Abstract
Objective/Hypothesis
Therapeutic agents that enhance mucociliary transport (via stimulation of transepithelial Cl- secretion) and inhibit inflammation could provide considerable advantages over conventional treatments for chronic rhinosinusitis (CRS). The objectives of the present study were to investigate whether the polyphenolic compound resveratrol promotes transepithelial Cl- transport and inhibits KC/IL-8 secretion in sinonasal epithelium.
Study Design
In vitro and in vivo study.
Methods
Transepithelial Cl- transport was investigated in primary murine nasal septal (MNSE) and human sinonasal epithelial (HSNE) cultures. In vivo activity was also measured using the murine nasal potential difference assay. CFTR R-domain phosphorylation and cAMP levels were examined as a test of cAMP/PKA-dependent activation. In vitro LPS-induced KC/IL-8 secretion was quantified and compared to a panel of intranasal steroids.
Results
Resveratrol(100μM) significantly increased CFTR-mediated Cl- transport(change in short-circuit current,ΔISC) in both MNSE[13.51+/-0.77 vs. 4.4+/-0.66(control); p<0.05] and HSNE[12.28+/-1.08 vs. 0.69+/-0.32(control); p<0.05]. Cl- secretion across in vivo murine nasal epithelium was also enhanced[-4+/-1.8 vs. -0.8+/-1.7mV(control), p<0.05]. There was no increase in cellular cAMP or CFTR R-domain phosphorylation detected. Resveratrol also significantly inhibited KC/IL-8 secretion in a dose-dependent fashion (pg/ml) in MNSE[181+/-39(100μM) vs. 94+/-16(200μM) vs. 16+/-22(500μM) vs. 1195+/-355(LPS control); p<0.001]. The compound robustly abrogated KC/IL-8 secretion when compared to ciclesonide (765+/-139), triamcinolone (561+/-124), and budesonide (742+/-428), but had similar activity to fluticasone proprionate (65+/-47). Similar effects were demonstrated in HSNE[975+/-244(100μM) vs. 1825+/-144(LPS control); p<0.001] with inhibition comparable to fluticasone proprionate (785+/-277).
Conclusions
These in vitro and in vivo findings indicate resveratrol is a potent Cl- secretagogue and anti-inflammatory agent. Future clinical trials for CRS are warranted.
Keywords: Transepithelial Ion Transport, Resveratrol, CFTR, Calcium-activated Chloride Channel, Chronic Sinusitis, KC, IL-8, Chloride Secretion, Murine Nasal Culture, Human Sinus Epithelium, Mucociliary Clearance
Introduction
Airway surface liquid (ASL) is a critical part of the mucociliary apparatus that is modulated by the epithelial transport of ions, such as chloride (Cl-) and sodium (Na+). One of the primary regulators of Cl- transport in sinonasal respiratory epithelium is the cystic fibrosis transmembrane conductance regulator (CFTR). Dysfunctional Cl- transport results in dehydrated ASL and mucus stasis as demonstrated in the global airway disease, cystic fibrosis (CF). Disrupted mucociliary clearance (MCC) places patients at risk for developing bacterial infections in the upper and lower airway leading to chronic inflammation and persistent problems with mucus transport.1,2 While chronic rhinosinusitis (CRS) is pervasive in the CF population, strategies designed to improve MCC in individuals without this disease are lacking.
Enhancing transepithelial Cl- secretion in the upper airway represents a new and leading edge approach to treatment that has recently been shown to activate MCC in human subjects3, but has not been investigated previously in CRS. Compounds that stimulate Cl- secretion, specifically those that activate CFTR are an area of active investigation for the treatment of CF and other respiratory diseases.4,5 Flavonoids, a group of ubiquitous plant molecules known for their antibacterial, antiviral, anti-inflammatory, and anti-allergic properties, are among the most potent activators of CFTR gating.6-8 Our prior studies indicate that these compounds may represent an effective therapeutic approach to enhancing transepithelial Cl- secretion in sinonasal respiratory epithelium.4,6
Resveratrol, a natural polyphenol related to flavonoid compounds, is found in various vegetables and fruits and is abundant in grapes.9 The molecule has well-known anti-inflammatory properties attributable to the inhibition of NF-κB 10, which may decrease the expression of many inflammatory proteins (granulocyte-macrophage colony stimulating factor (GM-CSF), IL-8 (or the murine homologue KC), COX-2, and inducible nitric oxide synthase (iNOS).11 Agents with an excellent safety profile that both inhibit inflammation and promote epithelial Cl- secretion could be of benefit in individuals suffering from inflammatory sinus disease. Due to the structural similarity to flavonoid compounds, we sought to determine whether resveratrol could serve as a Cl- secretagogue and anti-inflammatory agent in sinus and nasal epithelium.
Methods
University of Alabama at Birmingham Institutional Animal Care and Use Committee and Institutional Review Board approval were obtained prior to initiation of the study.
Tissue Culture
Techniques for murine nasal septal epithelia (MNSE) and human sinonasal epithelia (HSNE) culture on air-liquid interfaces have been described in previous studies.4,12-18 All MNSE cells were obtained from C57/BL6 wild type mice and from transgenic CFTR−/− knockout mice. Primary nasal epithelial cells were prepared and cultured according to previously established protocols.17 on collagen coated Costar 6.5-mm-diameter permeable filter supports (Corning, Lowell, MA) submerged in culture media. HSNE was obtained from nasal epithelial tissue removed during endoscopic skull base surgery from individuals without evidence of sinusitis. Differentiation and ciliogenesis occurred in all cultures within 10 to 14 days.
