Abstract
Background
Connective tissue growth factor (CTGF) is involved in the development and progression of kidney diseases including diabetic nephropathy and kidney fibrosis, but may also play a role in mesangial repair following injury. It is unknown whether, in the general population, urinary CTGF levels are associated with reduction of estimated glomerular filtration rate (eGFR) to less than 60 ml/min/1.73m2 (ie, development of chronic kidney disease [CKD] stage 3).
Study Design
Nested case-control.
Setting & Participants
100 cases of incident CKD stage 3 and 100 age-and sex-matched controls in the Framingham Heart Study (FHS); 141 cases and 135 age-, sexand race-matched controls in the Atherosclerosis Risk in Communities (ARIC) Study. Controls had eGFR ≥60 ml/min/1.73m2 at follow-up in both studies.
Predictors
Urinary CTGF concentrations.
Outcomes
Incident CKD stage 3, defined as eGFR <60 ml/min/1.73m2.
Measurements
Stored urine samples from Framingham Heart Study and ARIC were measured for CTGF. Covariates were obtained from Framingham Heart Study and ARIC participant examinations.
Results
In Framingham Heart Study, the median baseline urinary CTGF concentration was lower among cases (1.35 ng/mL) than controls (2.35 ng/mL; paired t-test P<0.0001). The multivariable-adjusted OR for incident CKD stage 3 was 0.33 (95% confidence interval [CI] 0.17–0.64; P<0.001) per 1-standard deviation increase in log urinary CTGF after adjustment for CKD risk factors, baseline eGFR and baseline log urinary albumin-creatinine ratio, with similar results among participants without diabetes (n=184). Results were not materially different when urinary CTGF was indexed to urinary creatinine (multivariable-adjusted OR, 0.34; 95% CI, 0.21–0.56; P<0.001). A similar, but non-significant, trend of risk of incident CKD stage 3 with lower baseline urinary CTGF concentration was observed in an independent case-control study conducted in the ARIC Study, with the strongest results observed among participants free of diabetes. This inverse relationship was robust in meta-analysis of both the overall and diabetes-free groups.
Limitations
Observational study; causality cannot be inferred.
Conclusions
Lower urinary CTGF concentrations precede the onset of CKD stage 3 in the general population. Further work is required to fully characterize how CTGF influences risk of CKD.
Chronic kidney disease (CKD) is a major worldwide public health problem, having reached epidemic proportions in the United States,1 Europe,2 Australia3 and Asia.4–6 Once established, CKD may result in progressive deterioration in kidney function,7 substantial morbidity,8–10 and excess mortality from both cardiovascular11 and non-cardiovascular causes.12 However, if detected early, kidney functional decline may be slowed13,14 or even reversed,15 and secondary complications can be averted. The currently available biomarkers of CKD and its progression, serum creatinine and urinary protein, tend to detect the later stages of injury, when kidney disease is already established and therapies are likely to be less effective. Hence, there is a need for novel biomarkers that may both identify individuals at risk for the development of reduced kidney function at the earliest possible stage.
Histologically, progressive kidney disease is characterized by matrix deposition within the glomerulus and interstitium, loss of functioning nephrons and the development of tubulointerstitial fibrosis.16 A central orchestrator of these maladaptive changes is transforming growth factor-ß (TGF-ß).17–21 Connective tissue growth factor (CTGF), an important downstream effector of TGF-ß, is a 38 kDa, heparin-binding cysteine-rich protein that has been shown to trigger cell proliferation, collagen synthesis, and chemotaxis in a number of cell lines.22 In vivo, CTGF exhibits pro-sclerotic properties in both animal models of fibrotic kidney disease23,24 and human kidney disease.25 However, CTGF has also been shown to play a role in mesangial repair in response to kidney injury in in-vitro models,26 suggesting that the balance of repair and fibrosis may be complex.
We hypothesized that higher urinary CTGF levels may be associated with an increased risk of developing eGFR < 60 ml/min/1.73m2 in both the general population and among individuals without diabetes. To address this question, we conducted a nested case-control study of incident CKD stage 3 in the Framingham Heart Study. The association between urinary CTGF concentrations was examined in the Framingham Heart Study sample and then in a case-control study of incident CKD stage 3 in Atherosclerosis Risk in Communities (ARIC) Study participants.
Methods
Framingham Heart Study Sample
The design and methods of the Framingham Offspring Study, from which the participants in this study were derived, have been described elsewhere.27 Briefly, it began in 1971 with the enrollment of 5124 men and women, and participants were examined approximately every four to seven years thereafter. This present study includes Offspring cohort participants assessed during the sixth examination cycle (1995 through 1998). All participants provided written informed consent, and the institutional review boards of the Boston University Medical Center approved the study.
ARIC Study and ARIC Carotid MRI Study Samples
The design of the ARIC (Atherosclerosis Risk in Communities) Study has been described in detail previously.28 It is a prospective, multicenter study of men and women in the general population. Overall, 15,792 participants aged from 45 to 64 years were enrolled between1987 and 1989, and attended 3 subsequent study visits approximately every 3 years. Participants at study visit 4 (1996 through 1998) were chosen for the present study, as urine samples were obtained at that visit. All participants provided written informed consent and the institutional review boards of each study center approved the study protocols.
The ARIC Carotid MRI Study exam served as the follow-up visit for this case-control analysis. Participants for the ARIC Carotid MRI Study were selected in 2004–2005 from the surviving ARIC Study participants under a stratified sampling plan, based on the most recent carotid intimal medial thickness (IMT) and field center.29 The goal was to recruit 1,200 participants with high values of maximum carotid artery IMT at their most recent ultrasound examination (ARIC exam 3 or 4, 1993–5 or 1996–8) and 800 individuals randomly sampled from the remaining eligible participants. Participants with contraindications to magnetic resonance imaging (MRI) or allergy to contrast media were excluded, as were those who could not provide informed consent. Participants who had a prior carotid endarterectomy on the side selected for imaging (for the group with high IMT) or either side (for the randomly sampled participants) were excluded. A total of 4,306 persons were invited: 1,403 refused, 837 were ineligible, and 2,066 participated.
