transition. J Endocrinol. 1985 Jun;105(3):357–62. doi: 10.1677/joe.0.1050357. [DOI] [PubMed] [Google Scholar]
- 34.Metcalf MG, Donald RA, Livesey JH. Pituitary-ovarian function before, during and after the menopause: a longitudinal study. Clin Endocrinol (Oxf) 1982 Nov;17(5):489–94. doi: 10.1111/j.1365-2265.1982.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 35.Metcalf MG. The approach of menopause: a New Zealand study. N Z Med J. 1988 Mar 9;101(841):103–6. [PubMed] [Google Scholar]
- 36.Metcalf MG, Livesey JH. Pregnanediol excretion in fertile women: age-related changes. J Endocrinol. 1988 Oct;119(1):153–7. doi: 10.1677/joe.0.1190153. [DOI] [PubMed] [Google Scholar]
- 37.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996 Mar;81(3):1038–45. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- 38.Santoro N, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, Thakur S, Jinnai H, Khosla N, Barad D. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003 Nov;88(11):5502–9. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- 39.Klein NA, Illingworth PJ, Groome NP, McNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996 Jul;81(7):2742–5. doi: 10.1210/jcem.81.7.8675606. [DOI] [PubMed] [Google Scholar]
- 40.Burger HG, Dudley E, Mamers P, Groome N, Robertson DM. Early follicular phase serum FSH as a function of age: the roles of inhibin B, inhibin A and estradiol. Climacteric. 2000 Mar;3(1):17–24. doi: 10.3109/13697130009167595. [DOI] [PubMed] [Google Scholar]
- 41.Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril. 1999 Apr;71(4):658–62. doi: 10.1016/s0015-0282(98)00529-9. [DOI] [PubMed] [Google Scholar]
- 42.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999 Jan;84(1):105–11. doi: 10.1210/jcem.84.1.5381. [DOI] [PubMed] [Google Scholar]
- 43.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996 Oct;11(10):2217–22. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 44.Santoro N, Crawford SL, Allsworth JE, Gold EB, Greendale GA, Korenman S, Lasley BL, McConnell D, McGaffigan P, Midgely R, Schocken M, Sowers M, Weiss G. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab. 2003 Mar;284(3):E521–30. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]
- 45.Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, Finkelstein JS, Greendale GA, Kelsey J, Korenman S, Luborsky JL, Matthews K, Midgley R, Powell L, Sabatine J, Schocken M, Sowers MF, Weiss G. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004 Jun;89(6):2622–31. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 46.Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect. 1996 Apr;104(4):408–13. doi: 10.1289/ehp.96104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004 Dec 22;292(24):2991–6. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 48.Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004 Jan-Feb;11(1):11–33. doi: 10.1097/01.GME.0000108177.85442.71. [DOI] [PubMed] [Google Scholar]
- 49.Crawford SL, Avis NE, Gold E, Johnston J, Kelsey J, Santoro N, Sowers M, Sternfeld B. Sensitivity and specificity of recalled vasomotor symptoms in a multiethnic cohort. Am J Epidemiol. 2008 Dec 15;168(12):1452–9. doi: 10.1093/aje/kwn279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skurnick JH, Weiss G, Goldsmith LT, Santoro N, Crawford S. Longitudinal changes in hypothalamic and ovarian function in perimenopausal women with anovulatory cycles: relationship with vasomotor symptoms. Fertil Steril. 2009 Apr;91(4):1127–34. doi: 10.1016/j.fertnstert.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 51.Burger H, van Tol H, Boersma AW, Brok M, Wiemer EA, Stoter G, Nooter K. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004 Nov 1;104(9):2940–2. doi: 10.1182/blood-2004-04-1398. [DOI] [PubMed] [Google Scholar]
- 52.Yessaian A, Magistris A, Burger RA, Monk BJ. Radical hysterectomy followed by tailored postoperative therapy in the treatment of stage IB 2 cervical cancer: feasibility and indications for adjuvant therapy. Gynecol Oncol. 2004 Jul;94(1):61–6. doi: 10.1016/j.ygyno.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Sowers M, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch M, Zhang D, Harlow S, Randolph JF. Anti-Mullerian Hormone and Inhibin B in the Definition of Ovarian Aging and the Menopause Transition. J Clin Endocrinol Metab. 2008;93(9):3478–83. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burger HG, Robertson DM, Baksheev L, Collins A, Csemiczky G, Landgren BM. The relationship between the endocrine characteristics and the regularity of menstrual cycles in the the approach to menopause. Menopause. 2004;12(3):267–74. doi: 10.1097/01.gme.0000147172.21183.86. [DOI] [PubMed] [Google Scholar]
- 55.Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009 Jul-Aug;16(4):797–802. doi: 10.1097/gme.0b013e318193e95d. [DOI] [PubMed] [Google Scholar]
- 56.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005 Apr;20(4):923–7. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 57.van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005 Apr;83(4):979–87. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 58.Taffe J, Caine K, Mitchell ES, Woods N, Crawford S, Harlow SD. "Persistence" improves the 60-day amenorrhea marker of entry to late-stage menopausal transition for women aged 40 to 44 years. Menopause. 2010;17(1):191–3. doi: 10.1097/gme.0b013e3181b5540e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santoro N, Brockwell S, Johnston J, Crawford SL, Gold EB, Harlow SD, Matthews KA, Sutton-Tyrrell K. Helping midlife women predict the onset of the final menses: SWAN, the Study of Women's Health Across the Nation. Menopause. 2007 May-Jun;14(3 Pt 1):415–24. doi: 10.1097/gme.0b013e31802cc289. [DOI] [PubMed] [Google Scholar]