Short Circuit (ISC) Measurements
Transwell inserts (Costar) were mounted in Ussing chambers in order to investigate pharmacologic manipulation of vectorial ion transport. Monolayers were continuously monitored under short circuit conditions following fluid resistance compensation using automatic voltage clamps (VCC 600; Physiologic Instruments, San Diego, CA). Batch solutions for the transwell filters were warmed to 37°C, and each solution continuously gas lifted with a 95%O2-5%CO2 mixture. DMSO control solutions were tested for comparison. The ISC was assessed at one current measurement per second. By convention, a positive deflection in ISC was defined as the net movement of anions in the serosal to mucosal direction.
Nasal potential difference assay
A 3-Step protocol was used, as described previously.4 Animals were anesthetized with ketamine (200 mg/kg), acepromazine (0.6 mg/kg), and xylazine (30 mg/kg) by intraperitoneal injection prior to testing. First, nasal cavities of anesthetized mice (C57/BL6) were perfused sequentially with 1) Ringer's solution containing 140mM NaCl, 5mM KCl, 1mM MgCl2, 2mM CaCl2, 10mM HEPES, and amiloride 50μM (pH 7.3); 2) amiloride + a low-Cl--containing solution (NMDG, 6 mM Cl-, pH 7.3); and 3) amiloride + low-Cl--containing solution + hesperidin, forskolin 20 mM, or DMSO control. Because of the continuous presence of amiloride (50μM) and the complete replacement of Na+ with a membrane-impermeant cation (140 mM NMDG in the perfusion solution), hyperpolarization in this setting is taken to reflect Cl- secretion rather than sodium absorption. Each condition was studied for 5 to 10 minutes until a stable signal was achieved. Activity of the test solution was measured from the stable baseline to the highest point of hyperpolarization. All traces were interpreted in a blinded fashion.
CFTR R-domain phosphorylation and cAMP levels
Because activation of CFTR anion transport requires protein kinase A (PKA)-dependent phosphorylation of the R-D, an ELISA-based detection kit (Cayman Chemicals, Ann Arbor, MI) was used to measure stimulation of cellular cAMP by resveratrol in MNSE cultures, as previously described.7 To confirm absence of a cAMP/PKA dependent mechanism, polyclonal NIH-3T3 cells expressing HA-tagged R-domain were treated with resveratrol for 20 minutes, and compared to forskolin (100 nM) × 5 minutes as a positive control and DMSO as negative control. Following lysis, equal amounts (50 μg) of total cell lysate were electrophoresed through a 12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE), and immunoblotted with antibody to the HA tag (Covance, Cumberland, VA). Phosphorylation of the R-domain was visualized as a 2-4 kD shift in migration, as previously described.7
KC and IL-8 ELISA
LPS (derived from E. Coli, Sigma) was dissolved in stock solutions of commonly prescribed topical steroids for allergic rhinitis (budesonide, triamcinolone acetonide, fluticasone propionate, and ciclesonide) in PBS solution at a final concentration of 100 ng/mL. Solutions were vortexed immediately prior to incubation to ensure adequate dissolution and homogenous application of steroids to each well. The positive and negative controls were LPS and DMSO (resveratrol vehicle), respectively. Steroid concentrations were derived from the total mcg per volume of spray as provided on product prescribing information (budesonide 32μL/spray, triamcinolone acetonide 55μL/spray, fluticasone propionate 67μL/spray, ciclesonide 50μL/spray). A four hour incubation time point was chosen based on initial experiments with MNSE. All cell plates were maintained at 37°C and 5% CO2 for 4 hours. Basal media from MNSE were used to measure mouse CXCL1/KC (functional homolog of human IL-8) ELISAs (R&D Systems, Minneapolis, MN). Media from incubated HSNE was run on a human CXCL8/IL-8 ELISA (R&D Systems, Minneapolis, MN). Absorbance analyses for all ELISAs were performed on a Bio-Rad (Hercules, CA) microplate absorbance spectrophotometer. Linear regression analysis of standard solutions to calculate sample concentrations was run on Microplate Manager 5.2.1 (BioRad Hercules, CA) software. Cilia beat frequency (CBF) and cellular integrity were analyzed to ensure the health of the cells before and after incubations.
Statistical analysis
Statistical analyses were performed using two-tailed unpaired t-tests, paired t-tests, and analysis of variance as indicated.
Results
Resveratrol is a robust CFTR-dependent Cl- Secretagogue
CFTR-mediated anion transport (change in short-circuit current, ΔISC) was analyzed using MNSE cultures in Ussing chambers (Figure 1A). Resveratrol activated CFTR dependent Cl- conductance in a dose dependent fashion up to 500 μM, but demonstrated inhibitory effects on total CFTR activation with post-treatment forskolin (100 nM) at concentrations > 100 μM (Figure 1B). Thus, 100 μM resveratrol [(ΔISC, 13.51+/-0.77 vs. 4.4+/-0.66(control); p<0.05] was considered the optimal dose for the remainder of the studies. Although total stimulated ΔISC was maximal at 100 μM (27.35 +/- 2.11), there was no significant difference when compared to forskolin alone (23.07 +/- 1.60) or when the total contribution of CFTR to the ΔISC was measured with the specific inhibitor INH-172 [(26.6 +/- 1.22 (resveratrol + forskolin) vs. 20.82 +/- 1.76 (forskolin)] (Figure 1C). While inhibition of total achievable ISC (resveratrol + forskolin) was noted at concentrations up to 10 mM resveratrol, stimulation of transepithelial Cl- secretion was still present at all concentrations (data not shown). CFTR-/- knockout MNSE were used to confirm that resveratrol-stimulated ΔISC is mediated through a CFTR-dependent mechanism (Figure 1D). Following application of amiloride (used to block sodium channels and support ΔISC attributable to Cl- transport), resveratrol (100μM) resulted in no measurable transepithelial conductance and, thus, no contribution from alternative Cl- transport pathways (i.e. calcium-activated Cl- channels) was implicated (see also below).