Selection of Cases and Controls
In the Framingham Heart Study, estimated GFR was calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) Study Equation,30 as recommended by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) working group31; incident CKD stage 3 was defined as estimated GFR<60 ml/min/1.73m2. Serum creatinine was measured using the modified Jaffé method and calibrated as previously described.32 Of a total of 3522 individuals who attended the sixth examination, 486 were missing UACR measurements, 26 were missing creatinine measurements, and 44 were missing both, leaving 2966 participants from whom we selected 200 for a nested case-control study of incident CKD stage 3; the design has previously been described.33 Participants with CKD or microalbuminuria (defined as UACR >25 mg/g in women and >17 mg/g in men)31 at baseline (offspring exam 6) were excluded from the nested case-control design. Those 100 participants with the lowest eGFR at follow-up (offspring exam 8; 2005 through 2008) formed the case group. Controls were matched to the cases by age (±1 yr) and gender, and were required to have a follow-up eGFR of at least 60 ml/min/1.73 m2.
In ARIC, cases and controls were selected from participants with data on serum creatinine and urinary albumin excretion at both exam 4 (baseline) and the ARIC Carotid MRI Study visit (follow-up). Participants with an estimated GFR < 60 mL/min/1.73 m2 or a UACR ≥ 30 mg/g at baseline were excluded. Cases of incident CKD stage 3 were identified as those participants with an eGFR < 60 ml/min/1.73 m2 at follow-up and a decrease in eGFR from baseline to follow-up of ≥ 25%. Controls were frequency matched to cases by race, sex, and 5-year age categories. As in the Framingham Heart Study, serum creatinine was measured using a modified Jaffé method and calibrated.32
Urinary CTGF Measurement
Urinary CTGF concentrations were measured in both Framingham Heart Study and ARIC using the Human Kidney Tox 1 assay (Rules-Based Medicine, Inc; www.rulesbasedmedicine.com), a microsphere-based panel using antigen-specific antibodies optimized in a capture-sandwich format, and a Luminex 100 Analyzer (Luminex Corp, www.luminexcorp.com), as per the manufacturer's instructions. This assay measures the full-length intact molecule of CTGF secreted protein. The inter-assay coefficient of variation is 9% at a mean concentration of 18 ng/mL and 14% at a mean concentration of 1.2 ng/ml. Overall, 18 samples in Framingham Heart Study and 21 in ARIC had CTGF concentrations below the lower limits of detection, and these values were set to the lowest value in each study.
Covariate Assessment
Participants in both Framingham Heart Study and ARIC underwent blood testing and were assessed for CKD risk factors. High-density lipoprotein cholesterol and blood glucose were measured on morning blood samples while fasting. Diabetes was defined as fasting blood glucose of 126 mg/dL or greater or use of medication for the treatment of diabetes; ARIC additionally included self-reported status in the definition of diabetes, as well as blood glucose of 200 mg/dL or greater if not fasting. Systolic and diastolic blood pressure measurements were taken as the mean of 2 physician readings using a mercury sphygmomanometer in Framingham Heart Study. In ARIC, three seated blood pressure measurements were taken using a random-zero sphygmomanometer, and the average of the second and third readings was recorded. Hypertension was defined as a systolic BP ≥140 mmHg or a diastolic BP ≥90 mmHg or self-reported use of medication for hypertension. Body mass index was defined as an individual's weight in kilograms divided by height in meters squared. Current smoking status was defined by self-report.
In Framingham Heart Study, spot urine samples collected at the baseline examination (1995 through 1998) were stored at −20°C and then transitioned to −80°C. The urinary albumin concentration was measured using immunoturbidimetry (Roche Diagnostics, www.roche.com) and urinary creatinine levels were measured using the Jaffé method.34 In ARIC, spot urine samples were collected and stored at −70°C. Urinary albumin levels were measured by nephelometry and urinary creatinine levels were measured using the Jaffé method.34
Statistical Analyses
In both studies, the distribution of basic demographic variables among participants was described using mean (SD) for continuous and proportions for categorical variables, respectively. Urinary CTGF concentrations were log-transformed to improve normality. A paired t-test was used to assess whether there was a difference in baseline log urinary CTGF concentrations between age and gender-matched pairs of cases and controls. Multivariable regression using conditional logistic regression was used to further adjust for systolic BP, hypertension treatment, diabetes, HDL, smoking, body mass index, baseline eGFR, and baseline UACR. These covariates were selected as they were predictive of incident CKD in previous studies.32 In ARIC, selection was stratified into 16 groups defined by race, sex and 5-year age windows. (i.e., frequency matching, rather than individual matching), and 10 of the 16 groups had equal numbers of cases and controls. Sampling and/or missing laboratory measures resulted in 6 of the 16 groups having one more case than control. We used conditional (fixed-effect) logistic regression for matched case-control groups, utilizing the “clogit” command in Stata, which accounts for the within-group correlation.
In order to normalize urinary CTGF concentrations and correct for variation in urinary volume between samples, we also indexed log urinary CTGF to urinary creatinine. In addition, we modeled the odds ratio of CKD by quartile of baseline urinary CTGF level using conditional logistic regression. In ARIC, additional sensitivity analyses examining the impact of sampling weights for the ARIC carotid MRI study were performed; sampling weights were found to be uncorrelated to CTGF levels. Framingham Heart Study and ARIC results were meta-analyzed using a study-level, fixed-effects meta-analysis. Finally, we considered the overall sample, and then limited our analyses to those without diabetes at baseline.
