Abstract
Adolescents with 22q11.2 Deletion Syndrome (22q11.2DS) and Schizotypal Personality Disorder (SPD) are at increased risk for the development of psychosis based, respectively, on genetic or behavioral factors. Thus both groups would be expected to manifest heightened rates of the prodromal signs that typically precede psychosis. Although there are now standardized procedures for assessing prodromal symptoms, there has been little research on the manifestation of these symptoms in 22q11.2DS patients, and no studies of differences in prodromal symptom patterns between genetically and behaviorally defined at-risk groups.
In this study, demographically-matched groups of 23 SPD, 23 22q11.2DS, and 23 control participants were administered the Structured Interview for Prodromal Syndromes (SIPS). Both risk groups showed elevated positive, negative, disorganized, and general prodromal symptoms, as well as elevations on 10 of the same individual symptom items, relative to the control group. Approximately 60% of individuals in the 22q11.2DS group and 70% of individuals in the SPD group met symptom criteria for a prodromal psychosis syndrome. The 22q11.2DS group scored significantly higher than the SPD group on the “decreased ideational richness” item and showed a trend toward greater motor abnormalities.
The results suggest that these two high-risk groups are similar in prodromal symptom presentation, possibly as a result of overlapping causal mechanisms, and that standardized measures of prodromal syndromes like the SIPS can be used to identify 22q11.2DS patients at greatest risk for conversion to psychosis.
Keywords: prodrome, psychosis, ultra high-risk, genetic deletion syndrome, Schizotypal Personality Disorder, 22q11.2 Deletion Syndrome
1. Introduction
Identifying populations at risk for psychosis has been a central aim of research on psychotic disorders for several decades. Early investigations focused on populations at genetic risk by studying the biological offspring of schizophrenia patients (Erlenmeyer-Kimling, 2000). While informative, these genetic high-risk studies were limited by relatively low positive predictive power; only about 12–15% of offspring eventually developed an Axis I psychotic disorder. Subsequently, attention shifted to clinical risk indicators, including schizotypal personality disorder (SPD). The defining criteria for SPD, which include both subclinical positive and negative signs, were based on research findings on the biological relatives of schizophrenia patients (Kendler et al., 1981; Webb & Levinson, 1993; Kendler et al., 1995). Thus, it is generally assumed that SPD shares some genetic determinants with schizophrenia (Kendler et al., 1995). Consistent with this assumption, data indicate that approximately 25–40% of adolescents/young adults who meet criteria for SPD will eventually develop an Axis I psychotic disorder (Klosterkotter, et al., 2001; Miller et al., 2002; Mittal et al., 2008; Woods et al., 2009; Yung et al., 2003).
More recently, investigators identified a relation between the 22q11.2 Deletion Syndrome (22q11.2DS) diagnosis and risk for schizophrenia and other psychoses. Adults with 22q11.2DS show rates of schizophrenia estimated as up to 25–30%, while estimated rates of broadly-defined psychosis are as high as 30–50%. Although only a small proportion of schizophrenia patients manifest 22q11.2DS (roughly .33–2%), this rate is nonetheless dramatically higher than that in the general population (i e., about .025%)(Shprintzen et al., 1992; Murphy et al., 1999; Gothelf et al., 2007; Pulver et al., 1994; Hoogendoorn et al., 2008). Together, these findings indicate that 22q11.2 deletion status confers high-risk for the development of psychosis (Ivanov et al., 2003; Karayiorgou et al., 1995; Horowitz et al., 2005; Goodship et al., 1998).
22q11.2DS results from an interstitial deletion of a segment on the long arm of the 22nd chromosome that, in the majority of cases, is ~3 megabases (mB) in size (Ivanov et al., 2003). This deletion is sporadic in most instances (Swillen et al., 1999), but transmitted as an autosomal dominant trait in 10–28% of cases (Goldberg et al., 1993; Ryan et al., 1997). Estimates of the prevalence of 22q11.2DS range from ~1/4000 to ~1/6000 (Botto et al., 2003; Oskarsdottir et al., 2004). However, the true prevalence may be higher because no molecular diagnosis population-based studies have been performed, and not all cases come to clinical attention.
Several studies have compared samples of psychotic patients with and without 22q11.2DS to determine whether the deletion syndrome is associated with a clinically distinct psychotic syndrome. Such studies provide no consistent evidence of unique symptomatic features in the presentation of 22q11.2DS-associated as compared to non-deletion-associated schizophrenia patients. For example, Murphy and colleagues (1999) found that their sample of patients with schizophrenia and Velo-Cardio-Facial Syndrome (and presumably 22q11.2DS) had less pronounced negative symptoms and a later age of onset than a demographically-matched group of schizophrenia patients without the deletion. Subsequently, Bassett et al. (2003) found that their samples of schizophrenia patients with and without 22q11.2 deletion did not differ on broad symptomatology or course of illness. The study did reveal less substance abuse and higher Positive and Negative Symptom Scale (PANSS) excitement subscale scores (i e., poorer impulse control, and greater uncooperativeness and hostility) in the 22q11.2DS group.
Very few studies, however, have investigated the prodromal period of psychotic illness in 22q11.2DS. The prodrome is defined by attenuated/subclinical manifestations of psychotic symptoms prior to the onset of psychosis (Klosterkotter, et al., 2001; Miller et al., 2002; Woods et al., 2009; Yung et al., 2003). This period can last from months to several years, and varies with respect to the severity and profile of symptoms. Although overwhelming evidence supports the hypothesis that 22q11.2DS patients represent a high-risk group for schizophrenia, there has been little research thus far comparing such patients to those at high-risk for schizophrenia for reasons other than the 22q11.2 deletion. Adolescent patients with SPD represent such a high-risk group.
A frequently-used measure of prodromal symptoms and syndromes is the Structured Interview for Prodromal Syndromes (SIPS; McGlashan et al., 2001), a diagnostic interview that assesses positive, negative, disorganized, and general symptom domains. Based on certain positive symptom criteria, this measure yields different prodromal syndrome designations, including the “attenuated positive symptom syndrome (APS)” or “brief intermittent psychotic syndrome (BIPS).” Researchers have found that individuals who meet prodromal criteria based on this instrument manifest a conversion rate to Axis I psychosis of approximately 30–40 % within two years (Cannon et al., 2008; Miller et al., 2003; Lemos et al., 2006). As would be expected, a substantial proportion (50–70%) of individuals with SPD show elevations on SIPS ratings that are used to index the prodrome (Woods et al., 2009). However, only three studies have used this instrument to investigate prodromal symptoms in 22q11.2DS (Rockers et al., 2009; Stoddard et al., 2010; Antshel, et al., 2010). No report has directly compared the phenomenology of these symptoms to those seen in other groups at high-risk for developing psychosis.
The purpose of the present study was to compare prodromal signs and symptoms in patients with 22q11.2DS (ascertained only on the basis of chromosomal diagnosis) to those in non-22q11.2DS patients meeting criteria for SPD. Previous research has found that individuals with 22q11.2DS show elevated positive, negative (Rockers et al., 2009; Stoddard et al., 2010; Antshel et al., 2010), disorganized (Stoddard et al., 2010; Antshel et al., 2010), and general (Stoddard et al., 2010) prodromal symptoms on the SIPS. Similarly, adolescents with SPD have been shown to have elevated symptoms on all four SIPS domains (Woods et al., 2009), as well as elevated positive and negative symptoms on other measures like the SAPS/SANS (Dickey et al., 2005). Therefore, it was hypothesized that subjects from both groups would show elevated scores on four SIPS symptom domains, when compared to healthy controls. Similarities in prodromal states and features between at-risk groups may suggest overlapping pathogenic factors, while differences may suggest that schizophrenia represents a common clinical syndrome that can arise from different antecedents. Second, given that both groups are at approximately equivalent risk for psychosis, with estimates at or exceeding 25%, it was predicted that roughly equivalent proportions would show prodromal-level symptom and meet criteria for a prodromal syndrome on the SIPS.