Figure 1. Resveratrol Stimulates CFTR-mediated Cl- Transport in MNSE in vitro.
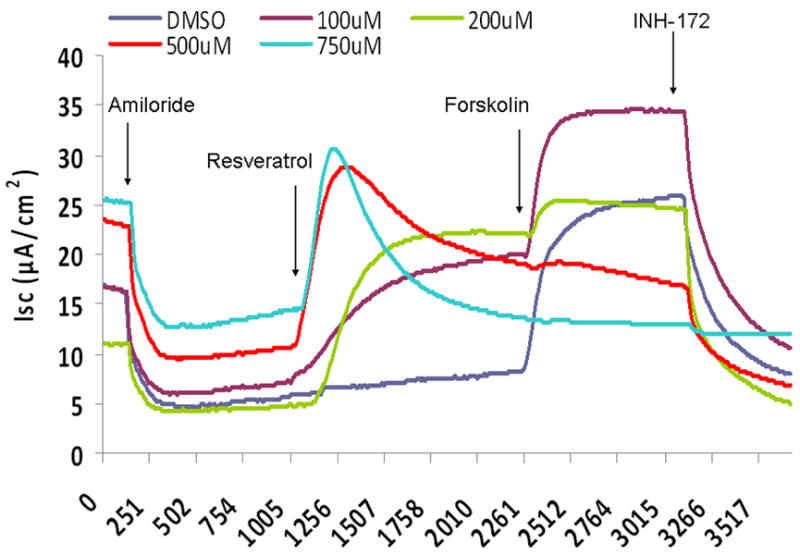
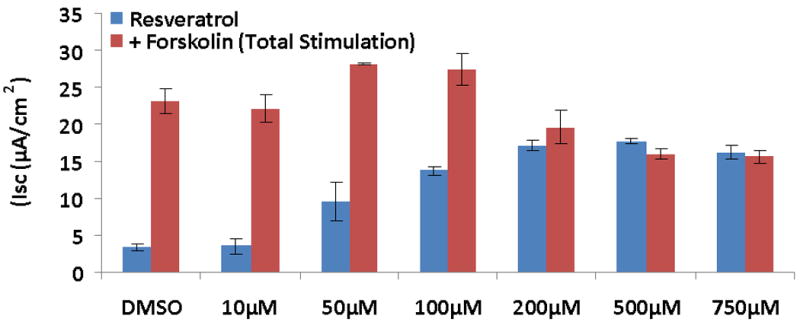
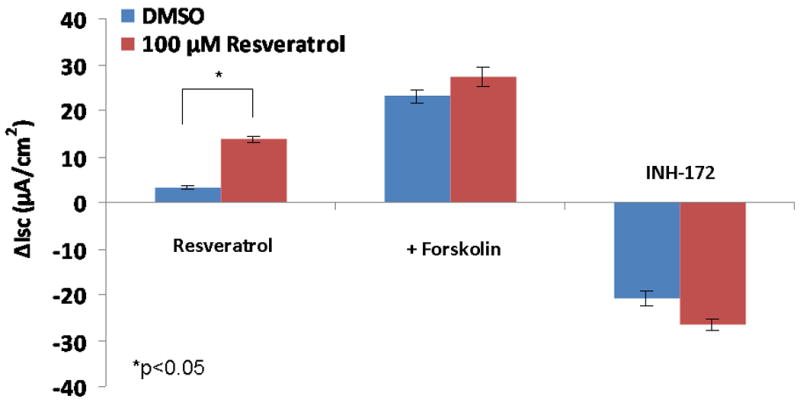
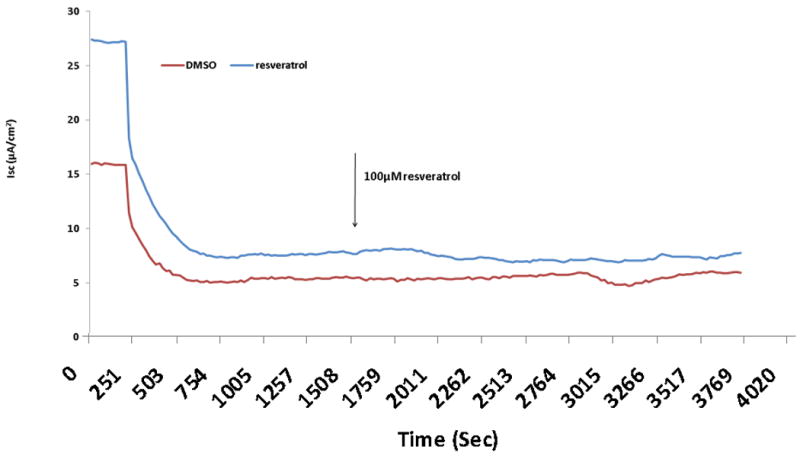
(A) Representative Ussing chamber tracings reveals resveratrol activation of Cl- transport in a dose-dependent fashion. By convention, a positive deflection in the tracing (ΔISC) represents movement of an anion (i.e. Cl-) from the serosal to mucosal direction. (B) Graphic representation of resveratrol stimulation vs. total stimulation [resveratrol + forskolin (100 nM)] of transepithelial Cl- conductance (n ≥ 3 per condition). Resveratrol concentrations > 100 uM result in decreased total stimulation. (C) Resveratrol (100 μM) significantly increased Cl- transport when compared to DMSO control (n ≥ 5; p<0.05) However, total stimulated ΔISC (resveratrol + forskolin) and inhibition of CFTR-mediated ISC following application of INH-172 were not statistically different when compared to the corresponding controls. (D) Ussing chamber tracings of CFTR-/- MNSE exposed to resveratrol (100 μM) following amiloride (100 nM) blockade shows no measurable response indicating apical CFTR dependence of Cl- secretagogue activity.