A type I error threshold of 0.05 was used to indicate statistical significance. All statistical analyses were performed using SAS 9.1 (SAS, www.sas.com) in Framingham Heart Study and Stata 10.1 (www.stata.com) and R 2.7.1. in ARIC.
Results
Framingham Heart Study Baseline Study Characteristics
Study sample characteristics appear in Table 1. Unadjusted correlation coefficients for urinary CTGF in the Framingham Heart Study are presented in Table 2. Log CTGF concentrations did not correlate with CKD risk factors, except for a negative correlation with log UACR (r=−0.16; p=0.01), which was somewhat attenuated after adjustment for age and sex (r=−0.14; p=0.06).
Table 1.
Baseline Characteristics by quartile of urinary CTGF concentration in the Framingham Heart Study and ARIC Study.
| Framingham Heart Study | ARIC | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| CTGF cut-points,* ng/ml | <1.41 | 1.41– 2.33 | 2.34 – 3.00 | ≥ 3.01 | <0.74 | 0.74–1.20 | 1.3–1.8 | ≥1.9 |
| no. of participants | 79 | 58 | 33 | 30 | 71 | 73 | 61 | 71 |
| Age* (years) | 64 +/− 7 | 64 +/− 8 | 64 +/− 6 | 63 +/− 7 | 67 +/− 5 | 65 +/− 6 | 63 +/− 6 | 63 +/− 6 |
| Women** | 55 (69.6) | 31 (53.5) | 18 (54.6) | 16 (53.3) | 31 (43.7) | 42 (57.5) | 41 (67.2) | 33 (46.4) |
| Black* | - | - | - | - | 20 (28.2) | 15 (20.5) | 13 (21.3) | 14 (19.7) |
| HTN | 42 (53.2) | 31 (53.5) | 14 (42.4) | 11 (36.7) | 41 (58.6) | 44 (61.1) | 31 (50.8) | 30 (42.2) |
| HTN Treatment | 33 (41.8) | 21 (36.2) | 10 (31.3) | 9 (30.0) | 34 (44.2) | 36 (40.9) | 18 (33.3) | 17 (29.8) |
| SBP, mmHg | 130 +/− 19 | 133 +/− 21 | 131 +/− 20 | 124 +/− 16 | 133 +/− 16 | 134 +/− 22 | 131 +/− 19 | 126 +/− 19 |
| BMI (kg/m2) | 27.2 +/− 4.9 | 27.7 +/− 4.8 | 27.0 +/− 4.1 | 29.3 +/− 4.9 | 29.2 +/− 5.7 | 28.1 +/− 5.2 | 29.0 +/− 4.8 | 27.3 +/− 4.7 |
| Diabetes | 5 (6.3) | 6 (10.3) | 2 (6.1) | 3 (10.0) | 12 (16.9) | 12 (16.4) | 11 (18.0) | 12 (16.9) |
| Current Smoking | 8 (10.1) | 5 (8.6) | 4 (12.1) | 4 (13.3) | 13 (18.3) | 5 (6.8) | 9 (14.8) | 10 (14.1) |
| HDL-cholesterol, mg/dL | 54.5 +/− 17.6 | 51.2 +/− 16.8 | 53.6 +/− 17.6 | 51.5 +/− 13.8 | 47.0 +/− 14.7 | 52.6 +/− 20.0 | 53.1 +/− 22.1 | 49.8 +/− 15.8 |
| UACR, mg/g | 6.16 (2.32, 11.35) | 3.80 (1.86, 6.67) | 4.56 (3.35, 11.08) | 3.97 (1.48, 9.60) | 3.57 (1.69, 7.84) | 3.09 (1.54, 7.85) | 4.10 (1.25, 6.67) | 3.06 (1.25, 6.67) |
| Baseline eGFR** | 84.6 +/− 23.3 | 88.0 +/− 21.8 | 93.7 +/− 31.6 | 86.2 +/− 17.4 | 83.0 +/− 15.0 | 80.9 +/− 12.1 | 82.9 +/− 14.2 | 83.5 +/− 18.3 |
| eGFR* at Follow-up | 59.3 +/− 18.3 | 64.3 +/− 17.0 | 75.1 +/− 15.7 | 74.2 +/− 17.3 | 62.5 +/− 20.9 | 62.7 +/− 25.7 | 66.5 +/− 10.7 | 59.3 +/− 18.3 |
| eGFR < 60 at follow-up | 54 (68.4) | 33 (56.9) | 8 (24.2) | 5 (16.7) | 44 (57.1) | 51 (58.0) | 20 (37.0) | 26 (45.6) |
| CTGF, ng/mL | 0.82 (0.14, 1.15) | 1.72 (1.57, 1.93) | 2.70 (2.49, 2.82) | 3.69 (3.29, 4.15) | 0.56 (0.39, 0.66) | 0.97 (0.86, 1.10) | 1.50 (1.40, 1.60) | 2.40 (2.00, 4.00) |
Data presented as mean +/− SD for normally distributed continuous variables or median (25th, 75th percentile) for continuous variables that are not normally distributed; categorical data presented as number (%). p-value for trend across quartiles. Quartiles were derived from controls only. eGFR was calculated given the 4-variable MDRD Study equation and is given in ml/min/1.73m2; factor for conversion to ml/s/1.73 m2, x0.01667.
Abbreviations: SBP: Systolic Blood Pressure; HDL: High Density Lipoprotein; eGFR: estimated glomerular filtration rate; BMI, body mass index; UACR, urinary albumin-creatinine ratio; HTN, hypertension; ARIC, Atherosclerosis Risk in Communities; CTGF, connective tissue growth factor
Quartiles were derived from controls only.