2. Materials and Methods
2.1 Participants and Procedure
Participants were drawn from two longitudinal studies at Emory University; one focusing on youth who meet criteria for SPD, the other on adolescents with 22q11.2DS. All participants 18 years of age or older provided written informed consent. When participants were younger than 18, parental written informed consent was also obtained. All consent/assent and study procedures were approved by the Emory University IRB.
Control and SPD individuals were recruited as part of the Emory University Adolescent Development Project, a five-year longitudinal study investigating the factors that predict conversion to psychosis. SPD subjects were recruited through announcements directed at clinicians and parents, with those directed at parents describing SPD in lay terms. The control group was comprised of individuals who did not meet criteria for any Axis I or II disorder. Some of these participants were originally screened for potential inclusion in the SPD group but found to be free of all Axis I and II disorders, while others were recruited from an Emory University database of control participants.
SPD diagnostic criteria were assessed via the Structured Interview for DSM-IV Personality Disorders (Pfohl et al., 1997). DSM-IV states that in individuals below 18 years of age, symptoms must persist for at least one year to make a diagnosis of SPD. Thus, this criterion was used. However, in previous studies that support the utility of SPD diagnostic criteria for predicting conversion to psychosis, this duration criterion was not strictly adhered to (e.g., Yung et al., 2003; Woods et al., 2009; Miller et al., 2003; Mittal et al., 2008). Individuals with the 22q11.2 deletion were included in the 22q11.2DS group, regardless of Axis II diagnostic status. Based on SCID and SIPS responses, only one individual included in the 22q11.2DS group met SPD diagnostic criteria. Participants in all groups were interviewed using the Structured Clinical Interview for DSM-IV (SCID IV; First et al., 1997) to assess for the presence of Axis I disorders. Exclusion criteria for all groups were the presence of previously diagnosed mental retardation, current substance abuse or addiction, and any current Axis I diagnosis. Exceptions were the presence of a history of learning disorders as well as attention-deficit and disruptive behavior disorders. In the control group, the parents of one participant reported a past diagnosis of ODD, two parents reported past concerns about ADHD, and one parent reported a learning disability. However, these participants had never been formally assessed for the presence of these conditions and did not appear to meet criteria for ADHD or ODD at the time of this study. Thus, no participant included in these analyses had ever achieved a diagnosis of a psychotic disorder. For a more detailed description of the ongoing study, see Mittal et al. (2007a).
22q11.2DS patients were ascertained in reverse-age order from a case registry of individuals diagnosed with 22q11.2DS, maintained at Children’s Healthcare of Atlanta since 1996. Presence of the 22q11.2 deletion was confirmed in each case by fluorescent in situ hybridization (FISH). Individuals were initially referred for FISH analysis either as children or adolescents, often due to the presence of heart defects, speech and language difficulties, and/or immunological problems. Individuals identified later in life were referred as part of clinical care within a Human Genetics Medical Clinic or Adult Heart Clinic. After recruitment, patients underwent assessment at the Emory University 22q11.2DS clinic, a collaborative center maintained by researchers and physicians from Children’s Healthcare of Atlanta and the departments of Human Genetics and Psychiatry and Behavioral Sciences at Emory University. (For more details on this sample, see Rockers et al. (2009)). SIPS data were collected from 23 individuals with 22q11.2DS, ranging in age from 14 to 22 at the time of their visits.
FISH analyses could not be performed on individuals in the control or SPD groups. However, individuals with 22q11.2 deletions are unlikely in these groups. First, as previously mentioned, low base rates of the 22q11.2 deletion in the general population, as well as in schizophrenia (Hoogendoorn et al., 2008), make it very unlikely that any individual in the SPD or control group would have the deletion. Further, as part of the Emory University Adolescent Development Project, two markers on the COMT genetic region of the 22q11.2 chromosomal region (RS4633 and RS4680) were investigated in a random subset of control and SPD participants. The original purpose of these analyses was to investigate whether genotype frequencies differed between the two groups. In total, 56.52% (13/23) of the SPD patients were genotyped. Of these, 61.54% (8/13) were heterozygous on at least one of these loci, precluding them from carrying the common forms of the deletion. Similarly, 65.22% (15/23) of the control participants were genotyped, 53.33% of which (8/15) were heterozygous on at least one of the two loci. Apparent homozygosity at each SNP occurred no more frequently than expected from the published allele frequencies of the SNPs. Thus, in more than half of the SPD and control participants, molecular evidence rules out either of the two most common 22q11 deletions, both of which delete the entire COMT locus and would thereby lead to apparent homozygosity at every SNP in the gene.
Age-matching was deemed important because the modal developmental period for the prodrome is adolescence/early adulthood. This is assumed to reflect neurodevelopmental processes that occur during this period (Walker et al., 2007). The onset of the prodrome prior to and following this period is atypical. Thus, in comparing symptom differences among risk groups, it is important that they represent the same age-range. Similarly, there may be differences in symptom frequency and severity between the sexes (Willhite et al., 2008). Thus, participants from the SPD, 22q11.2DS, and control groups were hand selected to match on age and sex, yielding 23 individuals in each group. Demographic characteristics of the samples are presented in Table 1.
Table 1.
Demographic Characteristics of Age, Sex, and Ethnicity Matched Groups
Schizotypal Personality Disorder (SPD) and control participants were hand matched to 22q11.2 Deletion Syndrome ( 22q11.2DS) participants on age and gender, as well as ethnicity, where possible, to attempt to control for potential group and developmental differences in symptom severity
| Controls
|
SPD
|
22q11.2DS
|
|
|---|---|---|---|
| Age1 | 17 (1.70) | 17.17 (2.02) | 17.48 (2.50) |
| range | 14–20 | 14–21 | 14–22 |
| Gender | |||
| Male | 11 | 11 | 11 |
| Female | 12 | 12 | 12 |
| Race/Ethnicity | |||
| African American | 9 | 4 | 3 |
| Hispanic | 0 | 1 | 2 |
| Caucasian | 13 | 18 | 17 |
| Asian | 0 | 0 | 1 |
| Mixed Race or other | 1 | 0 | 0 |
Value (SD)
2.2 Structured Interview for Prodromal Syndromes (SIPS)
The SIPS (McGlashan et al., 2001) is a semi-structured diagnostic interview with good psychometric properties (Miller et al., 2003), designed to assess and diagnose the severity of prodromal symptoms of schizophrenia and other psychotic disorders. It is composed of 19 symptom-items, each rated on a 0–6 scale, grouped into four symptom scales: positive, negative, disorganized, and general symptoms. Scores of 0 indicate the absence of a symptom while scores of 1–2 (“questionably present” and “mild”) indicate the non-prodromal presence of a symptom. Scores between 3 and 5 (“moderate,” “moderately severe,” and “severe but not psychotic”) are considered to be within the prodromal range and a score of 6 is in the psychotic range. Each item is comprised of a number of questions that allow the interviewer to accurately rate the severity of each symptom.