Resveratrol (100μM) increases transepithelial Cl- transport in HSNE
Once an optimal dose was determined in MNSE, confirmation in primary HSNE cultures was performed using the 100 uM concentration (Figure 2A). CFTR-mediated transepithelial Cl- transport promoted by resveratrol was significantly increased in HSNE when compared to DMSO control [(15.69 +/- 2.66 vs. 2.49 +/-0.98, respectively (p<0.0001). Total stimulated ISC [(26.29 +/- 1.62 vs. 20.67 +/-2.5, (p<0.0001) and INH-172 CFTR blockade [(26.29 +/- 1.62 vs. 20.67 +/-2.5, (p<0.01) were also significantly increased when compared to forskolin alone (Figure 2B).
Figure 2. Resveratrol (100μM) Increases Transepithelial Cl- Conductance in Human Sinonasal Epithelium In Vitro.

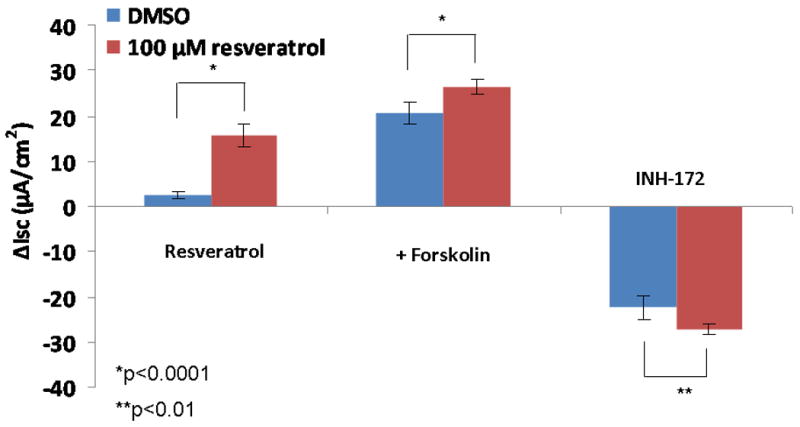
(A) Representative Ussing chamber tracings demonstrating robust CFTR-mediated Cl- transport in human sinonasal epithelial cell cultures compared with corresponding DMSO control. (B) Resveratrol stimulated Cl- transport when compared to DMSO controls (n ≥ 5 per condition; p<0.0001). Total stimulation (ISC shown in Figure 2A immediately prior to INH-172) and CFTR blockade with INH-172 were also significantly different from controls (p<0.01).
Resveratrol activates CFTR-dependent Cl- conductance across in vivo murine nasal epithelium
We next examined the effects of resveratrol on Cl- transport in vivo via standardized murine NPD measurements. Mice demonstrated significant hyperpolarization following perfusion with resveratrol (100 uM) in the presence of amiloride (100 μM) and a Cl- secretory gradient (-4 ± 1.87 mV) compared with continued depolarization seen in mice perfused with vehicle and otherwise identical conditions [-0.93 ± 1.69 mV, p<0.01). Although increased when compared to forskolin (-1.65 +/- 1.78 mV), this was not statistically significant. (Figure 3)
Figure 3. Murine Nasal Potential Difference Measurements Demonstrate Activation of Cl- Transport In Vivo.