Matching characteristics: eGFR only matched in ARIC.
Table 2.
Correlation coefficients for log urinary CTGF in the Framingham Heart Study and ARIC Study*
| Age | BMI | SBP | HDL | Glucose | Baseline eGFR | Log UACR | ||
|---|---|---|---|---|---|---|---|---|
| Framingham Heart Study | Log CTGF r | −0.07 | 0.10 | −0.04 | −0.04 | 0.07 | 0.08 | −0.16 |
| p-value | 0.3 | 0.1 | 0.6 | 0.6 | 0.3 | 0.3 | 0.01 | |
| ARIC | Log CTGF r | −0.26 | −0.13 | −0.14 | 0.07 | −0.009 | −0.01 | 0.00 |
| p-value | <0.001 | 0.03 | 0.02 | 0.2 | 0.9 | 0.8 | 0.9 |
Data presented as Pearson correlation coefficient above with associated p-value below for each variable
Abbreviation: BMI: Body mass index; SBP: systolic blood pressure; HDL: High Density Lipoprotein; eGFR: estimated glomerular filtration rate UACR: Urinary albumin-creatinine ratio; ARIC, Atherosclerosis Risk in Communities; CTGF, connective tissue growth factor
CTGF Levels in Framingham Heart Study Cases and Controls
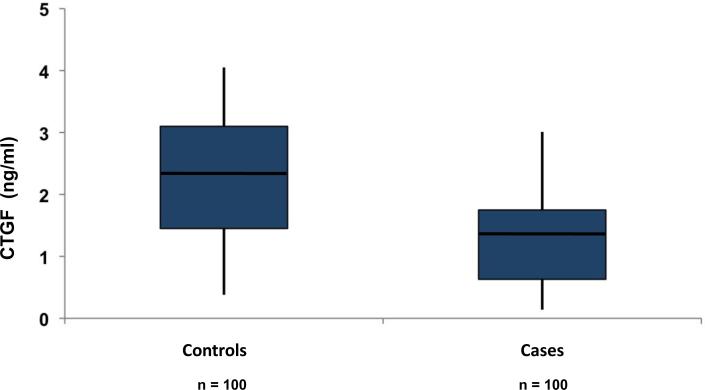
The distribution of urinary CTGF levels by case/control status is shown in Figure 1; baseline median concentrations of CTGF were lower among cases (1.35 ng/mL) as compared to controls (2.35 ng/mL; paired t-test P < 0.001). Higher CTGF levels were associated with a decreased risk for incident CKD stage 3: the multivariable-adjusted odds ratio for incident CKD stage 3 was 0.31 (95% confidence interval [CI], 0.16–0.59; P < 0.001; Table 3) per 1-SD higher log CTGF. Additional adjustment for baseline UACR did not materially affect the results, and results were similar among participants without diabetes (n=184; Table 3). Results were not materially different after indexing log urinary CTGF concentrations to urinary creatinine (OR, 0.34; 95% CI, 0.21–0.56; P < 0.001 per 1-SD higher log ratio of CTGF to urinary creatinine).
Figure 1.
CTGF concentrations in case and control participants at baseline in the Framingham Heart Study are shown, with medians marked (line). Box represents 25th to 75th percentile values; whiskers represent 5th to 95th percentile values. Controls: Mean 2.32 ng/mL median 2.35 ng/mL . Cases: Mean 1.42 ng/mL median 1.35 ng/mL.
Table 3.
Association of incident CKD stage 3 with log urinary CTGF in the Framingham Heart Study and ARIC Study.
| Framingham Heart Study | ARIC | Meta-analysis | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Overall Sample | ||||||
| Unadjusted | 0.42 (0.27–0.63) | <0.001 | 0.84 (0.65, 1.09) | 0.2 | 0.70 (0.56, 0.87) | 0.001 |
| multivariable-adjusted* | 0.31 (0.16–0.59) | <0.001 | 0.90 (0.68, 1.17) | 0.4 | 0.77 (0.60, 0.99) | 0.04 |
| multivariable -adjusted* + log UACR | 0.33 (0.17–0.64) | <0.001 | 0.89 (0.68, 1.17) | 0.4 | 0.77 (0.60, 0.99) | 0.04 |
| Non-diabetes sample | ||||||
| Unadjusted | 0.39 (0.25–0.62) | <0.001 | 0.82 (0.60, 1.12) | 0.2 | 0.65 (0.50, 0.84) | 0.001 |
| multivariable -adjusted* | 0.28 (0.14–0.58) | <0.001 | 0.89 (0.64, 1.23) | 0.5 | 0.73 (0.54, 0.98) | 0.04 |
| multivariable -adjusted* + log UACR | 0.30 (0.14–0.62) | 0.001 | 0.89 (0.64, 1.23) | 0.5 | 0.75(0.55, 1.01) | 0.06 |
Shown are ORs for incident CKD stage 3 per 1-standard deviation increase of log urinary CTGF using conditional logistic regression in the Framingham Heart Study and ARIC Study.
adjusted for estimated glomerular filtration rate, systolic blood pressure, hypertension treatment, diabetes, high density lipoprotein-cholesterol, body mass index, smoking; matched for age and sex
Abbreviation: UACR: Urinary albumin-creatinine ratio; ARIC, Atherosclerosis Risk in Communities; CTGF, connective tissue growth factor; CKD, chronic kidney disease; OR, odds ratio; CI, confidence interval
We observed a significant trend of decreasing risk of incident CKD stage 3 for each incremental quartile of CTGF concentration compared with the lowest (referent) quartile (Table 4), with similar results among those without diabetes. Results were similar following adjustment for prevalent CVD (OR for incident CKD stage 3 per 1 SD increase in log urinary CTGF, 0.43; 95% CI, 0.31–0.58; p<0.001).