The positive symptom scale includes items that assess unusual thought content and delusional ideas, suspiciousness and persecutory ideas, grandiosity, perceptual abnormalities and hallucinations, and disorganized communication. The negative symptom scale includes items that assess social anhedonia, avolition, reduced expression of emotion, decreased experience of emotion and self, decreased ideational richness, and deterioration of role functioning. Items on the disorganized symptom scale assess odd behavior or appearance, bizarre thinking, trouble with focus and attention, and impairment in personal hygiene. Finally, the general symptom scale contains items that assess sleep disturbance, dysphoric mood, motor disturbances, and impaired tolerance to stress. Each symptom scale also yields a factor score, comprised of the average of all the items within that scale.
2.3 Prodromal Syndromes
SIPS symptom dimension scores are used to determine whether individuals meet criteria for one or more prodromal syndromes—the attenuated positive syndrome (APS), or the brief intermittent psychotic syndrome (BIPS). APS is characterized by the presence of at least one subthreshold positive symptom (i e., one symptom rated 3–5) and no psychotic level positive symptoms (i.e. a rating of 6). In BIPS, an individual experiences at least one psychotic level positive symptom which must have developed or increased to psychotic intensity within the past three months. In addition to these prodromal syndromes, the SIPS can also classify individuals as meeting criteria for Presence of Psychotic Syndrome (POPS). Criteria for POPS are similar to those of APS except that individuals have at least one positive symptoms rated as psychotic.
2.4 Cognitive Function
Cognitive ability is not a focus of the current research; symptom assessment was prioritized in the data collection process. However, given that cognitive function is often affected in both 22q11.2DS and SPD, intellectual ability may be relevant for the interpretation of SIPS scores. This may be particularly true in the interpretation of items that relate to thought content or richness, and the quality of expressed language or communication. Thus, brief estimates of overall cognitive ability were collected for descriptive and, in the non-deletion groups, exclusionary purposes. Participants under the age of 17 were administered the Wechsler Intelligence Scale for Children—Third Edition (WISC-III) and participants 17 and older were administered the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III). Time and research burden did not allow for administration of the standard subtest battery in the 22q11.2DS group, so the vocabulary, similarities, and block design tasks were administered to provide estimated verbal and performance IQs (Ryan, 1981; 1983; Ringe et al., 2002). In total, WAIS-III data were collected on 6, 7, and 8 participants in the control, SPD, and 22q11.2DS groups, respectively, and WISC-III data were collected for 17, 16, and 15 participants, respectively.
2.5 Analyses
All analyses were conducted using SPSS 16. Inspection of the raw data suggested likely floor effects in the SIPS scores of the normal control group, as well as positive skew across all groups. Neither square root nor logarithmic transformations (both run with raw scores and with a constant added) successfully equalized the variances between diagnostic groups on the majority of the SIPS scales. Therefore, the Games-Howell MANOVA post hoc contrast procedure was used to investigate group differences within each dependent variable. Games-Howell, a modification of the Tukey test, minimizes potential Type I error when all possible comparisons are being run on groups with unequal variances (Ramsey & Ramsey, 2009). To further control for the inflated chances of Type I error that accompanies multiple comparisons, an alpha of .01 was used. SIPS means and standard deviations for all groups are listed in Table 2.
Table 2.
SIPS Scores by Diagnostic Group
Significant group contrasts are noted, as are the effect sizes for contrasts between the two high-risk groups.
| Controls | SPD | 22q11.2DS | SPD v. 22q11.2DS | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Cohen’s d | |
| POSITIVE SYMPTOMS ‡† | 0.652 | 0.616 | 2.070 | 1.193 | 1.446 | 0.577 | 0.666 |
| unusual thought content/delusional ideas ‡† | 0.652 | 0.982 | 2.435 | 1.879 | 1.478 | 0.665 | 0.679 |
| suspiciousness/persecutory ideas ‡† | 0.652 | 1.027 | 2.348 | 1.695 | 1.652 | 1.152 | 0.480 |
| grandiosity | 0.739 | 0.915 | 1.304 | 1.428 | 0.739 | 1.096 | 0.444 |
| perceptual abnormalities/hallucinations ‡† | 0.696 | 0.926 | 2.435 | 1.830 | 1.783 | 1.204 | 0.421 |
| disorganized communication † | 0.609 | 0.891 | 2.000 | 1.537 | 1.478 | 1.123 | 0.388 |
| NEGATIVE SYMPTOMS ‡† | 0.574 | 0.735 | 1.808 | 1.352 | 2.058 | 0.830 | −0.223 |
| social anhedonia ‡† | 0.826 | 1.370 | 2.826 | 2.059 | 3.000 | 1.651 | −0.093 |
| avolition ‡ | 0.609 | 1.340 | 1.913 | 1.730 | 1.957 | 1.522 | −0.027 |
| reduced expression of emotion | 0.826 | 1.114 | 1.696 | 1.941 | 1.696 | 1.550 | 0.000 |
| decreased experience of emotion/self | 0.391 | 0.722 | 1.000 | 1.537 | 1.043 | 1.186 | −0.031 |
| decreased ideational richness ‡† * | 0.130 | 0.344 | 1.348 | 1.229 | 2.913 | 1.676 | −1.065 |
| deterioration of role functioning ‡ | 0.609 | 1.373 | 1.826 | 1.946 | 1.913 | 1.443 | −0.0508 |
| DISORGANIZED SYMPTOMS ‡† | 0.326 | 0.449 | 1.630 | 0.935 | 1.576 | 1.001 | 0.056 |
| odd behavior/appearance ‡† | 0.130 | 0.344 | 1.696 | 1.608 | 1.435 | 1.273 | 0.180 |
| bizarre thinking † | 0.174 | 0.650 | 1.174 | 1.193 | 0.783 | 1.043 | 0.349 |
| trouble with focus/attention ‡† | 0.826 | 1.267 | 2.304 | 1.396 | 2.652 | 0.935 | −0.2929 |
| problems with hygiene ‡† | 0.174 | 0.491 | 1.391 | 1.500 | 1.304 | 1.608 | 0.056 |
| GENERAL SYMPTOMS ‡† | 0.467 | 0.524 | 1.859 | 1.290 | 1.826 | 0.831 | 0.030 |
| sleep disturbance | 1.043 | 1.331 | 2.391 | 1.751 | 1.609 | 1.406 | 0.493 |
| dysphoric mood ‡† | 0.565 | 0.896 | 2.696 | 2.204 | 2.391 | 1.500 | 0.162 |
| motor disturbance ‡ | 0.087 | 0.417 | 0.696 | 1.490 | 1.826 | 1.557 | −0.742 |
| impaired tolerance to stress ‡ | 0.174 | 0.388 | 1.696 | 2.077 | 1.478 | 1.201 | 0.129 |
Significant difference between Schizotypal Personality Disorder (SPD) and 22q11.2 Deletion Syndrome (22q11.2DS) groups at p <.01
Significant difference between control and SPD groups at p <.01
Significant difference between control and 22q11.2DS groups at p <.01
To analyze symptom “profiles” in the two high risk groups, repeated measures ANOVAs were conducted to determine whether non-parallel patterns were evident as group × subscale interactions. Symptom items were entered as within-subjects factors and diagnostic status as the between subjects factor. Items on which the two groups differed were excluded from these models.
