Abstract
Polyunsaturated fatty acids (PUFA), a lipid family comprised of omega-3 (n-3) and n-6 fatty acids, are a critical component of cellular membranes, and recent in vitro studies have found that antipsychotic medications up-regulate genes responsible for PUFA biosynthesis. To evaluate this effect in vivo, rats were treated with risperidone (1.5, 3, 6 mg/kg/d), paliperidone (1.5, 3, 6 mg/kg/d), olanzapine (2.5, 5, 10 mg/kg/d), quetiapine (5, 10, 20 mg/kg/d), haloperidol (1, 3 mg/kg/d) or vehicle through their drinking water for 40 d. Effects on liver Fads1, Fads2, Elovl2, and Elovl5 mRNA expression, plasma indices of n-3 (plasma 22:6/18:3 & 20:5/18:3 ratios) and n-6 (plasma 20:4/18:2 & 20:3/18:2 ratios) biosynthesis, and peripheral (erythrocyte, heart) and central (frontal cortex) membrane PUFA composition were determined. Only risperidone and its metabolite paliperidone significantly and selectively up-regulated liver delta-6 desaturase (Fads2) mRNA expression, and robustly increased plasma indices of n-3 and n-6 fatty acid biosynthesis. In risperidone- and paliperidone-treated rats, plasma indices of n-3 and n-6 fatty acid biosynthesis were all positively correlated with liver Fads2 mRNA expression, but not Fads1, Elovl2, or Elovl5 mRNA expression. All antipsychotics at specific doses increased erythrocyte docosahexaenoic acid (DHA, 22:6n-3) composition, and all except quetiapine increased arachidonic acid (AA, 20:4n-6) composition. Risperidone, paliperidone, and olanzapine increased heart DHA and AA composition, and no antipsychotic altered frontal cortex DHA or AA composition. These in vivo data demonstrate that augmentation of PUFA biosynthesis is not common to all antipsychotic medications, and that risperidone and paliperidone uniquely increase delta-6 desaturase (Fads2) mRNA expression and most robustly increase PUFA biosynthesis and peripheral membrane composition.
Keywords: Polyunsaturated fatty acids; docosahexaenoic acid; arachidonic acid; delta6-desaturase (Fads2); delta5-desaturase (Fads1); elongase (Elovl2,Elovl5); plasma; erythrocytes; heart; frontal cortex; rat
1. Introduction
Polyunsaturated fatty acids (PUFA), a lipid family comprised of omega-3 (n-3) and omega-6 (n-6) fatty acids, are critical components of cellular membranes and play a vital role in normal brain development and function (Innis, 2008; Maekawa et al., 2009; McNamara & Carlson, 2006; Ryan et al., 2010; Salem et al., 2001). The long-chain n-3 PUFA docosahexaenoic acid (DHA, 22:6n-3) and long-chain n-6 PUFA arachidonic acid (AA, 20:4n-6) exert opposing effects on synaptic, immune, and inflammatory signaling pathways (Calder, 2008; Groeger et al., 2010; McNamara et al., 2006, 2010a). The biosynthesis of DHA from the short-chain fatty acid precursor α-linolenic acid (ALA, 18:3n-3), and AA from linoleic acid (LA, 18:2n-6), are mediated by a common biosynthetic pathway. Principle biosynthetic enzymes include delta6-desaturase (FADS2, Cho et al., 1999a), delta5-desaturase (FADS1, Cho et al., 1999b), and elongases (Elovl2, Elovl5, Jakobsson et al., 2006). The genes encoding these enzymes have been cloned and are predominantly expressed in liver and brain (Cho et al., 1999a,b; Marquardt et al., 2000; Matsuzaka et al., 2002). Pharmacological (Harmon et al., 2003; Obukowicz et al., 1999), mutant mouse (Guillou et al., 2010; Stoffel et al., 2008) and human genomic (Martinelli et al., 2008; Schaeffer et al., 2006) studies have confirmed the critical role of these enzymes in regulating PUFA homeostasis.
Emerging evidence from basic and clinical studies suggest that antipsychotic medications may augment PUFA biosynthesis (McNamara, 2009). Specifically, in vitro studies have found that different antipsychotic medications up-regulate the expression of multiple lipogenic genes regulated by the sterol regulatory element-binding protein (SREBP)(Ferno et al., 2005; Raeder et al., 2006), and both FADS1 (delta5-desaturase) and FADS2 (delta6-desaturase) promoters are positively regulated by SREBP (Matsuzaka et al., 2002). Indeed, a microarray study found that typical and atypical antipsychotic medications up-regulate FADS1 and FADS2 mRNA expression in human cell lines (Polymeropoulos et al., 2009). However, FADS1 and FADS2 mRNA expression and associated enzymatic activity are regulated by multiple physiological factors that could be altered by antipsychotic medications in vivo but not in vitro, including insulin/glucose (Brenner, 2003), gonadal hormones (Childs et al., 2010; McNamara et al., 2009a), and long-chain PUFAs (Igarashi et al., 2007). Clinical studies have found that chronic treatment with risperidone or olanzapine significantly increase plasma indices of delta6-desaturase activity (Kaddurah-Daouk et al., 2007) and erythrocyte DHA and AA composition (Evans et al., 2003) in schizophrenic patients. However, because these studies did not control for dietary n-3 and n-6 PUFA intake, the etiology of these effects cannot be determined.