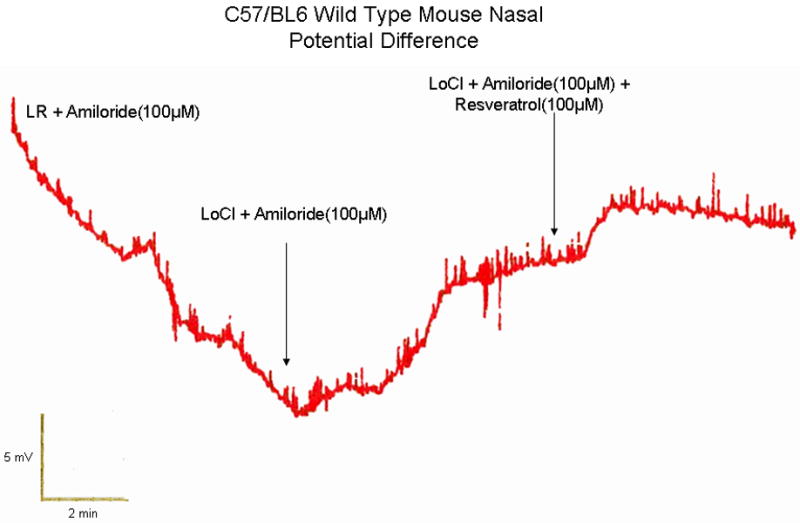
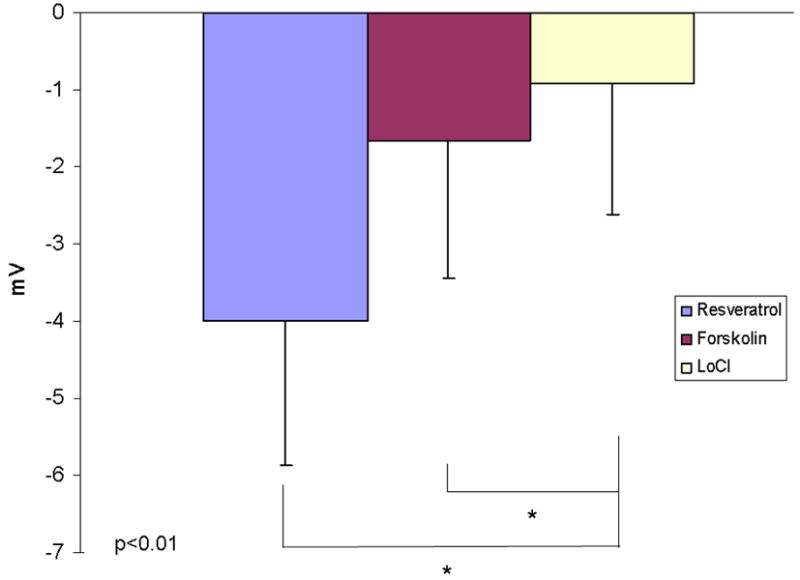
(A) Mice underwent a standardized nasal potential difference (NPD) protocol with the addition of 100 uM Resveratrol (or vehicle control) in the final perfusion solution (20 μg/mL in Cl- free gluconate solution and amiloride (100 μM)). This is a representative tracing from C57 mice stimulated with resveratrol in the final perfusate. (B) Resveratrol perfusion resulted in a -4 mV mean NPD polarization that was significantly different than in mice receiving vehicle alone (p<0.05).
Resveratrol activates CFTR in the absence of R-domain phosphorylation
PKA/cAMP-dependent phosphorylation of the CFTR R-Domain (R-D) is a crucial step in channel activation, but it has been previously demonstrated that flavonoid compounds, including genistein19 and hesperidin6, activate CFTR through a mechanism independent of PKA/cAMP-dependent phosphorylation of the R-D. To examine the mechanistic basis of CFTR activation by resveratrol, we measured the effects of resveratrol on cAMP levels and R-D phosphorylation. As opposed to the cAMP agonist forskolin, resveratrol had no detectable effect on the phosphorylation-dependent mobility shift of recombinant CFTR R-D and did not confer a significant elevation in cAMP. (Figure 4)
Figure 4. Resveratrol does not Increase Cellular cAMP or Phosphorylate the CFTR R-Domain.
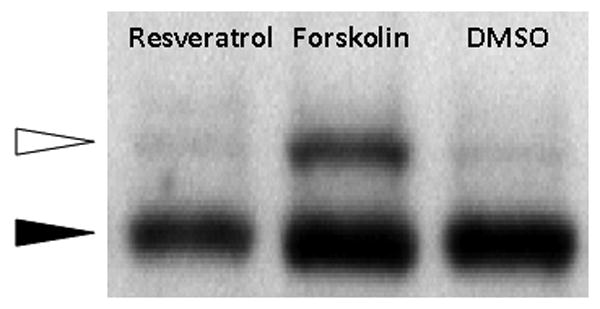
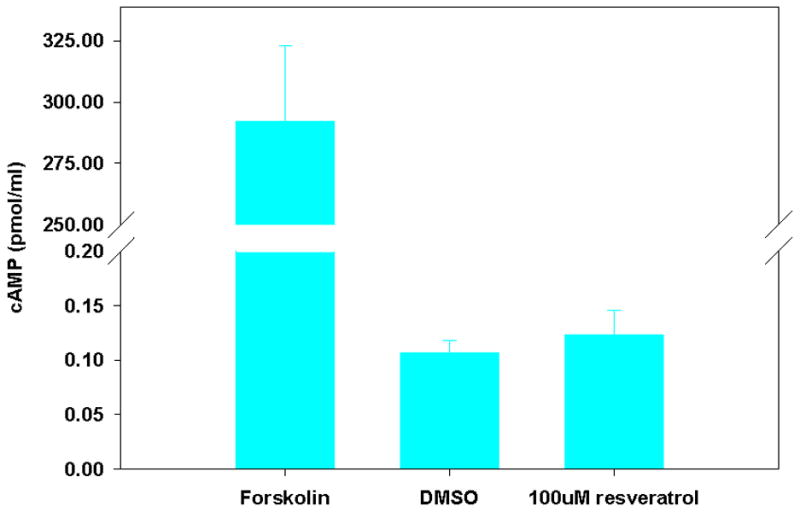
(A) Cellular cAMP was minimal in the presence of resveratrol and not significantly changed when compared to DMSO controls. The adenylate cyclase agonist forskolin was used as a positive control. (B) Polyclonal NIH-3T3 cells expressing HA-tagged R-domain were treated with resveratrol for 20 minutes, and compared to forskolin (100 nM) or DMSO control in an assay of R-D phosphorylation. Phosphorylation results in a 2-4 kD shift in migration (white arrow).