WISC-III and WAIS-III composite scores were computed for the control and SPD groups, using age-based norms. Subtest scores in the 22q11.2DS group were also standardized using age norms and converted to scaled scores. Kolmogorov-Smirnov z-tests indicated that, of the six scaled scores in the 22q11.2DS group and four in the other two groups, only the distribution of the WISC-III block design scores in the 22q11.2DS group deviated from normality. Scaled scores were then transformed to z-scores with a mean of 100 and standard deviation of 15, as suggested by Palmer et al. (2003). One sample t-tests were computed within each diagnostic group to determine whether scores deviated from the the mean raw scaled score of 100. Because there is no widely accepted non-parametric equivalent of the one sample z or t-test, this same statistic was used for block design in the 22q11.2DS group, but should be interpreted with caution.
3. Results
3.1 Intellectual functioning
As mentioned, cognitive variables were investigated for descriptive purposes—meaningful direct comparisons could not be conducted with the available data. In the control group, WAIS-III scores (M = 108.333, SD = 16.008) were within the average range ( t(5) = 1.275, p = .258), as were WISC- III scores (M = 102.563, SD = 14.624; t(16) = −.500, p = .624). WAIS-III (M = 107.143, SD = 28.250) and WISC-III (M = 97.357, SD = 16.003) scores were also within the average range in the SPD group (WAIS-III: t(6) = .669, p = .528; WISC-III: t(15) = −1.626, p = .125). Conversely, z-tests in the 22q11.2DS group were significant on all three subtests for both the WAIS-III (vocabulary: t(14) = −2.766, p = .015; similarities: t(14) = −2.997, p = .010; block design: t(14) = −5.608), p < .001) and WISC-III (vocabulary: t(7) = −8.079, p < .001; similarities: t(7) = −3.435, p = .011; block design: t(7) = −17.045, p < .001). However, these mean scores were still in the average range for the WAIS-III on the vocabulary (M = 86.67, SD = 18.663) and similarities (M = 86.330, SD = 17.667) tasks. They were below average for the block designs (M = 80.670, 13.350) task. Scores on the WISC-III were below average for the similarities task (M = 78.756) and in the lower extreme range on the vocabulary (M = 68.76, SD = 10.937) and block designs task (M = 57.494, SD = 7.053).
3.2 SIPS Scores
3.2.1 Differences between the risk groups and healthy controls
Games-Howell contrasts were first conducted to test for group differences in symptom severity. As hypothesized, both the SPD and 22q11.2DS groups had higher scores than controls on the positive symptom factor (p < .0001 and p = .0001, respectively), the negative symptom factor (p = .0014 and p < .0001, respectively), the disorganized factor (p < .0001 for both), and the general symptoms factor (p = .0001 and p<.0001).
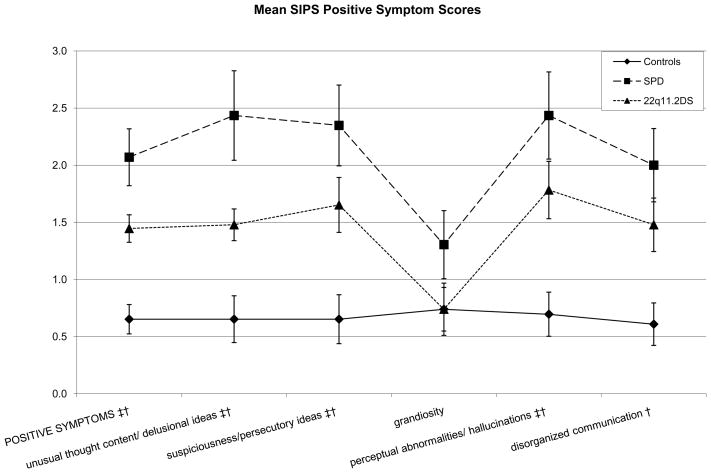
Next, group differences in symptom items within symptom factors were tested. Figures 1–4 show SIPS group means with significant group differences noted. Within the positive symptom domain, the 22q11.2DS group had higher scores than the control group on the unusual thought content/delusional ideas (p = .0052), suspiciousness/persecutory ideas (p = .0092), and perceptual abnormalities/hallucinations items (p = .0039). Mean differences were marginally significant for the disorganized communication item (p = .0156). In the same domain, the SPD group had higher scores than the control group on the unusual thought content/delusional ideas (p = .0009), suspiciousness/persecutory ideas (p = .0006), perceptual abnormalities/hallucinations (p = .0008), and disorganized communication items (p = .0018).
Figure 1.
Schizotypal Personality Disorder (SPD) and 22q11.2 Deletion Syndrome (22q11.2DS) participants show what appear to be parallel patterns of positive symptom score elevations; the SPD group shows non-significantly higher scores than the 22q11.2DS group; Cohen’s d values for the SPD-22q11.2DS contrasts range from .388 to .679.
* Significant difference between SPD and 22q11.2DS groups at p <.01
† Significant difference between control and SPD groups at p <.01
‡ Significant difference between control and 22q11.2DS groups at p <.01
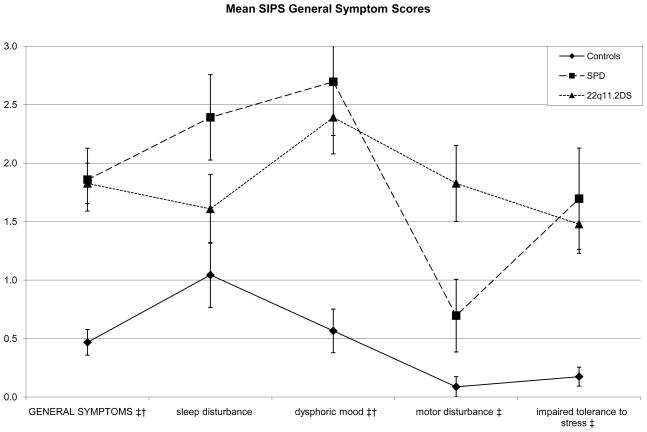
Figure 4.
Both risk groups again show elevations on the same items, with the exception of the motor disturbance item, where only the 22q11.2 Deletion Syndrome (22q11.2DS) group has an elevated score.
* Significant difference between Schizotypal Personality Disorder (SPD) and (22q11.2DS groups at p <.01
† Significant difference between control and SPD groups at p <.01
‡ Significant difference between control and 22q11.2DS groups at p <.01
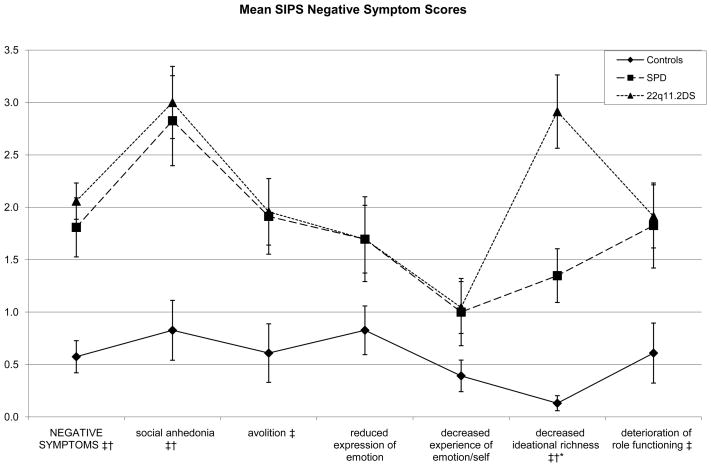
In the negative symptom domain, the 22q11.2DS group had higher scores than controls on the social anhedonia (p < .0001), avolition (p = . 0074), decreased ideational richness (p < .0001), and deterioration of role functioning items (p = .0083). The SPD group had higher scores than controls on the social anhedonia (p = .0011) and decreased ideational richness (p = .0003) items. Results were marginally significant for the avolition item (p = .0178), with the SPD group again showing higher scores than controls.