To begin to characterize the effects of chronic antipsychotic exposure on PUFA biosynthesis in vivo under controlled dietary conditions, we recently examined the effects of chronic risperidone treatment on erythrocyte and frontal cortex PUFA composition in rats maintained on ALA-fortified diet and ALA-free diets (McNamara et al., 2009b). It was found that risperidone-treated rats maintained on ALA-fortified diet exhibited significantly greater erythrocyte and frontal cortex DHA composition, and that these elevations were not observed in risperidone-treated rats maintained on ALA-free diet. These data suggest chronic risperidone augments ALA→DHA biosynthesis. To extend these findings, in the present study we investigated the effects of chronic treatment with multiple doses of different antipsychotic drugs (risperidone, paliperidone, olanzapine, quetiapine, haloperidol) on liver Fads1, Fads2, Elovl2, and Elovl5 mRNA expression and indices of desaturase- and elongase-mediated long-chain n-3 (plasma 22:6/18:3 & 20:5/18:3 ratios) and n-6 (plasma 20:4/18:2 & 20:3/18:2 ratios) fatty acid biosynthesis. We also investigated effects on peripheral (erythrocyte & heart) and central (frontal cortex) membrane DHA and AA composition.
2. Materials and methods
2.1. Animals and diet
Adult (P56) male Long-Evans hooded rats were purchased from Harlan-Farms Indianapolis, IN. Upon arrival, all rats were maintained on the same custom research diet (TD.04285, Harlan-TEKLAD, Madison, WI). This diet contained casein (vitamin-free) 200 g/kg, L-cystine 3 g/kg, sucrose 270 g/kg, dextrose monohydrate 99.5 g/kg, corn starch 200 g/kg, maltodextrin 60 g/kg, cellulose 50 g/kg, mineral mixture AIMN-93G-MX 35 g/kg, vitamin mixture AIN-93-VX 10 g/kg, choline bitartrate 2.5 g/kg, TBHQ (antioxidant) 0.02 g/kg). Analysis of diet fatty acid composition by gas chromatography found that it contained the short-chain n-6 fatty acid precursor linoleic acid (18:2n-6, 22% of total fatty acid composition) and the short-chain n-3 fatty acid precursor α-linolenic acid (ALA, 18:3n-3, 4.6% of total fatty acid composition)(for complete diet lipid composition, see Table 1 in McNamara et al., 2008). Neither diet contained preformed long-chain n-3 or n-6 fatty acids including DHA and AA, respectively. Rats were housed 2 per cage under standard vivarium conditions, and food and fluids were available ad libitum. Changes in food consumption (g/kg/d), fluid intake (ml/kg/d), and body weight (kg) were recorded. Rats were sacrificed by decapitation on P99–101 in counterbalanced manner. Trunk blood was collected into EDTA-coated tubes, plasma isolated by centrifugation, and erythrocytes washed 3x with 4°C 0.9% NaCl. The brain was dissected on ice to isolate the frontal cortex (olfactory tubercle and residual striatal tissue were removed) and heart and liver samples were collected and flash frozen in liquid nitrogen. All samples were stored at −80°C deg. All experimental procedures were approved by the University of Institutional Animal Care and Use Committee, and adhere to the guidelines set by the National Institutes of Health.
2.2. Drug administration
On P60, rats (n=122) were randomly assigned to receive chronic treatment with drug vehicle (0.1 M acetic acid diluted in deionized water), risperidone (1.5, 3, 6 mg/kg/d; supplied by Ortho-McNeil Janssen Scientific Affairs LLC), paliperidone (1.5, 3, 6 mg/kg/d, supplied by Ortho-McNeil Janssen Scientific Affairs LLC), olanzapine (2.5, 5, 10 mg/kg/d, supplied by Eli Lilly and Company), quetiapine (5, 10, 20 mg/kg/d, supplied by AstraZeneca Pharmaceuticals), or haloperidol (1, 3 mg/kg/d, Sigma-Aldrich Chemicals) through their drinking water for 40 d (n=8/drug dose). Drug doses were selected based on prior studies finding that they produce therapeutically-relevant plasma concentrations in rats following oral administration (Andersson et al., 2002; McNamara et al., 2009b; Terry et al., 2005). Doses of quetiapine were selected based on prior findings of significant effects on behavioral and neurochemical variables within this dose range (Migler et al., 1993; Tarazi et al., 2002), and to avoid significant sedative effects observed at higher doses (≥40 mg/kg, Betz et al., 2005). Drugs were administered through the rat’s drinking water to avoid daily injection stress and surgical implantation of mini-pumps, to mimic oral administration in human patients, and to permit maintenance of drug dose in accordance with age-related increases in body weight. For three days prior to drug delivery, 24 h water consumption was determined for each cage using bottle weights (1 g water = 1 ml water), and ml water intake/mean kg body weight calculated. All drugs were dissolved and diluted in 0.1 M acetic acid to prepare a stock solution (stored at 4 deg) which was added to tap water in a volume required to deliver the targeted daily dose. To maintain intake of the targeted daily dose, drug concentrations were adjusted to daily fluid intake and mean body weight (ml/kg/day) every 3 days. Red opaque drinking bottles were used to protect drug from light degradation. Rats were maintained on their respective drug and dose until being sacrificed on P99–101 (39–41 days of treatment).