Resveratrol inhibition of KC/IL-8 secretion compares favorably to topical intranasal steroids
Resveratrol significantly suppressed LPS-induced KC production [181±39pg/mL(100μM), 94±16pg/mL(200μM), vs. 16±22pg/mL(500μM) vs. 1195±355pg/mL (LPS control); p<0.001] in MNSE cell cultures in a dose dependent fashion (Figure 5A). Resveratrol also abrogated LPS-induced KC secretion when compared to selected topical steroids applied at pharmacologic concentrations. KC was significantly decreased (p<0.001) with application of 100, 200, and 500 uM concentrations of resveratrol when compared to budesonide (742±428pg/mL), triamcinolone acetonide (561±124pg/mL), and ciclesonide (765±139pg/mL)). Resveratrol had similar activity on KC inhibition compared to fluticasone propionate (94±16pg/mL). Strong anti-inflammatory activity was also demonstrated in HSNE cultures as judged by release of the KC homolog IL-8 (Figure 5B). Resveratrol (100μM) significantly suppressed LPS-induced IL-8 production [975±244pg/ml (100μM) vs. 1825±144pg/ml (LPS control); p<0.001] and was not significantly different from fluticasone proprionate (785±277). Because DMSO also significantly stimulates IL-8 secretion (958±216pg/ml vs. 504±284pg/ml; PBS; p<0.05), the corresponding amount of DMSO required for 100μM resveratrol was used for each condition. Resveratrol without LPS (757±42) did not suppress IL-8 secretion below constitutive levels. Fluticasone without LPS or DMSO decreased IL-8 constitutive expression (349±25) but was not significantly different than PBS controls.
Figure 5. Resveratrol has Superior Anti-Inflammatory Activity Compared to Intranasal Steroids.
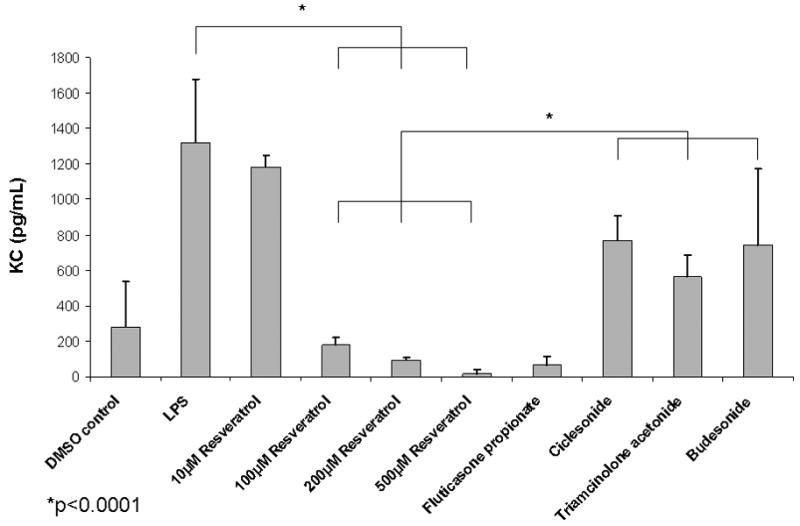
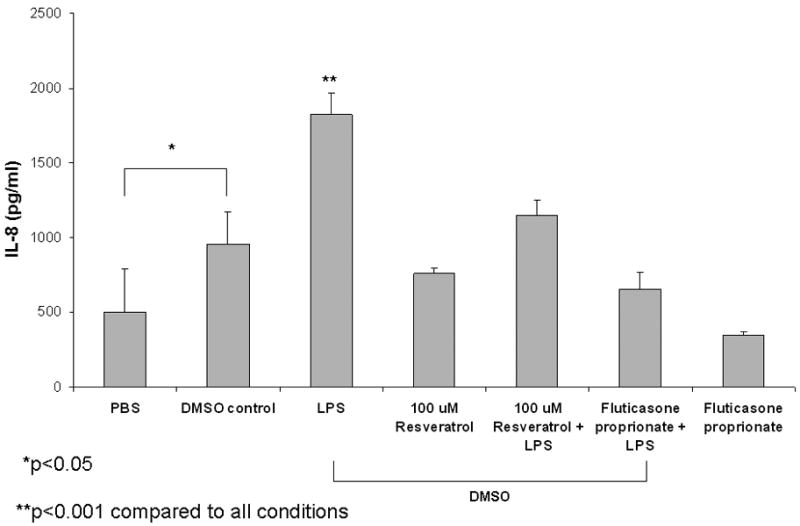
(A) Lipopolysaccharide (LPS) -stimulated KC production was inhibited in a dose-dependent fashion and strongly decreased at doses ≥ 100 μM in murine nasal septal epithelium (n ≥ 5 per condition) p<0.0001). Resveratrol ≥ 100 μM suppressed KC more strongly than three major intranasal steroids (budesonide, triamcinolone acetonide, and ciclesonide) (p<0.0001). (B) LPS-stimulated IL-8 production was significantly abrogated (n ≥ 3 per condition, p<0.001) in human sinonasal epithelial cultures exposed to resveratrol (100 μM) and was comparable to fluticasone. DMSO significantly stimulates IL-8 secretion by itself (p<0.05) compared to PBS.