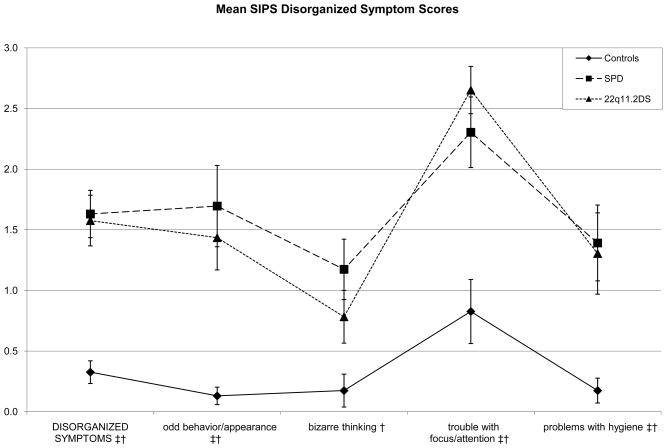
Within the disorganized symptom factor, the 22q11.2DS group had higher scores than the control group on the odd behavior or appearance (p = .0002), trouble with focus/attention (p < .0001), and impairment in personal hygiene items (p = .0092). Here, the SPD group had higher scores than controls on the odd behavior or appearance (p = .0004), bizarre thinking (p = .0034), trouble with attention (p = .0014), and impairment in personal hygiene (p = .0027) items.
In the general symptom domain, the 22q11.2DS group had higher scores than the control group on the dysphoric mood (p < .0001), motor disturbance (p < .0001), and impaired stress tolerance (p = .0001) items. The SPD group had higher scores than the control group on the dysphoric mood (p=.0005) and impaired tolerance to stress (p = .0057) items. Results were marginally significant for the sleep disturbance item (p = .0146), with the SPD group again showing higher scores.
3.2.2 Differences between the 22q11.2DS and SPD groups
Games-Howell contrasts were also conducted to determine whether the two high-risk groups differed in SIPS scores. The 22q11.2DS and SPD groups did not differ on any of the positive symptom items. Within the negative symptoms factor, the 22q11.2DS scored higher than the SPD group on the decreased ideational richness item (p = .0024). The two groups did not differ on any of the other negative symptom items or on any of the disorganized symptoms. In the general symptom domain, the 22q11.2DS subjects showed marginally significant elevations in impairment on the motor disturbance (p = .0406) item compared to the SPD group, but the two groups did not differ on any other item.
Finally, power analyses suggest that current study has low power (24.75%) for detecting a significant moderate effect size in comparisons with two groups of 23 participants each. Thus, Cohen’s d effect sizes were calculated for the high-risk group contrasts (Table 2). While figures 1–3 suggest similar profiles of peaks and valleys in the scores for the clinical groups in the individual SIPS positive, negative, and disorganized symptom items, effect sizes suggest group differences that may yield statistical significance in higher-powered studies—the SPD group showed elevations in positive symptom item scores over the 22q11.2DS group that varied from small in size to moderate to large in size. Contrasts suggested that in the negative symptoms domain, the 22q11.2DS group had elevated scores with respect to the SPD group that were negligible on all items except the decreased ideational richness item, where the contrast yielded a large Cohen’s d of 1.0650. In the disorganized symptoms domain, the difference between the SPD group and the 22q11.2DS group was small in size for the bizarre thinking item (Cohen’s d = .349), while the 22q11.2DS group showed an elevation over the SPD group on the trouble with focus and attention item that was roughly small in size (Cohen’s d = .2929). Scores in the general symptoms domain were slightly more variable, with the SPD group showing elevations over the 22q11.2DS group that were small to moderate in size for the sleep disturbance item (Cohen’s d = .493) and the 22q11.2DS group showing an elevation on the motor disturbances item that was moderate to large in size (Cohen’s d = 742).
Figure 3.
Both risk groups again show elevations on the same items, except on the bizarre thinking item, where only the Schizotypal Personality Disorder (SPD) group was elevated. Their profiles again appear to roughly parallel each other.
* Significant difference between (SPD and 22q11.2 Deletion Syndrome (22q11.2DS) groups at p <.01
† Significant difference between control and SPD groups at p <.01
‡ Significant difference between control and 22q11.2DS groups at p <.01
3.2.3 Symptom profiles
To investigate parallelism in profiles between the two risk groups, repeated measures ANOVAs were conducted for the items in each symptom domain, with diagnostic status entered as the between group factor. No group × symptom interaction was found for the positive symptoms model ( F(4,176) = .233, p = .920), supporting the absence of an interaction. Similarly, excluding the decreased ideational richness item, no interaction was found for the negative symptoms model ( F(4,176) = .032, p = .001) or for the disorganized symptoms model ( F(3,132) = .919, p = .434), again suggesting parallel profiles. The distribution of general symptoms in the two risk groups violated the assumption of sphericity (Mauchly’s W = .724, Approximate Chi square = 13.814, p = .017). Thus, the Greenhouse-Geisser procedure was used to evaluate the model, yielding a significant interaction term ( F(3,132) = 7.754, p = .017). model ( F(3,132) = 7.754, p = .017). These results further suggest similar profiles of positive, negative, and disorganized prodromal symptoms in the SPD and 22q11.2DS groups.
3.2.4 Prodromal Syndromes
Using the SIPS symptom ratings, the proportion of subjects in each group meeting criteria for two prodromal syndromes—BIPS and APS—were derived. APS designations were based only on symptom severity criteria. 5 controls (21.74%), 16 participants with SPD (69.57%), and 13 22q11.2DS individuals (56.52%) met symptom criteria for APS, but no participants met BIPS criteria. Additionally, 2 SPD individuals (8.70%) met POPS criteria at some point in their follow-ups. Kruskal-Wallis Chi-Square tests including all three diagnostic groups revealed that rates of APS differed between groups (χ2(2) = 11.086, p = .004). Post hoc analyses indicated that the rate did not differ between the two risk groups (χ2(1) = .822, p = .365), but did differ between the control and 22q11.2DS groups (χ2(1) = 5.841, p = .016). Rates of POPS did not differ across risk groups (χ2(2) = 4.06, p = .131).
4. Discussion
The findings of the present study support the hypothesis that both the SPD and 22q11.2DS groups manifest elevated positive, negative, disorganized, and general prodromal symptoms, relative to controls. These results replicate those of previous studies that have found elevated SIPS scores in both high-risk groups. Specifically, three previous studies have examined SIPS scores in individuals with 22q11.2DS (Rockers et al., 2009; Stoddard et al., 2010; Antshel et al., 2010); all found scores comparable to those of the present sample. Using a somewhat older sample of 20 22q11.2DS patients, Rockers and colleagues (2009) reported mean SIPS positive (1.68) and negative (2.19) symptom scores that were very similar to those of the 22q11.2DS patients in the present study—some of these patients were also included in the current study. Antshel et al. (2010) compared a slightly younger sample of 70 youths with Velo-Cardio-Facial Syndrome with a healthy control and sibling group. These authors also reported elevated positive (1.3), negative (2.0), and disorganized (1.0) symptom scores which were similar to those found in the current analyses. Stoddard and co-authors (2010) reported median SIPS symptom scale item scores for 20 individuals with 22q11.2DS. Averaging their median scores on the symptom domains yielded an average of .6 for positive symptoms, which is slightly lower than that found in the current study, and a score of 2.875 on the general symptoms scale, which is higher than the mean found in the current study. Their scores of 1.917 for negative symptoms and 1.25 on the disorganized symptom scale are similar to those reported here for 22q11.2DS patients. Taken together, these results lend support to the validity of the SIPS. They also suggest that the severity of elevated prodromal symptoms in the current 22q11.2DS sample is comparable to previous reports.