2.3. Fatty acid composition
The gas chromatography procedure used to determine plasma, erythrocyte, heart, and frontal cortex fatty acid composition has been described in detail previously (McNamara et al., 2009a,b). Briefly, total fatty acid composition was determined with a Shimadzu GC-2014 (Shimadzu Scientific Instruments Inc., Columbia MD). Analysis of fatty acid methyl esters was based on area under the curve calculated with Shimadzu Class VP 4.3 software. Fatty acid identification was based on retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). All analyses were performed by a technician blinded to treatment.
2.4. Liver mRNA expression
Frozen liver was homogenized (BioLogics Model 300 V/T ultrasonic homogenizer, Manassas, VA) in Tri Reagent, and total RNA isolated and eluted according to the manufacturer’s instructions (RNeasy Lipid Tissue Mini Kit, Qiagen, Valencia, CA). RNA was quantified using a Nanodrop instrument (Nanodrop Instruments, Wilmington, DE). RNA quality was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). cDNA was prepared from total RNA using a high-capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). mRNA levels of delta-6 desaturase (Fads2, Rn00580220_m1), delta-5 desaturase (Fads1, Rn00584915_m1), elongase-2 (Elovl2, Rn01450661_m1), and elongase-5 (Elovl5, Rn00592812_m1) were measured in triplicate by real-time quantitative PCR using an ABI 7500 Real Time PCR System (Applied Biosystems, Foster City, CA). Data were analyzed by comparing the difference between target and endogenous control (GAPDH, Rn99999916_s1) cycle thresholds using the comparative ΔCt method (Livak et al., 2001), and expressed as fold difference from the control group.
2.5. Statistical analysis
Within-drug differences in fatty acid composition (vehicle vs. drug doses) and mRNA expression were evaluated with a one-way ANOVA, and pairwise comparisons made with unpaired t-tests (2-tail, α=0.05). Homogeneity of variance was evaluated using Bartlett’s test. Parametric correlation analyses were used to determine the relationship between fatty acid and hepatic gene expression (2-tail, α=0.05). Analyses were performed with GB-STAT (V.10, Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Food/fluid intake and body weight
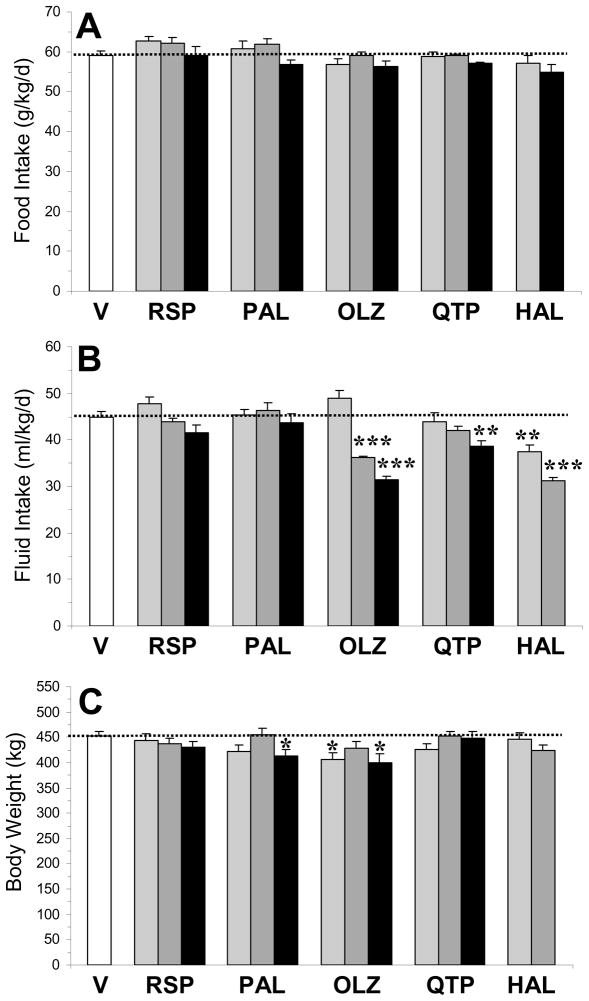
For food intake (g/kg/d)(Fig 1A), the main effect was not significant for risperidone, F(3,16)=1.8, p=0.19, paliperidone, F(3,16)=2.3, p=0.13, olanzapine, F(3,16)=1.5, p=0.25, quetiapine, F(3,16)=1.1, p=0.37, or haloperidol, F(2,14)=1.8, p=0.21. For fluid intake (ml/kg/d)(Fig 1B), the main effect was not significant for risperidone, F(3,16)=1.2, p=0.33, or paliperidone, F(3,16)=0.5, p=0.69, and there was a significant main effect for olanzapine, F(3,16)=47.6, p≤0.0001, quetiapine, F(3,16)=3.9, p=0.04, and haloperidol, F(2,14)=30, p≤0.0001. There were no significant group differences in body weight at baseline (mean ± SD: 276 ± 11 kg). For weight gain (endpoint-baseline), the main effect was significant for paliperidone, F(3,33)=2.9, p=0.04, and olanzapine, F(3,33)=3.7, p=0.02, but not for risperidone, F(3,33)=2.1, p=0.12, quetiapine, F(3,33)=0.8, p=0.49, or haloperidol, F(2,25)=2.2, p=0.14. For endpoint body weight (kg) (Fig 1C), the main effect was significant for paliperidone, F(3,33)=3.0, p=0.04, and olanzapine, F(3,33)=3.5, p=0.03, but not for risperidone, F(3,33)=0.7, p=0.57, quetiapine, F(3,33)=1.2, p=0.31, or haloperidol, F(2,25)=1.4, p=0.26.