Table 4.
Association of incident CKD stage 3 with quartile of log urinary CTGF in the Framingham Heart Study and ARIC Study
| OR (95% CI) | P-value for trend | |||
|---|---|---|---|---|
| Q2 vs. Q1 | Q3 vs. Q1 | Q4 vs. Q1 | ||
| Framingham Heart Study | ||||
| Overall sample | ||||
| Unadjusted | 0.44 (0.19–1.04) | 0.10 (0.03–0.35) | 0.10 (0.03–0.32) | <.0001 |
| multivariable-adjusted* | 0.56 (0.25–1.28) | 0.13 (0.04–0.37) | 0.06 (0.02–0.20) | <.0001 |
| multivariable-adjusted* + UACR | 0.54 (0.23–1.24) | 0.13 (0.04–0.38) | 0.06 (0.02–0.20) | <.0001 |
| Non-Diabetes sample | ||||
| Unadjusted | 0.66(0.32–1.37) | 0.16(0.06–0.42) | 0.11(0.04–0.33) | <.0001 |
| multivariable-adjusted* | 0.55(0.24–1.30) | 0.13(0.04–0.40) | 0.06(0.02–0.22) | <.0001 |
| multivariable-adjusted* + UACR | 0.57(0.24–1.36) | 0.14(0.05–0.42) | 0.06(0.02–0.23) | <.0001 |
| ARIC | ||||
| Overall sample | ||||
| Unadjusted | 1.05 (0.56–2.00) | 0.44 (0.21–0.91) | 0.60 (0.28–1.26) | 0.04 |
| multivariable-adjusted* | 1.09 (0.56–2.12) | 0.45 (0.21–0.97) | 0.74 (0.34–1.61) | 0.1 |
| multivariable-adjusted* + UACR | 1.09 (0.56–2.13) | 0.45 (0.21–0.97) | 0.74 (0.34–1.61) | 0.1 |
| Non-Diabetes sample | ||||
| Unadjusted | 1.16 (0.57–2.37) | 0.37 (0.16–0.84) | 0.57 (0.25–1.30) | 0.03 |
| multivariable-adjusted* | 1.23 (0.58–2.63) | 0.34 (0.14–0.84) | 0.70 (0.29–1.67) | 0.07 |
| multivariable-adjusted* + UACR | 1.23 (0.57–2.64) | 0.34 (0.14–0.84) | 0.70 (0.29–1.67) | 0.07 |
Odds ratio of CKD by quartiles of urinary CTGF concentration in the Framingham Heart Study and ARI
Multivariable adjustment included the following variables: estimated glomerular filtration rate, systolic blood pressure, hypertension treatment, diabetes, high density lipoprotein-cholesterol, body mass index, smoking
Abbreviation: UACR: Urinary albumin-creatinine ratio; ARIC, Atherosclerosis Risk in Communities; CTGF, connective tissue growth factor; CKD, chronic kidney disease; OR, odds ratio; CI, confidence interval
Replication in the ARIC Study
Baseline characteristics of the ARIC sample are presented in Table 1. In ARIC, the overall median CTGF concentration was 1.3 ng/ml. Unadjusted correlation coefficients for urinary CTGF in the ARIC are presented in Table 2. Log CTGF concentrations were inversely correlated with age (r = −0.26; p<0.001), as well as weakly correlated with BMI and systolic blood pressure. Although the direction of effect was consistent with the Framingham Heart Study, the results of the continuous CTGF analysis in ARIC were not statistically significant (Table 3). However, in quartile analysis we observed an inverse relationship between CTGF concentration and incident CKD stage 3 (p-value for trend=0.04, Table 4), which was attenuated in multivariable analysis (P=0.1). Stronger results were observed amongst ARIC participants without diabetes (Table 4). Results in the subset of white ARIC participants (n=214) were similar to those observed in the overall sample (multivariable-adjusted odds ratio of 0.96 for Q2 vs. Q1 (95% CI, 0.44–2.07); 0.62 for Q3 vs. Q1 (95% CI 0.28–1.39); 0.50 for Q4 vs. Q1 (95% CI 0.21–1.19); P for trend=0.09). The number of participants of African ancestry was too small to analyze separately (n=62).
Meta-analysis of Framingham Heart Study and ARIC
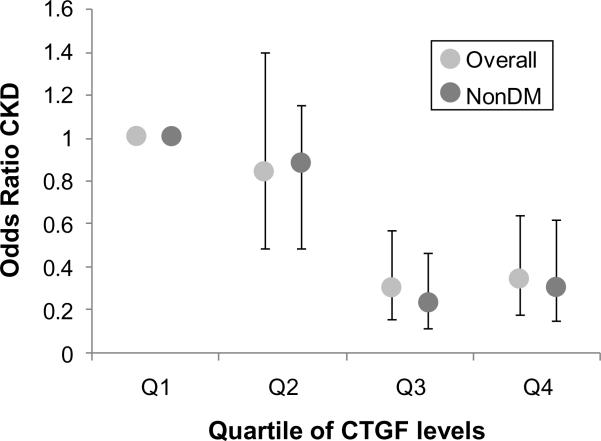
Results were robust in meta-analysis of both studies, with a significant trend of decreasing risk of incident CKD stage 3 for each incremental quartile of CTGF concentration, compared with the referent (lowest) quartile in the overall sample (multivariable-adjusted odds ratio of 0.34 for 4th quartile vs. 1st quartile (95% CI, 0.18–0.64; P for trend<0.001) and among those free of diabetes (multivariable-adjusted odds ratio of 0.30 for Q4 vs. Q1 (95% CI 0.15–0.62; P for trend<0.001; Figure 2). Consistent with this, meta-analysis of continuous log CTGF results also demonstrated an inverse relationship with risk of incident CKD stage 3 in analyses of the overall sample (p=0.001) and participants free of diabetes (p=0.001; Table 3).