Elevated SIPS ratings for the SPD group in the present study were also comparable to those of the one previous report in the literature. Woods et al. (2009) found that their sample of 49 youths with SPD had average positive, negative, disorganized, and general symptoms scores of 2.14, 2.283, 1.975, and 1.65, respectively. All of these scores are similar to those found in the present study, with the exception of the negative symptom score which is somewhat higher than that observed in the present SPD sample.
The second finding of the current study is that the SPD and 22q11.2DS groups show remarkable similarity in their SIPS profiles, illustrated in Figures 1–4. Relative to controls, both risk groups showed elevated scores on the positive, negative, disorganized, and general symptoms domains, but did not differ from each other. There were also no significant symptom × risk group interactions within the positive, negative, and disorganized symptoms domains. These results suggest that the psychosis prodrome in these groups is likely to be similar, with substantive differences seen only in thought quality/content, likely movement abnormalities (discussed below), and some of the non-specific symptoms included in the general symptoms domain. Thus, it is plausible that etiologic mechanisms leading to the prodromal phenomenology in 22q11.2DS overlap with those involved in SPD. For example, genes in the 22q11.2 chromosomal region may confer risk for the development of psychosis in subgroups of both SPD and 22q11.2DS patients.
Only a few areas of symptom score divergence between the two high-risk groups were identified. Although no statistically significant differences between the SPD and 22q11.2DS groups were found on positive symptom ratings, Cohen’s d values for the SPD/22q11.2DS contrasts in the positive symptom domain ranged from .388 to .679 (i.e., moderate to large effect sizes), suggesting that positive symptoms may be more pronounced in the SPD group, and that studies of larger samples with greater statistical power might detect significant differences. However, these differences are likely due to selection criteria, given that subjects must have some elevated positive symptoms to qualify for a diagnosis of SPD. The two groups also differed significantly on one negative symptom scale item (decreased ideational richness) and the difference on a general symptom item, motor disturbance, was marginally significant with an effect size that was moderate to large in size. In addition, when compared to the control group, the SPD group showed elevations on two unique symptoms (bizarre thinking and disorganized communication) and the 22q11.2DS group showed elevations on three unique symptoms (avolition, motor disturbance, and deterioration of role functioning).
Given the pronounced motor abnormalities reported in 22q11.2DS (Swillen et al., 1999; Van Aken et al., 2007; Sobin et al., 2006; Chow et al., 2006), it is not surprising that this group would manifest higher scores than the other groups on the motor disturbance item. At the same time, the SPD group did show moderate, nonsignificant elevations when compared with controls on this item (Cohen’s d = .556). This accords well with the literature on movement abnormalities in schizophrenia (Walker & Lewine, 1994), which typically finds subtle, but significant motor problems in individuals who later develop illness, while the abnormalities seen in 22q11.2DS tend to be more pronounced. Interestingly, reports on movement abnormalities in SPD have also found that these are often correlated with the severity of other prodromal symptoms (Neumann & Walker, 1999; Mittal et al., 2007b), suggesting that risk factors for motor problems and later psychosis may overlap.
The two clinical groups also differed significantly on decreased ideational richness, where the 22q11.2DS group had greater deficits than the SPD group (with a large effect size of 1.065), who in turn had more impairment than the control group. This item addresses some basic cognitive abilities, namely the ability to understand the meaning of what others say and to explain proverbs and sayings to the examiner. In addition, the 22q11.2DS group performed more poorly on brief cognitive tasks that tapped both verbal and nonverbal cognitive abilities, with more consistent deficits seen on tasks assessing nonverbal abilities. These findings are consistent with previous reports that describe 22q11.2DS patients as having pronounced general intellectual and executive function deficits (Henry et al., 2002, Kiley-Brabeck & Sobin, 2006; Zinkstok et al., 2005). Thus, greater deficits in ideational richness in the 22q11.2DS subjects may reflect the cognitive impairments and brain morphometric abnormalities found in 22q11.2DS (i.e. Zinkstok et al., 2005; Eliez et al., 2000; Simon et al., 2005; Van Amelsvoort et al., 2004)—further investigation is needed to differentiate between thought problems due to prodromal- and deletion-related processes.
Finally, the present investigation revealed that the high-risk groups are similar in the proportions of participants who meet symptom severity criteria for a prodromal syndrome. Results are consistent with the prediction that at least 25% of the individuals in the 22q11.2DS and SPD groups would meet criteria for a prodromal syndrome. Because data on the duration of symptoms were not available for all participants, the rates of prodromal syndromes were assessed by focusing only on the symptom severity criteria. According to these criteria, 69.57% of the SPD group and 56.52% of the 22q11.2DS group met criteria for either APS or BIPS. Thus, according to these estimates, a similar proportion of 22q11.2DS and SPD individuals are likely to meet criteria for a prodromal syndrome. As noted, past research using the SIPS/SOPS indicates that the conversion rate to Axis 1 psychosis within 2–3 years from baseline ranges from 30–40% in those who meet criteria for the prodrome (Miller et al., 2003; Yung et al., 2003; Lemos et al., 2006; Cannon et al., 2008). Thus, if 30–40% of those in the present study who meet prodromal criteria eventually convert to psychosis, the rate of psychotic outcomes in the SPD and 22q11.2DS groups would be 20.87–27.83% and 16.96–22.61%, respectively. These figures are at the lower end of the range of estimated eventual rates of psychosis in the two groups, but syndrome rates are comparable to those reported in the Stoddard et al. (2010) study of the prodrome in 22q11.2DS.
A significantly smaller proportion of the healthy controls (21.74%, n=5) also met symptom criteria for one of the prodromal syndromes. Given that the base rate of schizophrenia in the general population is approximately 1% and approximately 30–40% of those classified as prodromal develop illness, this estimate seemed high. Four individuals in this control group may have met criteria for ADHD, LD, or ODD in the past, reflecting the fact that some controls were help seeking. While there is some indication of elevated levels of these diagnoses in samples of prodromal adolescents (Meyer et al., 2005), they are relatively common in the general population, suggesting that the control group in the current study is representative of this population. Further, there is a growing literature on the presence of psychotic-like experiences in healthy adolescents. This literature suggests that as many as 13–38% of late-adolescent controls report prodromal-level unusual ideations (Morgan et al., 2009; Rossler et al., 2007). Further, Loewy and colleagues (2007) found that 25% of their general college sample self-reported the presence of a prodromal-level symptom, which is similar to the symptom-severity criteria used in the current study. It therefore appears that the present counterintuitive findings in the healthy control group converge with previous reports. These results raise questions about the validity of the SIPS/SOPs criteria for non-clinical or help-seeking control populations.