Fig. 1.
Effects of chronic treatment with drug vehicle (V)(n=10), risperidone (RSP)(1.5, 3, 6 mg/kg/d), paliperidone (PAL)(1.5, 3, 6 mg/kg/d), olanzapine (OLZ)(2.5, 5, 10 mg/kg/d), quetiapine (QTP)(5, 10, 20 mg/kg/d), or haloperidol (HAL)(1, 3 mg/kg/d)(n=8/drug dose) on food intake (g/kg/d)(A), fluid intake (ml/kg/d)(B), and endpoint body weight (kg)(C). Note that none of the antipsychotic medications significantly altered food intake. Values are group mean ± S.E.M. *p≤0.05, **p≤0.01, ***p≤0.0001 vs. Vehicle.
3.2. Plasma indices of n-3 PUFA biosynthesis
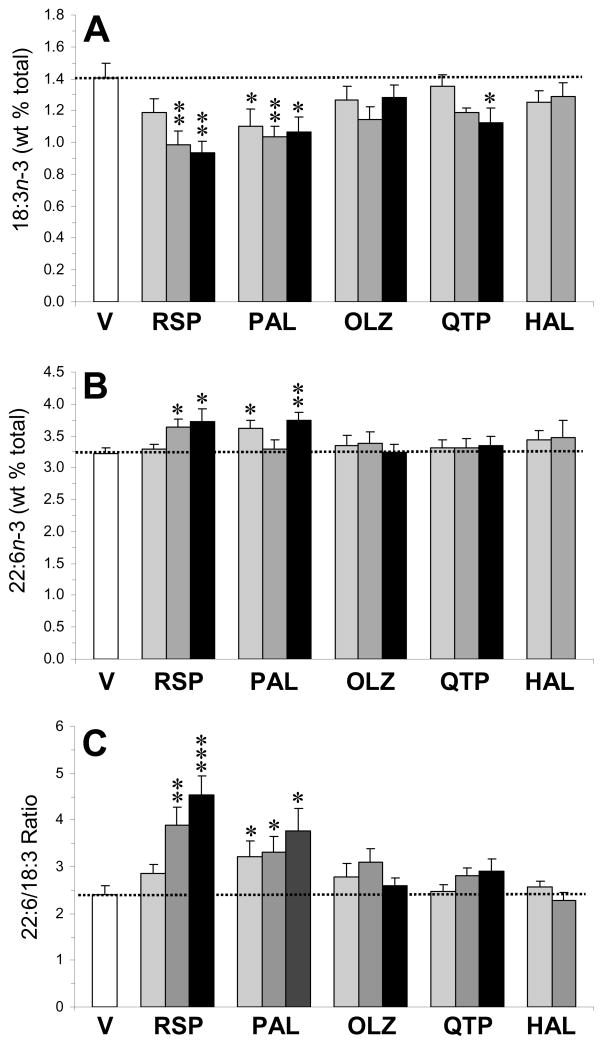
For plasma ALA (18:3n-3)(Fig. 2A), the main effect of treatment was significant for risperidone, F(3,33)=8.2, p=0.0004, paliperidone, F(3,33)=3.8, p=0.01, quetiapine, F(3,33)=2.9, p=0.04, but not for olanzapine, F(3,33)=1.6, p=0.23, or haloperidol, F(2,25)=0.9, p=0.41. For the plasma DHA (22:6n-3) composition (Fig. 2B), the main effect of treatment was significant for risperidone, F(3,33)=6.6, p=0.001, and paliperidone, F(3,33)=4.0, p=0.01, but not for olanzapine, F(3,33)=0.3, p=0.79, quetiapine, F(3,33)=0.2, p=0.88, or haloperidol, F(2,25)=0.5, p=0.58. For the plasma 22:6/18:3 ratio, an index of ALA→DHA biosynthesis (Fig. 2C), the main effect of treatment was significant for risperidone, F(3,33)=11.1, p≤0.0001, and paliperidone, F(3,33)=3.2, p=0.04, but not for olanzapine, F(3,33)=1.6, p=0.23, quetiapine, F(3,33)=1.7, p=0.19, or haloperidol, F(2,25)=0.5, p=0.63. For the plasma 20:5/18:3 ratio, an index of ALA→EPA biosynthesis, the main effect of treatment was significant for risperidone, F(3,33)=6.7, p=0.001, and paliperidone, F(3,33)=9.5, p=0.0002, but not for olanzapine, F(3,33)=1.5, p=0.23, quetiapine, F(3,33)=0.75, p=0.53, or haloperidol, F(2,25)=0.2, p=0.79 (Supplemental Fig. 1A).
Fig. 2.
Effects of chronic treatment with drug vehicle (V)(n=10), risperidone (RSP)(1.5, 3, 6 mg/kg/d), paliperidone (PAL)(1.5, 3, 6 mg/kg/d), olanzapine (OLZ)(2.5, 5, 10 mg/kg/d), quetiapine (QTP)(5, 10, 20 mg/kg/d), or haloperidol (HAL)(1, 3 mg/kg/d)(n=8/drug dose) on plasma 18:3n-3 (ALA) composition (A), plasma DHA (22:6n-3) composition (B), and the plasma 22:6/18:3 ratio (an index of ALA→DHA biosynthesis)(C). Values are group mean ± S.E.M. *p≤0.05, **p≤0.01, ***p≤0.0001 vs. Vehicle.