Figure 2.
Meta-analysis results of the Framingham Heart Study and the ARIC study. Bars represent 95% confidence intervals. P-value for trend across the quartiles <0.001 for both the overall and non-diabetes groups. nonDM, group without diabetes
Discussion
Our data demonstrate a significant inverse association between lower urinary CTGF concentrations and the development of CKD stage 3 in this case-control study. A similar, but non-significant, trend was observed in a complementary analysis of the ARIC study, and results were robust in meta-analysis of both studies.
CTGF belongs a family of matricellular proteins known as CCN, an acronym derived from three prototypic members: Cysteine-rich protein 61, CTGF, and Nephroblastoma overexpressed protein. These proteins interact with cell surface receptors in the extracellular matrix, growth factors, proteases, cytokines and extracellular matrix proteins35 and stimulate mitosis, adhesion, apoptosis, extracellular matrix deposition, and the migration of several cell types.36 In health, CTGF appears to play an essential role in embryogenesis,37angiogenesis (via modulation of vascular endothelial growth factor [VEGF] function),37,38 wound healing and tissue repair,37 including mesangial repair following kidney injury.26 However, CTGF has mostly been studied in the setting of disease, particularly fibrotic kidney disease and diabetic nephropathy.39 In vitro, CTGF is a highly up-regulated protein in glucose-exposed kidney mesangial cells.40 In vivo, increased expression of CTGF is observed in the renal cortex of rats with streptozotocin-induced diabetes mellitus.41 CTGF is an essential downstream mediator of the pro-fibrotic effects of TGF-ß,42 and has been shown to mediate advanced glycation end product–induced epithelial–mesenchymal transition and kidney fibrosis in rodents.43 Furthermore, treatment with antisense molecules directed against CTGF has been shown to attenuate the development of tubulointerstitial fibrosis in animal models of fibrotic kidney disease.44 In cross-sectional human studies, urinary CTGF excretion correlates with disease severity in diabetic nephropathy45,46 and was shown to predict progression of albuminuria, but not decline in GFR, in a small prospective study of patients with diabetic nephropathy.47
The present study extends knowledge in this area in several key ways. First, we demonstrate a longitudinal relationship between urinary CTGF concentration and subsequent kidney disease in a population-based setting. We also demonstrate that, in this disease-free cohort, the direction of the relationship with risk of kidney disease appears reversed, with lower urinary CTGF concentrations associating with worse kidney outcomes. These observations in the low and normal CTGF range may appear to contradict the prevailing understanding of CTGF biology in diabetic nephropathy, where levels are typically markedly higher,47 and merit further discussion.
Despite its name, CTGF is not a true growth factor, but rather a contextual modulator of cell function whose effects depend on the local cellular microenvironment, and whose functions are complex and cannot be easily generalized.38 For example, transient physiologic expression of CTGF in response to injury results in wound healing,48 and CTGF is released in response to a wide variety of injurious agents, including glucose, advanced glycation end-products, angiotensin II, endothelin, glucose, thrombin, stretch, and oxidative stress.48 Conversely, constitutive over-expression of CTGF promotes an environment where such stimuli may induce potent fibrotic responses,36 and such over-expression is associated with the development of fibrosis.49 In addition, the local level of expression of CTGF markedly influences its ultimate effects at a tissue level. For example, CTGF at low concentrations is a promoter of angiogenesis, whereas at high concentrations CTGF causes inhibition of this process via modulation of VEGF function.38 As such, CTGF may be pro-angiogenic, anti-angiogenic, or may not be involved in angiogenesis at all depending on the local level of expression. Many other concentration-dependent effects of CTGF also have been described, including its induction by TGF-ß, its release in response to mechanical forces, and its modulation by chromatin modification.38
Although the mechanism of the observed inverse association between CTGF and risk of kidney disease is unclear, a similar differential effect of CTGF concentration may be responsible. Our most robust findings were observed in participants without diabetes, an understudied population in terms of CTGF biology, and must be interpreted in that context. It is notable that urinary concentrations of CTGF are typically 100-fold higher in patients with diabetic nephropathy when compared with unaffected diabetic controls47 and, as such, the literature on diabetic nephropathy may not apply to the present population. It is possible that at these basal levels of expression, CTGF is necessary for mesangial health via the mediation of mesangial repair following injury. This hypothesis is supported by in-vitro studies demonstrating CTGF to be an essential pro-resolution factor following mesangial injury.26 These studies demonstrate that mesangial cells demonstrate little or no capacity to heal in the absence of CTGF, and that the pace of mesangial repair is a function of local CTGF concentration.26
This proposed hypothesis, whereby CTGF performs distinct functions in different contexts, mirrors exactly the `homeostatic challenge model' described for TGF-ß, a closely-related cytokine.50 Itself a powerful trigger of tubular cell injury and fibrosis, TGF-ß has recently been shown to also have an essential role in maintaining epithelial cell homeostasis, such that a deficiency in signaling paradoxically triggers tubular cell injury and fibrosis.51 Tight regulation of TGF-ß signaling appears vital for kidney epithelial cell structure and health, with the cytokine apparently performing an essential custodial function under basal conditions.52 Studies with larger sample sizes, spanning later and more severe CKD, are needed to confirm whether a similar paradigm might explain the present observations, stemming as they do from this small, pilot study.