The major limitation of the present study is small sample size; the study may have been underpowered for detecting moderate effect sizes. The low base-rate of 22q11.2DS makes subject ascertainment and recruitment challenging. Nonetheless, the sample size herein is in the range of many previous reports on 22q11.2DS. Additionally, the absence of FISH data for the SPD and control groups made it impossible to verify the absence of the 22q11.2 deletion in every participant. However, the low base rates of this deletion in both the overall population and in samples of schizophrenic patients, combined with the genetic data that were available, make it unlikely that any additional cases would have this deletion. It was also not possible to control for differences in cognitive abilities in the current analyses. The use of an IQ-matched control sample may be beneficial in future studies. Finally, the current study is, to our knowledge, the first to compare 22q11.2DS individuals with a clinically-defined high-risk group. Further research is needed to compare prodromal syndromes in 22q11.2DS with individuals who are at heightened genetic risk, for example, due to psychosis in first degree relatives.
Figure 2.
The two risk groups show elevations on the same items, with the exception of the deterioration of role functioning and avolition items, where only the 22q11.2 Deletion Syndrome (22q11.2DS) group showed elevated scores. These groups only differed on the decreased ideational richness item. Otherwise, their scores again appear roughly parallel.
* Significant difference between Schizotypal Personality Disorder (SPD) and 22q11.2DS groups at p <.01
† Significant difference between control and SPD groups at p <.01
‡ Significant difference between control and 22q11.2DS groups at p <.01
Acknowledgments
The authors of this article gratefully thank the staff and research team at the Emory University Autism Center and the Emory University Mental Health and Development Program, as well as the National Institutes of Mental Health, the Robert W. Woodruff Fund, and the Predictive Health Initiative of Emory University. In addition, we are grateful to Karlene Coleman, CGC, MSN for assistance ascertaining patients with 22q11.2DS, and to Amanda MacMillan for assistance with assessments. Thank you also to Dr. Irwin Waldman and Dr. Jocelyn Bachevalier for feedback and comments on earlier versions of this manuscript. Finally, we are grateful to the participants and their families for their generous gifts of time and effort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. Cognitive and psychiatric predictors to psychosis in Velocardiofacial Syndrome: A 3-year follow-up study. J Am Acad Child Psy. 2010;49(4):333–344. [PMC free article] [PubMed] [Google Scholar]
- Basset AS, Chow EWC, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The Schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry. 2003;160:1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, Merritt RK, O’Leary LA, Wong LY, Elixson EM, Mahle WT, Campbell RM. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112(1 pt 1):101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EWC, Watson M, Young DA, Bassett AS. Neurocognitive profile in 22q11 deletion syndrome and Schizophrenia. Schizophr Res. 2006;87:270–278. doi: 10.1016/j.schres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Niznikiewicz MA, Voglmaier MM, Seidman LJ, Kim S, Shenton ME. Clinical, cognitive, and social characteristics of a sample of neuroleptic-naïve persons with schizotypal personality disorder. Schizophr Res. 2005;78:297–308. doi: 10.1016/j.schres.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Reiss AL. Children and adolescents with velocardiofacial syndrome: a volumetric MRI study. Am J Psychiatry. 2000;157(3):409–415. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L. Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. Am J Med Genet. 2000;97(1):65–71. doi: 10.1002/(sici)1096-8628(200021)97:1<65::aid-ajmg9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV—clinical version (SCID-CV) (User’s Guide and Interview) Washington, D.C: American Psychiatric Press, Inc; 1997. [Google Scholar]
- Goldberg R, Motzkin B, Marion R, Scambler PJ, Shprintzen RJ. Velo-cardio-facial syndrome: a review of 120 patients. Am J Med Genet. 1993;45:313–319. doi: 10.1002/ajmg.1320450307. [DOI] [PubMed] [Google Scholar]
- Goodship J, Cross I, LiLing J, Wren C. A population study of chromosome 22q11 deletions in infancy. Arch Dis Child. 1998;79:348–351. doi: 10.1136/adc.79.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, Kwon H, Eliez S, Reiss AL. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry. 2007;164(4):663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- Henry JC, van Amelsvoort T, Morris RG, Owen MJ, Muphry DGM, Muphy KC. An investigation of the neuropsychological profile in adults with velo-cardio-facial syndrome (VCFS) Neuropsychologia. 2002;40:471–478. doi: 10.1016/s0028-3932(01)00136-1. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn ML, Vorstman JA, Jalali GR, Selten JP, Sinke RJ, Emanuel BS, Kahn RS. Prevalence of 22q11.2 deletions in 311 Dutch patients with schizophrenia. Schizophr Res. 2008;98:84–8. doi: 10.1016/j.schres.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, Shifman S, Rivlin N, Pisante A, Darvasi A. A survey of the 22q11 microdeletion in a large cohort of Schizophrenia patients. Schizophr Res. 2005;73:263–267. doi: 10.1016/j.schres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Ivanov D, Kirov G, Norton N, Williams HJ, Williams NM, Nikolov I, Tzwetkova R, Stambolova SM, Murphy KC, Toncheva D, Thapar A, O’Donovan MC, Owen MJ. Chromosome 22q11 deletions, velo-cardio-facial syndrome and early-onset psychosis; Molecular genetic study. Br J Psychiatry. 2003;183:409–413. doi: 10.1192/bjp.183.5.409. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, Eisen H, Childs B, Kazazian HH, Kucherlapati R, Kiley-Brabeck K, Sobin C. Social skills and executive function deficits in children with the 22q11 deletion syndrome. Appl Neuropsychol. 2006;13(4):258–268. doi: 10.1207/s15324826an1304_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gruenberg AM, Strauss JS. An independent analysis of the Copenhagen sample of the Danish adoption study of Schizophrenia. II. The relationship between schizotypal personality disorder and Schizophrenia. Arch Gen Psychiatry. 1981;38(9):982–984. doi: 10.1001/archpsyc.1981.01780340034003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Walsh D. Evaluating the Spectrum Concept of Schizophrenia in the Roscommon Family Study. Am J Psychiatry. 1995;152(5):749–54. doi: 10.1176/ajp.152.5.749. [DOI] [PubMed] [Google Scholar]
- Kiley-Brabeck K, Sobin C. Social skills and executive function deficits in children with the 22q11 Deletion Syndrome. Appl Neuropsychol. 2006;13(4):258–268. doi: 10.1207/s15324826an1304_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58(2):158–64. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Lemos S, Vallina O, Fernandez P, Ortega JA, Garcia P, Gutierrez A, Bobes J, Garcia A, Miller T. Predictive validity of the scale of prodromal symptoms (SOPS) Actas Esp Psiquiatr. 2006;34(4):216–223. [PubMed] [Google Scholar]
- Loewy RL, Johnson JK, Cannon TD. Self-report of attenuated psychotic experiences in a college population. Schizophr Res. 2007;93:144–151. doi: 10.1016/j.schres.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Rosen JL, Hoffman RE, Davidson L. Structured Interview for Prodromal Syndromes (SIPS) Yale University; New Haven: 2001. [Google Scholar]