3.3. Plasma indices of n-6 PUFA biosynthesis
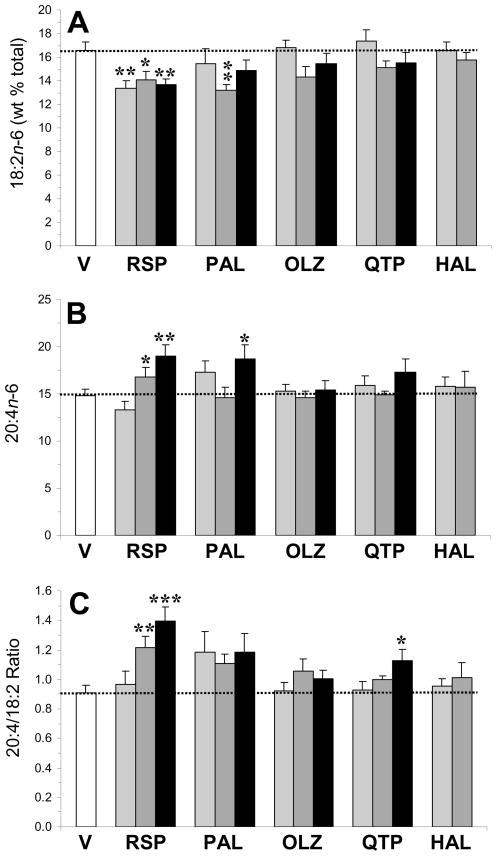
For plasma LA (18:2n-6) composition (Fig. 3A), the main effect of treatment was significant for risperidone, F(3,33)=3.5, p=0.03, and paliperidone, F(3,33)=2.8, p=0.04, but not for olanzapine, F(3,33)=2.1, p=0.12, quetiapine, F(3,33)=1.6, p=0.2, or haloperidol, F(2,25)=0.4, p=0.7. For plasma AA (20:4n-6) composition (Fig. 3B), the main effect of treatment was significant for risperidone, F(3,33)=6.7, p=0.001, and paliperidone, F(3,33)=3.2, p=0.03, but not for olanzapine, F(3,33)=0.2, p=0.87, quetiapine, F(3,33)=1.8, p=0.17, or haloperidol, F(2,25)=0.3, p=0.71. For the plasma 20:4/18:2 ratio, an index of LA→AA biosynthesis (Fig. 3C), the main effect of treatment was significant for risperidone, F(3,33)=8.3, p=0.0003, and quetiapine, F(3,33)=2.9, p=0.048, but not for paliperidone, F(3,33)=1.9, p=0.14, olanzapine, F(3,33)=1.2, p=0.34, or haloperidol, F(2,25)=0.6, p=0.53. For the plasma 20:3/18:2 ratio, an index of LA→homo-γ-LA biosynthesis, the main effect of treatment was significant for risperidone, F(3,33)=2.9, p=0.04, paliperidone, F(3,33)=3.4, p=0.03, and olanzapine, F(3,33)=4.2, p=0.01, but not for quetiapine, F(3,33)=0.6, p=0.62, or haloperidol, F(2,25)=0.6, p=0.56 (Supplemental Fig. 1B).
Fig. 3.
Effects of chronic treatment with drug vehicle (V)(n=10), risperidone (RSP)(1.5, 3, 6 mg/kg/d), paliperidone (PAL)(1.5, 3, 6 mg/kg/d), olanzapine (OLZ)(2.5, 5, 10 mg/kg/d), quetiapine (QTP)(5, 10, 20 mg/kg/d), or haloperidol (HAL)(1, 3 mg/kg/d)(n=8/drug dose) on the plasma 18:2n-6 (LA) composition (A), plasma AA (20:4n-6) composition (B), and the 20:4/18:2 ratio (an index of LA→AA biosynthesis)(C). Values are group mean ± S.E.M. *p≤0.05, **p≤0.01, ***p≤0.0001 vs. Vehicle.
3.4. Liver desaturase and elongase mRNA expression
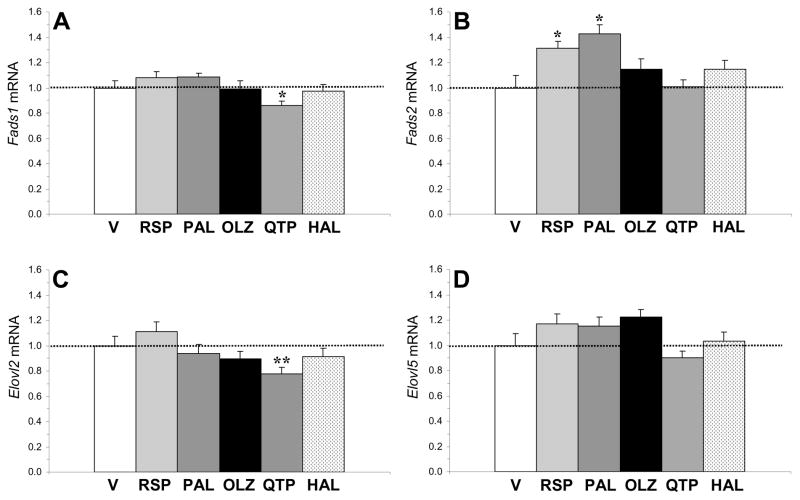
Liver Fads1, Fads2, Elovl2, and Elovl5 mRNA expression were determined in controls and groups receiving the middle dose of atypical antipsychotic (risperidone: 3 mg/kg; paliperidone: 3 mg/kg; olanzapine: 5 mg/kg; quetiapine: 10 mg/kg) and low dose of haloperidol (1 mg/kg)(n=48). For Fads1 mRNA expression, there was a significant main effect of treatment, F(5,47)=2.6, p=0.04, and rats treated with quetiapine exhibited lower Fads1 mRNA expression relative to controls (p=0.02)(Fig. 4A). For Fads2 mRNA expression, there was a significant main effect of treatment, F(5,47)=4.1, p=0.004, and rats treated with risperidone (p=0.04) and paliperidone (p=0.02) exhibited greater Fads2 mRNA expression relative to controls (Fig. 4B). For Elovl2 mRNA expression, there was a significant main effect of treatment, F(5,47)=2.6, p=0.039, and rats treated with quetiapine exhibited lower Elovl2 mRNA expression relative to controls (p=0.009)(Fig. 4C). For Elovl5 mRNA expression, the main effect of treatment was not significant, F(5,47)=1.1, p=0.39 (Fig. 4D).