Of note, it is also possible that CTGF per se may not be directly responsible for these observations. For example, the results may relate to the form of CTGF that was measured. As CTGF is an adherent matricellular protein, tissue levels (which are the likely mediator of fibrosis) may not correlate with secreted/excreted protein. Further studies incorporating measures of plasma CTGF would help address this issue.
The findings from the present work suggest that CTGF levels may predict the development of CKD stage 3 in the general population and in participants without diabetes up to a decade before clinical diagnosis. However, this work also highlights the complex biology of CTGF, and efforts to elucidate the mechanisms underlying the association between higher basal CTGF expression and lower risk of kidney disease require further study. Furthermore, those involved in current efforts to develop anti-CTGF therapies for the treatment of fibrotic diseases should be aware of the attendant possibility that there may be an optimal level of CTGF expression, below which harm may occur, possibly due to inadequate repair of ongoing damage. This concern is supported by a recent study of TGF-ß blockade, which unexpectedly resulted in increased fibronectin deposition in renal tubular cells.53
This study has several strengths, including the long follow-up, the richness of the dataset and consistent findings in an independent, external analysis. Limitations pertain to the observational nature of the study, such that causality cannot be inferred from our results. Given that this is a nested case-control study, the results may not be generalized to the Framingham Heart Study as a whole. Also, although every effort was made to adjust for confounding by baseline proteinuria and eGFR, the possibility of residual confounding cannot be completely eliminated. Furthermore, results were stronger in the Framingham cohort compared to ARIC. It is possible this observation may be explained by a `winner's curse' i.e. upward bias of effect sizes in early studies. Nonetheless, further studies in larger, population-based cohorts are desirable to confirm the findings of this exploratory, pilot study.
In summary, the presence of lower urinary CTGF concentrations is associated with the development of CKD stage 3 in two independent study samples. Further work is required to fully characterize how CTGF influences risk of kidney disease.
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Support: The Framingham Heart Study is supported by grant N01-HC-25195 from the National Heart, Lung, and Blood Institute (NHLBI); Framingham Heart Study UACR measurements were supported by an American Diabetes Association Research Grant. The ARIC sample assays were supported by the National Institute of Diabetes and Digestive and Kidney Diseases (1 R01 DK076770-01). ARIC is carried out as a collaborative study supported by NHLBI contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022, with the ARIC carotid MRI examination funded by U01HL075572-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
N section: Because the Editor-in-Chief recused himself from consideration of this manuscript, the Deputy Editor (Daniel E. Weiner, MD, MS) served as Acting Editor-in-Chief. Details of the journal's procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol. 2003 Jul;14(7 Suppl 2):S131–138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Wildman RP, Gu D, et al. Prevalence of decreased kidney function in Chinese adults aged 35 to 74 years. Kidney Int. 2005 Dec;68(6):2837–2845. doi: 10.1111/j.1523-1755.2005.00757.x. [DOI] [PubMed] [Google Scholar]
- 5.Perkovic V, Cass A, Patel AA, et al. High prevalence of chronic kidney disease in Thailand. Kidney Int. 2008 Feb;73(4):473–479. doi: 10.1038/sj.ki.5002701. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, Shiohira Y, Uezu Y, Higa A, Iseki K. Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int. 2006 Jan;69(2):369–374. doi: 10.1038/sj.ki.5000050. [DOI] [PubMed] [Google Scholar]
- 7.McClellan WM, Flanders WD. Risk factors for progressive chronic kidney disease. J Am Soc Nephrol. 2003 Jul;14(7 Suppl 2):S65–70. doi: 10.1097/01.asn.0000070147.10399.9e. [DOI] [PubMed] [Google Scholar]
- 8.Muntner P, Vupputuri S, Coresh J, Uribarri J, Fox CS. Metabolic abnormalities are present in adults with elevated serum cystatin C. Kidney Int. 2009 Jul;76(1):81–88. doi: 10.1038/ki.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002 Feb;13(2):504–510. doi: 10.1681/ASN.V132504. [DOI] [PubMed] [Google Scholar]
- 10.Ensrud KE, Lui LY, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007 Jan 22;167(2):133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 11.Fried LF, Shlipak MG, Crump C, et al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003 Apr 16;41(8):1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 12.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004 May;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 13.de Zeeuw D, Ramjit D, Zhang Z, et al. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int. 2006 May;69(9):1675–1682. doi: 10.1038/sj.ki.5000326. [DOI] [PubMed] [Google Scholar]
- 14.Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999 Jul 31;354(9176):359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 15.Makino H, Nakamura Y, Wada J. Remission and regression of diabetic nephropathy. Hypertens Res. 2003 Jul;26(7):515–519. doi: 10.1291/hypres.26.515. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl. 2005 Dec;(99):S82–86. doi: 10.1111/j.1523-1755.2005.09915.x. [DOI] [PubMed] [Google Scholar]
- 17.Wardle N. Glomerulosclerosis: the final pathway is clarified, but can we deal with the triggers? Nephron. 1996;73(1):1–7. doi: 10.1159/000188990. [DOI] [PubMed] [Google Scholar]
- 18.Border WA, Noble NA. TGF-beta in kidney fibrosis: a target for gene therapy. Kidney Int. 1997 May;51(5):1388–1396. doi: 10.1038/ki.1997.190. [DOI] [PubMed] [Google Scholar]
- 19.Kuncio GS, Neilson EG, Haverty T. Mechanisms of tubulointerstitial fibrosis. Kidney Int. 1991 Mar;39(3):550–556. doi: 10.1038/ki.1991.63. [DOI] [PubMed] [Google Scholar]