- Meyer SE, Bearden CE, Lux SR, Gordon JL, Johnson JK, O’Brien MP, Niendam TA, Loewry RL, Ventura J, Cannon TD. The psychosis prodrome in adolescent patients viewed through the lens of DSM-IV. J Child Adolesc Psychopharmacol. 2005;15(3):434–451. doi: 10.1089/cap.2005.15.434. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Dhruv S, Tessner KD, Walder D, Walker EF. The relations among putative bio risk markers in Schizotypal Adolescents: minor physical anomalies, movement abnormalities and salivary cortisol. Biol Psychiatry. 2007a;61(10):1179–86. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Tessner KD, Trottman HD, Esterberg M, Dhrub SH, Simeonova DI, McMillan AL, Murphy E, Saczawa ME, Walker EF. Movement abnormalities and the progression of prodromal symptomatology in adolescents at risk for psychotic disorders. J Abnorm Psychol. 2007b;116(2):260–267. doi: 10.1037/0021-843X.116.2.260. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Saczawa ME, Walder D, Willhite R, Walker EF. Prenatal exposure to viral infection and conversion among male adolescents at high-risk for psychotic disorders. Schizophr Res. 2008;99(1–3):375–376. doi: 10.1016/j.schres.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Fisher H, Hutchinson G, Kirkbride J, Craig TK, Morgan K, Dazzan P, Boydell J, Doody GA, Jones PB, Murray RM, Leff J, Fearon P. Ethnicity, social disadvantage and psychotic-like experiences in a healthy population based sample. Acta Psychiatr Scand. 2009;119(3):226–235. doi: 10.1111/j.1600-0447.2008.01301.x. [DOI] [PubMed] [Google Scholar]
- Muphy KC, Jones LA, Owen MJ. High rates of Schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56(10):940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Neumann CS, Walker EF. Motor dysfunction in schizotypal personality disorder. Schizophr Res. 1999;38(2–3):159–168. doi: 10.1016/s0920-9964(99)00011-0. [DOI] [PubMed] [Google Scholar]
- Oskarsdottir S, Vujic M, Fasth A. Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Arch Dis Child. 2004;89(2):148–151. doi: 10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BW, Taylor MJ, Heaton RK. Accuracy of WAIS-III—WMS-III joint factor scores when one or more subtests is omitted or an alternate subtest is employed. In: Tulsky DS, Saklofske DH, Chelune GJ, Heaton RK, Ivnik RJ, Bornstein R, Prifitera A, Ledbetter MF, editors. Clinical interpretation of the WAIS-III and WMS-III. Academic Press; San Diego: 2003. pp. 391–450. [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured interview for DSM-IV personality: SIDP-IV. Washington DC: American Psychiatric Press; 1995. [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, Morrow B, Karayiorgou M, Antonarakis SE, Housman D, Kucheriapati R. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- Ramsey PH, Ramsey PP. Power and type I errors for pairwise comparisons of means in the unequal variances case. Br J Math Stat Psychol. 2009;62:263–281. doi: 10.1348/000711008X291542. [DOI] [PubMed] [Google Scholar]
- Ringe WK, Saine KC, Lacritz LH, Hynan LS, Munro Cullum C. Dyadic short forms of the Wechsler Adult Intelligence Scale—III. Assessment. 2002;9(3):254–260. doi: 10.1177/1073191102009003004. [DOI] [PubMed] [Google Scholar]
- Rockers K, Ousley O, Sutton T, Schoenberg E, Coleman K, Walker E, Cubells JF. Performance on the Modified Card Sorting Test and its relation to psychopathology in adolescents and young adults with 22q11.2 deletion syndrome. J Intellect Disabil Res. 2009;53(7):665–676. doi: 10.1111/j.1365-2788.2009.01178.x. [DOI] [PubMed] [Google Scholar]
- Rossler W, Riecher-Rossler A, Angst J, Murray R, Gamma A, Eich D, van Os J, Gross VA. Psychotic experiences in the general population: a twenty-year prospective community study. Schizophr Res. 2007;92(1–3):1–14. doi: 10.1016/j.schres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: A European collaborative study. J Med Genet. 1997;42:1141–142. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JJ. Clinical utility of a WISC-R short form. J Clin Psychol. 1981;37(2):389–391. [PubMed] [Google Scholar]
- Ryan JJ. Clinical utility of a WAIS-R short form. J Clin Psychol. 1983;39(2):261–262. [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg R, Golding-Kushner KJ, Marion RW. Late-onset psychosis in the velo-cardio-facial syndrome (letter) Am J Med Genet. 1992;42:141–142. doi: 10.1002/ajmg.1320420131. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. NeuroImage. 2005;25(1):169–180. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Sobin C, Monk SH, Kiley-Brabeck K, Khuri J, Karayiorgou MV. Neuromotor deficits in children with the 22q11 deletion syndrome. Mov Disord. 2006;21(12):2082–2089. doi: 10.1002/mds.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Niendam T, Hendren R, Carter C, Simon TJ. Attenuated positive symptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndrome. Schizophr Res. 2010;118:118–121. doi: 10.1016/j.schres.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Prinzie P, Vogels A, Ghesquiere P, Fryns JP. The behavioural phenotype in velo-cardio-facial syndrome (VCFS): from infancy to adolescence. Genetic Couns. 1999;10(1):79–88. [PubMed] [Google Scholar]
- Van Aken K, De Smedt B, Van Roie A, Gewillig M, Devriendt K, Fryns JP, Simons J, Swillen A. Motor develop ment in school-aged children with 22q11 deletion (velocardiofacial/DiGeorge syndrome) Dev Med Child Neurol. 2007;49(3):210–213. doi: 10.1111/j.1469-8749.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- Van Amelsvoort T, Daly E, Henry J, Robertson D, Ng V, Owen M, Murphy KC, Murphy DGM. Brain anatomy in adults with velocardiofacial syndrome with and without Schizophrenia: preliminary results of a structured magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:1085–1096. doi: 10.1001/archpsyc.61.11.1085. [DOI] [PubMed] [Google Scholar]
- Walker E, Lewine RJ. Prediction of Adult-Onset Schizophrenia From Childhood Home Movies of the Patients. Am J Psychiatry. 1990;147:1052–56. doi: 10.1176/ajp.147.8.1052. [DOI] [PubMed] [Google Scholar]
- Walker E, McMillan A, Mittal V. Neurohormones, neurodevelopment and the prodrome of psychosis in adolescence. In: Romber D, Walker E, editors. Adolescent Psychopathology and the Developing Brain: Integrating Brain and Prevention Science. New York: Oxford University Press; 2007. [Google Scholar]
- Webb CT, Levinson DF. Schizotypal and paranoid personality disorder in the relatives of patients with Schizophrenia and affective disorders: a review. Schizophr Res. 1993;11(1):81–92. doi: 10.1016/0920-9964(93)90041-g. [DOI] [PubMed] [Google Scholar]
- Willhite RK, Niendam TA, Bearden CE, Zinberg J, O’Brien MP, Cannon TD. Gender differences in symptoms, functioning and social support in patients at ultra-high risk for developing a psychotic disorder. Schizophr Res. 2008;104:237–245. doi: 10.1016/j.schres.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW, Addington J, Cadenhead KS, Cannot TD, Cornblatt BA, Heinssen R, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, McGlashan TH. Validity of the prodromal risk syndrome for first psychosis: Findings from the North American Prodrome Longitudinal study. Schizophr Bull. 2009;35(5):841–843. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Pan Yuen H, Francey SM, McFarlane CA, Hallgren M, McGorry PD. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60(1):21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- Zinkstok J, van Amelsvoort T. Neuropsychological profile and neuroimaging in patients with 22q11.2 deletion syndrome: a review. Child Neuropsychol. 2005;11:21–37. doi: 10.1080/09297040590911194. [DOI] [PubMed] [Google Scholar]