Fig. 4.
Effects of chronic treatment with drug vehicle (V)(n=8), risperidone (RSP)(3 mg/kg/d), paliperidone (PAL)(3 mg/kg/d), olanzapine (OLZ)(5 mg/kg/d), quetiapine (QTP)(10 mg/kg/d), or haloperidol (HAL)(1 mg/kg/d)(n=8/drug) on liver Fads1 (A), Fads2 (B), Elovl2 (C), and Elovl5 (D) mRNA expression. Values are group mean ± S.E.M. *p≤0.05, **p≤0.01 vs. Vehicle.
3.5. Correlations with liver mRNA expression
Among all rats for which liver gene expression data were collected (n=48), the plasma 22:6/18:3 (n-3) ratio was positively correlated with Fads2 (r = +0.37, p=0.01) and Elovl2 (r = +0.38, p=0.008), but not Fads1 (r = −0.05, p=0.73) or Elovl5 (r = +0.19, p=0.39). The plasma 20:5/18:3 (n-3) ratio was positively correlated with Fads1 (r = +0.29, p=0.02) and Elovl2 (r = +0.35, p=0.009), and was not significantly correlated with Fads2 (r = +0.16, p=0.28) or Elovl5 (r = −0.13, p=0.55). The plasma 20:4/18:2 (n-6) ratio was positively correlated with Fads1 (r = +0.32, p=0.03), but not Fads2 (r = +0.16, p=0.29), Elovl2 (r = +0.25, p=0.07), or Elovl5 (r = +0.22, p=0.22). The plasma 20:3/18:2 (n-6) ratio was positively correlated with Elovl2 (r = +0.34, p=0.02), but not Fads2 (r = +0.02, p=0.89), Fads1 (r = +0.09, p=0.54), or Elovl5 (r = +0.09, p=0.54).
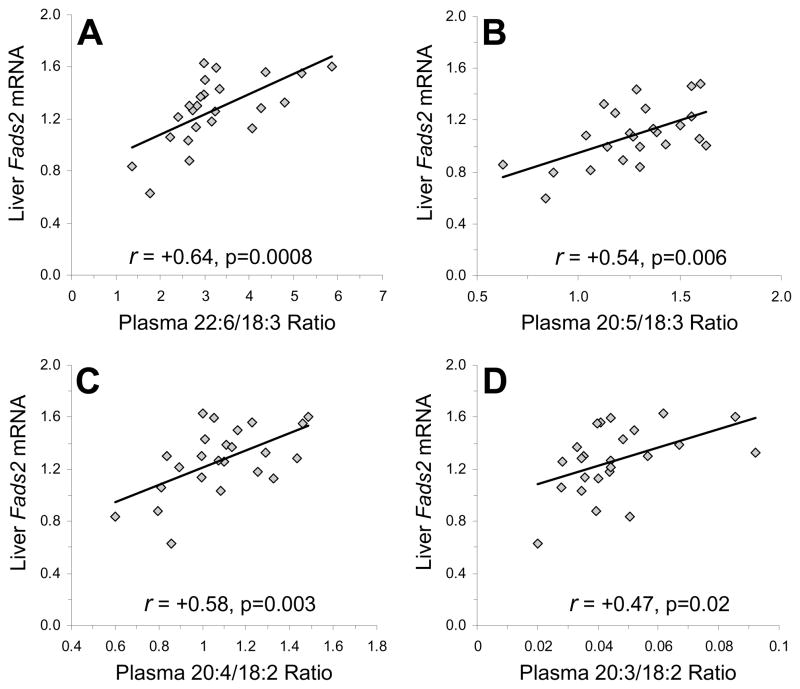
In view of the finding that risperidone and paliperidone uniquely up-regulated liver Fads2 mRNA expression, we performed a sub-analysis restricted to controls and risperidone- (3 mg/kg) and paliperidone-treated (3 mg/kg) rats (n=24). Fads2 expression was positively correlated with the plasma 22:6/18:3 (r = +0.64, p=0.0008), 20:5/18:3 (r = +0.54, p=0.006), and 20:4/18:2 (r = +0.58, p=0.003) and 20:3/18:2 ratio (r = +0.47, p=0.02), ratios (Fig. 5). In contrast, liver Fads1 expression was not significantly correlated with plasma 22:6/18:3 (r = +0.39, p=0.06), 20:5/18:3 (r = +0.27, p=0.29), 20:2/18:2 (r = +0.30, p=0.15), or 20:4/18:2 (r = +0.34, p=0.10) ratios. Neither Elovl2 nor Elovl5 mRNA expression were correlated with plasma 22:6/18:3, 20:5/18:3 or 20:4/18:2 ratios, and Elovl2 was positively correlated with the plasma 20:3/18:2 ratio (r = +0.63, p=0.001).