Discussion
Flavonoids are a group of small molecules structurally derived from plant-based compounds of the common flavone (2-phenyl-γ-benzopyrone).5 Numerous flavonoid compounds have garnered attention over the last decade based upon a variety of health benefits and favorable biological activities. Although resveratrol lacks the central ring of the common three ring flavone backbone, it is otherwise structurally similar to flavonoids as it is a polyphenolic molecule. Resveratrol is found in the skins of red fruits, trees, some flowering plants, and peanuts.20,9 Prior studies have demonstrated antioxidative, antiproliferative, anti-inflammatory, cardioprotective, anticancer, and even chemopreventative properties. 20,21 Although not an inhibitor of inducible gene transcription directly,20 the compound appears to play a role in numerous cellular pathways including the inhibition of the transcription factor NF-κB and activator protein-1(AP-1).10
The present studies revealed that resveratrol is a robust activator of CFTR-dependent transepithelial Cl- transport in vitro (murine and human primary cell culture models of sinonasal epithelium) and in vivo (murine NPD assay). CFTR is the major Cl- channel in the apical membranes of respiratory epithelia and is responsible for modulating ASL via salt and water secretion and absorption.4 Dysfunctional Cl- transport may be genetic (i.e. cystic fibrosis) or acquired (cigarette smoke exposure, high altitude/hypoxemia, inflammation, and infectious agents).2,6 The loss of CFTR causes dehydration of the ASL and interference with MCC, resulting in widespread respiratory disease. Novel Cl- secretagogues, such as VX-770, have shown considerable clinical promise in CF, and have generated a great deal of recent excitement.22,23 Because molecules such as these activate Cl- secretion in the upper as well as lower airways, the agents may benefit subjects with CRS and impaired nasal mucus clearance. Certain flavonoids24-26 and related compounds, such as resveratrol in the present investigations, have demonstrated robust activation of CFTR-mediated anion transport and, thus, represent an excellent prototypic resource for investigating the mechanisms underlying CRS and as part of therapeutic development for acquired defects in CFTR.
Resveratrol activation of transepithelial Cl- secretion was robustly inhibited by the specific CFTR blocker INH-172, indicating that activation of transepithelial Cl- secretion in nasal airway epithelium is largely dependent upon CFTR. When resveratrol was administered in CFTR-/- MNSE in the presence of amiloride, no measurable ΔISC was detected further establishing reliance on apical CFTR for activation of ion transport. While stimulation of CFTR is dependent upon the phosphorylation of the R domain by the cAMP/PKA dependent pathway, our results provide evidence that resveratrol promotes CFTR activation in a manner distinct from that produced by classic PKA dependent stimuli. Resveratrol did not produce elevated cAMP levels and no R-domain phosphorylation was detected following treatment. Previous work from our group has shown that CFTR channel activation by hesperidin6, quercetin7, and the bioflavonoid mixture Sinupret® 4 can be blocked by inhibiting PKA. This implies that CFTR requires some level of PKA activity, (and thus PKA dependent R-domain phosphorylation) for compounds in this class to mediate Cl- transport in intact cells from sinonasal epithelium. Thus, we speculate that resveratrol increases channel opening in CFTR that has already entered the phosphorylated state – a mechanism currently under investigation in our laboratory by membrane patch analysis of CFTR single channels and macropatches pre and post-PKA administration.
Interleukin-8 (IL-8) gene expression levels have been shown to correlate with disease severity in CRS.27 In the murine model, KC has been suggested to be a functional homolog of IL-8, as it is known to mediate neutrophilic infiltration in association with acute and chronic airway inflammation.28 Resveratrol has been shown to inhibit IL-8 and GM-CSF release by alveolar macrophages from patients with COPD under conditions where steroids were largely ineffective.20,29 The current study demonstrated that resveratrol significantly suppressed LPS-induced KC production in a dose dependent fashion in MNSE and inhibited IL-8 production in HSNE. In addition, the anti-inflammatory activity of resveratrol with regard to KC suppression was superior to several commonly prescribed intranasal steroids (budesonide, triamcinolone acetonide, and ciclesonide) and similar to fluticasone propionate (in MNSE and HSNE) after 4 hours of application. This data, in combination with evidence indicating strong topical activation of Cl- transport in vivo, suggests that intranasal resveratrol could play an important role in drug discovery efforts aiming to activate or potentiate CFTR in respiratory diseases. While topical administration of resveratrol has been investigated as an antioxidant supplement in sunscreen,30 to our knowledge, this is the first study demonstrating the applicability of an intranasal topical formulation of the compound as a possible therapy in chronic rhinosinusitis.
Conclusion
These in vitro and in vivo findings indicate resveratrol is both a potent Cl- secretagogue and anti-inflammatory agent – the combination of such properties make resveratrol a unique and promising topical therapeutic agent for inflammatory sinus and nasal disease. Future human clinical trials investigating resveratrol preparations in CRS and other airway diseases are warranted.
Acknowledgments
Research Support: This research was funded by the American Rhinologic Society New Investigator Award (2009) and Flight Attendant's Medical Research Institute Young Clinical Scientist Award (072218) to B.A.W.; and NIH/NIDDK (5P30DK072482-03) to E.J.S.
Footnotes
Presented at the Triological Society's Combined Section meeting, Scottsdale, Az., January 30, 2011.
Dr. Sorscher and Dr. Woodworth are inventors on a patent submitted regarding the possible activity of chloride secretagogues for therapy of sinus disease (Provisional Patent Application Under 35 U.S.C. §111(b) and 37 C.F.R. § 1.53(c) in the United States Patent and Trademark Office). Dr. Woodworth is a consultant for Gyrus ENT, ArthroCare ENT, and was on the GlaxcoSmithKline speaker's bureau.