- 20.Schnaper HW, Jandeska S, Runyan CE, et al. TGF-beta signal transduction in chronic kidney disease. Front Biosci. 2009;14:2448–2465. doi: 10.2741/3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002 Oct;13(10):2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 22.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997 Sep;8(3):171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Goldschmeding R, Bende R, et al. Kinetics of connective tissue growth factor expression during experimental proliferative glomerulonephritis. J Am Soc Nephrol. 2001 Mar;12(3):472–484. doi: 10.1681/ASN.V123472. [DOI] [PubMed] [Google Scholar]
- 24.Yokoi H, Mukoyama M, Sugawara A, et al. Role of connective tissue growth factor in fibronectin expression and tubulointerstitial fibrosis. Am J Physiol Renal Physiol. 2002 May;282(5):F933–942. doi: 10.1152/ajprenal.00122.2001. [DOI] [PubMed] [Google Scholar]
- 25.Ito Y, Aten J, Bende RJ, et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998 Apr;53(4):853–861. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]
- 26.Blom IE, van Dijk AJ, Wieten L, et al. In vitro evidence for differential involvement of CTGF, TGFbeta, and PDGF-BB in mesangial response to injury. Nephrol Dial Transplant. 2001 Jun;16(6):1139–1148. doi: 10.1093/ndt/16.6.1139. [DOI] [PubMed] [Google Scholar]
- 27.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979 Sep;110(3):281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 28.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989 Apr;129(4):687–702. [PubMed] [Google Scholar]
- 29.Wasserman BA, Astor BC, Sharrett AR, Swingen C, Catellier D. MRI measurements of carotid plaque in the atherosclerosis risk in communities (ARIC) study: methods, reliability and descriptive statistics. J Magn Reson Imaging. Feb;31(2):406–415. doi: 10.1002/jmri.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 31.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 32.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004 Feb 18;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 33.Kottgen A, Hwang SJ, Larson MG, et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J Am Soc Nephrol. Feb;21(2):337–344. doi: 10.1681/ASN.2009070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu CC, Brancati FL, Astor BC, et al. Blood pressure, atherosclerosis, and albuminuria in 10,113 participants in the atherosclerosis risk in communities study. J Hypertens. 2009 Feb;27(2):397–409. doi: 10.1097/hjh.0b013e32831aede6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal. 2009 Dec;3(3–4):163–165. doi: 10.1007/s12079-009-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006 Dec 1;119(Pt 23):4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 37.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008 Apr;19(2):133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Cicha I, Goppelt-Struebe M. Connective tissue growth factor: context-dependent functions and mechanisms of regulation. Biofactors. 2009 Mar–Apr;35(2):200–208. doi: 10.1002/biof.30. [DOI] [PubMed] [Google Scholar]
- 39.Sakharova OV, Taal MW, Brenner BM. Pathogenesis of diabetic nephropathy: focus on transforming growth factor-beta and connective tissue growth factor. Curr Opin Nephrol Hypertens. 2001 Nov;10(6):727–738. doi: 10.1097/00041552-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Murphy M, Godson C, Cannon S, et al. Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem. 1999 Feb 26;274(9):5830–5834. doi: 10.1074/jbc.274.9.5830. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Denichilo M, Brubaker C, Hirschberg R. Connective tissue growth factor in tubulointerstitial injury of diabetic nephropathy. Kidney Int. 2001 Jul;60(1):96–105. doi: 10.1046/j.1523-1755.2001.00776.x. [DOI] [PubMed] [Google Scholar]
- 42.Qi W, Chen X, Poronnik P, Pollock CA. Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int J Biochem Cell Biol. 2008;40(1):9–13. doi: 10.1016/j.biocel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathway. Am J Pathol. 2004 Dec;165(6):2033–2043. doi: 10.1016/s0002-9440(10)63254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoi H, Mukoyama M, Nagae T, et al. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol. 2004 Jun;15(6):1430–1440. doi: 10.1097/01.asn.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]
- 45.Gilbert RE, Akdeniz A, Weitz S, et al. Urinary connective tissue growth factor excretion in patients with type 1 diabetes and nephropathy. Diabetes Care. 2003 Sep;26(9):2632–2636. doi: 10.2337/diacare.26.9.2632. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen TQ, Tarnow L, Andersen S, et al. Urinary connective tissue growth factor excretion correlates with clinical markers of renal disease in a large population of type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006 Jan;29(1):83–88. doi: 10.2337/diacare.29.1.83. [DOI] [PubMed] [Google Scholar]
- 47.Tam FW, Riser BL, Meeran K, Rambow J, Pusey CD, Frankel AH. Urinary monocyte chemoattractant protein-1 (MCP-1) and connective tissue growth factor (CCN2) as prognostic markers for progression of diabetic nephropathy. Cytokine. 2009 Jul;47(1):37–42. doi: 10.1016/j.cyto.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993 Jun;4(6):637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002 Oct;21(6):473–482. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- 50.Wakefield LM, Stuelten C. Keeping order in the neighborhood: new roles for TGFbeta in maintaining epithelial homeostasis. Cancer Cell. 2007 Oct;12(4):293–295. doi: 10.1016/j.ccr.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Gewin L, Bulus N, Mernaugh G, et al. TGF-beta receptor deletion in the renal collecting system exacerbates fibrosis. J Am Soc Nephrol. Aug;21(8):1334–1343. doi: 10.1681/ASN.2010020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kopp JB. TGF-beta signaling and the renal tubular epithelial cell: too much, too little, and just right. J Am Soc Nephrol. Aug;21(8):1241–1243. doi: 10.1681/ASN.2010060676. [DOI] [PubMed] [Google Scholar]
- 53.Qi W, Chen X, Holian J, et al. Transforming growth factor-beta1 differentially mediates fibronectin and inflammatory cytokine expression in kidney tubular cells. Am J Physiol Renal Physiol. 2006 Nov;291(5):F1070–1077. doi: 10.1152/ajprenal.00013.2006. [DOI] [PubMed] [Google Scholar]