Fig. 5.
Relationship between liver Fads2 mRNA expression and the plasma 22:6/18:3 (A) and 20:5/18:3 (B) ratios (indices of n-3 fatty acid biosynthesis), and the plasma 20:4/18:2 (C) and 20:3/18:2 (D) ratios (indices of n-6 fatty acid biosynthesis) in rats treated with drug vehicle, RSP (3 mg/kg/d), or PAL (3 mg/kg/d)(n=24). Pearson correlation coefficients and associated p-values (two-tailed) are presented.
3.6. Membrane PUFA composition
3.6.1. Erythrocytes
For DHA composition (Fig. 6A), the main effect of treatment was significant for risperidone, F(3,33)=3.7, p=0.02, paliperidone, F(3,33)=4.3, p=0.014, and olanzapine, F(3,33)=3.1, p=0.041, quetiapine, F(3,33)=3.0, p=0.044, and haloperidol, F(2,25)=3.6, p=0.046. For EPA composition, the main effect of treatment was significant for risperidone, F(3,33)=6.7, p=0.001, paliperidone, F(3,33)=73.0, p=0.04, and haloperidol, F(2,25)=11.6, p=0.0004, but not for olanzapine, F(3,33)=0.9, p=0.45, or quetiapine, F(3,33)=1.3, p=0.29 (Supplemental Fig. 2A). For AA composition (Fig. 6B), the main effect of treatment was significant for risperidone, F(3,33)=4.8, p=0.008, paliperidone, F(3,33)=7.9, p=0.0005, olanzapine, F(3,33)=6.2, p=0.002, and haloperidol, F(2,25)=4.4, p=0.03, but not for quetiapine, F(3,33)=2.0, p=0.13. Among all rats (n=122), the plasma 20:5/18:3 ratio was positively correlated with erythrocyte EPA (20:5n-3) composition (r = +0.40, p≤0.0001)(Supplemental Fig. 3A), but not erythrocyte DHA composition (r = +0.10, p=0.26). The plasma 20:4/18:2 ratio was not correlated with erythrocyte arachidonic acid (20:4n-6) composition (r = +0.15, p=0.10)(Supplemental Fig. 3C).
Fig. 6.
Effects of chronic treatment with drug vehicle (V)(n=10), risperidone (RSP)(1.5, 3, 6 mg/kg/d), paliperidone (PAL)(1.5, 3, 6 mg/kg/d), olanzapine (OLZ)(2.5, 5, 10 mg/kg/d), quetiapine (QTP)(5, 10, 20 mg/kg/d), or haloperidol (HAL)(1, 3 mg/kg/d)(n=8/drug dose) on erythrocyte DHA (22:6n-3)(A) and AA (20:4n-6)(B) compositions, heart DHA (22:6n-3)(C) and AA (20:4n-6)(D) compositions, and frontal cortex DHA (22:6n-3)(E) and AA (20:4n-6)(F) compositions. Values are group mean ± S.E.M. *p≤0.05, **p≤0.01, ***p≤0.0001 vs. Vehicle.
3.6.2. Heart
For DHA composition (Fig. 6C), the main effect of treatment was significant for risperidone, F(3,33)=5.4, p=0.004, paliperidone, F(3,33)=5.8, p=0.003, and olanzapine, F(3,33)=5.0, p=0.006, but not for quetiapine, F(3,33)=1.2, p=0.32, or haloperidol, F(2,25)=2.3, p=0.12. For EPA composition, the main effect of treatment was significant for paliperidone, F(3,33)=3.0, p=0.04, and haloperidol, F(2,25)=3.9, p=0.03, but not for risperidone, F(3,33)=1.4, p=0.26, olanzapine, F(3,33)=0.7, p=0.55, or quetiapine, F(3,33)=1.2, p=0.31 (Supplemental Fig. 2B). For AA composition (Fig. 6D), the main effect of treatment was significant for risperidone, F(3,33)=3.0, p=0.047, paliperidone, F(3,33)=3.9, p=0.017, and olanzapine, F(3,33)=3.3, p=0.03, but not for quetiapine, F(3,33)=0.3, p=0.79, or haloperidol, F(2,25)=3.2, p=0.06. The plasma 20:5/18:3 ratio was positively correlated with heart DHA (22:5n-6) composition (r = +0.32, p=0.0004)(Supplemental Fig. 3B), and the plasma 20:4/18:2 ratio was positively correlated with heart arachidonic acid (20:4n-6) composition (r = +0.41, p≤0.0001)(Supplemental Fig. 3D).