References
- 1.Moller W, Haussinger K, Ziegler-Heitbrock L, Heyder J. Mucociliary and long-term particle clearance in airways of patients with immotile cilia. Respir Res. 2006;7:10. doi: 10.1186/1465-9921-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virgin FW, Azbell C, Schuster D, et al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope. 2010;120:1465–1469. doi: 10.1002/lary.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones AM, Helm JM. Emerging treatments in cystic fibrosis. Drugs. 2009;69:1903–1910. doi: 10.2165/11318500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Virgin F, Zhang S, Schuster D, et al. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. Laryngoscope. 2010;120:1051–1056. doi: 10.1002/lary.20871. [DOI] [PubMed] [Google Scholar]
- 5.Illek B, Lizarzaburu ME, Lee V, Nantz MH, Kurth MJ, Fischer H. Structural determinants for activation and block of CFTR-mediated chloride currents by apigenin. Am J Physiol Cell Physiol. 2000;279:C1838–1846. doi: 10.1152/ajpcell.2000.279.6.C1838. [DOI] [PubMed] [Google Scholar]
- 6.Azbell C, Zhang S, Skinner D, Fortenberry J, Sorscher EJ, Woodworth BA. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol Head Neck Surg. 2010;143:397–404. doi: 10.1016/j.otohns.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyle LC, Fulton JC, Sloane PA, et al. Activation of CFTR by the Flavonoid Quercetin: Potential Use as a Biomarker of {Delta}F508 CFTR Rescue. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer H, Illek B. Activation of the CFTR Cl- channel by trimethoxyflavone in vitro and in vivo. Cell Physiol Biochem. 2008;22:685–692. doi: 10.1159/000185552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee M, Kim S, Kwon OK, Oh SR, Lee HK, Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 2009;9:418–424. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell D, Collins J, Coles R. Summary health statistics for U.S. adults: National Health Interview Survey, 1997. National Center for Health Statistics. Vital Health Stat. 2002;10:15. [PubMed] [Google Scholar]
- 12.Woodworth BA, Zhang S, Tamashiro E, Bhargave G, Palmer JN, Cohen NA. Zinc increases ciliary beat frequency in a calcium-dependent manner. Am J Rhinol Allergy. 2010;24:6–10. doi: 10.2500/ajra.2010.24.3379. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Fortenberry JA, Cohen NA, Sorscher EJ, Woodworth BA. Comparison of vectorial ion transport in primary murine airway and human sinonasal air-liquid interface cultures, models for studies of cystic fibrosis, and other airway diseases. Am J Rhinol Allergy. 2009;23:149–152. doi: 10.2500/ajra.2009.23.3285. [DOI] [PubMed] [Google Scholar]
- 14.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009 doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 15.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol. 2008;22:234–238. doi: 10.2500/ajr.2008.22.3178. [DOI] [PubMed] [Google Scholar]
- 16.Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am J Rhinol. 2008;22:7–12. doi: 10.2500/ajr.2008.22.3125. [DOI] [PubMed] [Google Scholar]
- 17.Woodworth BA, Antunes MB, Bhargave G, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43:195–204. doi: 10.2144/000112531. [DOI] [PubMed] [Google Scholar]
- 18.Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: A comparative study. Am J Rhinol. 2007;21(5):533–537. doi: 10.2500/ajr.2007.21.3068. [DOI] [PubMed] [Google Scholar]
- 19.Moran O, Zegarra-Moran O. A quantitative description of the activation and inhibition of CFTR by potentiators: Genistein. FEBS Lett. 2005;579:3979–3983. doi: 10.1016/j.febslet.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Donnelly LE, Newton R, Kennedy GE, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–783. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- 21.Leung PS, Chan HC, Chung YW, Wong TP, Wong PY. The role of local angiotensins and prostaglandins in the control of anion secretion by the rat epididymis. J Reprod Fertil Suppl. 1998;53:15–22. [PubMed] [Google Scholar]
- 22.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayre C. A Brighter Outlook for Cystic Fibrosis Patients. The New York Times. 2009 April 24; [Google Scholar]
- 24.Illek B, Fischer H. Flavonoids stimulate Cl conductance of human airway epithelium in vitro and in vivo. Am J Physiol. 1998;275:L902–910. doi: 10.1152/ajplung.1998.275.5.L902. [DOI] [PubMed] [Google Scholar]
- 25.Illek B, Fischer H, Machen TE. Alternate stimulation of apical CFTR by genistein in epithelia. Am J Physiol. 1996;270:C265–275. doi: 10.1152/ajpcell.1996.270.1.C265. [DOI] [PubMed] [Google Scholar]
- 26.Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol. 1995;268:C886–893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- 27.Rhyoo C, Sanders SP, Leopold DA, Proud D. Sinus mucosal IL-8 gene expression in chronic rhinosinusitis. J Allergy Clin Immunol. 1999;103:395–400. doi: 10.1016/s0091-6749(99)70462-8. [DOI] [PubMed] [Google Scholar]
- 28.Farone A, Huang S, Paulauskis J, Kobzik L. Airway neutrophilia and chemokine mRNA expression in sulfur dioxide-induced bronchitis. Am J Respir Cell Mol Biol. 1995;12:345–350. doi: 10.1165/ajrcmb.12.3.7873201. [DOI] [PubMed] [Google Scholar]
- 29.Culpitt SV, Rogers DF, Fenwick PS, et al. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58:942–946. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols D, Chmiel J, Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol. 2008;34:146–162. doi: 10.1007/s12016-007-8039-9. [DOI] [PubMed] [Google Scholar]