3.6.3. Frontal cortex
For DHA composition (Fig. 6E), the main effect of treatment was not significant for risperidone, F(3,33)=0.5, p=0.68, paliperidone, F(3,33)=1.0, p=0.41, olanzapine, F(3,33)=0.5, p=0.66, quetiapine, F(3,33)=1.8, p=0.17, or haloperidol, F(2,25)=0.9, p=0.39. For AA composition (Fig. 6F), the main effect of treatment was not significant for risperidone, F(3,33)=1.7, p=0.18, paliperidone, F(3,33)=1.1, p=0.38, olanzapine, F(3,33)=0.9, p=0.96, quetiapine, F(3,33)=0.3, p=0.83, or haloperidol, F(2,25)=0.01, p=0.99.
4. Discussion
The main finding of this study is that chronic treatment with either risperidone or its primary metabolite 9-OH-risperidone (paliperidone) up-regulate expression of liver Fads2 mRNA (delta-6 desaturase), the rate-limiting step in PUFA biosynthesis, and robustly increased plasma indices of n-3 (22:6/18:3 & 20:5/18:3 ratios) and n-6 (20:4/18:2 & 20:3/18:2 ratios) fatty acid biosynthesis. Among risperidone- and paliperidone-treated rats, plasma indices of both n-3 and n-6 fatty acid biosynthesis were positively correlated with liver Fads2 mRNA, but not Fads1, Elovl2, or Elovl5 mRNA expression. However, chronic treatment with the atypical antipsychotics olanzapine and quetiapine, and the typical antipsychotic haloperidol, did not significantly up-regulate liver Fads2 mRNA expression or plasma indices of n-3 fatty acid biosynthesis. Nevertheless, among all rats plasma indices of n-3 and n-6 fatty acid biosynthesis were positively correlated with liver Fads2 and/or Fads1 mRNA expression, and all antipsychotic medications increased erythrocyte DHA and/or AA composition at specific doses. Risperidone, paliperidone, and olanzapine increased heart DHA and AA compositions, and none of the antipsychotics significantly altered frontal cortex DHA or AA composition. These data demonstrate that augmentation of PUFA biosynthesis is not common to all antipsychotic medications, and that risperidone and paliperidone uniquely increase delta-6 desaturase (Fads2) mRNA expression and most robustly increase PUFA biosynthesis and peripheral membrane composition.
This study has four notable limitations. First, rats treated with higher doses of olanzapine and quetiapine, and both doses of haloperidol, exhibited significant reductions in fluid intake which may have reduced drug intake. However, because drug concentrations were adjusted every 3 days to mean daily fluid intake and body weight (ml/kg/day), these reductions would not substantially alter daily drug intake. Nevertheless, in the absence of plasma drug concentration data it remains possible that greater changes in PUFA measures may have been observed using a different mode of administration. Second, this study examined one duration of drug exposure (40 d), and shorter or longer treatment durations may have yielded different results. However, the 40 d treatment duration was based in part on our prior finding that a similar duration (30 d) of risperidone treatment increased indices of PUFA biosynthesis (McNamara et al., 2009b). Third, only male rats were employed, precluding evaluation of gender effects. However, male rats were selected to obviate potential interactions with ovarian hormones previously found to influence primary outcome measures (Childs et al., 2010; McNamara et al., 2009a). Fourth, we did not directly evaluate enzyme activity, and used plasma product/precursor ratios as an estimate. However, the pattern of changes in plasma fatty acids observed in risperidone and paliperidone rats, reductions in short-chain precursors and reciprocal elevations in long-chain products, are consistent with elevated delta-6 desaturase activity (Guillou et al., 2010; Harmon et al., 2003; Obukowicz et al., 1998; Stoffel et al., 2008).
Consistent with our prior study (McNamara et al., 2009b), the present study also found that chronic treatment with risperidone (3 mg/kg/d) significantly increased erythrocyte long-chain n-3 fatty acid composition. In our earlier study, we found that rat plasma concentrations of 9-OH-risperidone (paliperidone), the principle metabolite of risperidone, following chronic risperidone treatment was approximately 4-fold greater than risperidone concentrations (McNamara et al., 2009b). In the present study, we further demonstrate that chronic treatment with paliperidone alone is sufficient to increase erythrocyte DHA composition, and that both risperidone and paliperidone up-regulate liver delta6-desaturase expression and indices of enzyme activity. In our prior study (McNamara et al., 2009b), we also found that chronic risperidone treatment produced a small (7%) but statistically significant increase in frontal cortex DHA composition (McNamara et al., 2009b). In the present study, chronic treatment with risperidone, or other antipsychotics, did not significantly altered frontal cortex DHA or AA composition. The reason for this discrepancy may be related to differences in the presence of long-chain fatty acids in the diets used during perinatal (E0-P60) development (TD.04285 diet vs. rodent chow in the present study). Nevertheless, this finding is consistent with prior rat studies finding that chronic treatment with atypical antipsychotics do not alter whole brain DHA or AA composition (Levant et al., 2006; Parikh et al., 2003). It may be relevant that blockade of phospholipase A2 (PLA2)-coupled serotonin 5-HT2A/C (Qu et al., 2003) and dopamine D2 (Myers et al., 2001) receptors, which are blocked by atypical antipsychotic medications, down-regulate PUFA turnover in rat brain.
A prior in vitro study found that exposure to different antipsychotic medications up-regulate FADS1 and FADS2 mRNA expression in human cell lines (Polymeropoulos et al., 2009). In the present study, we did not observe a uniform up-regulation of Fads1 or Fads2 mRNA expression in rat liver following treatment with different antipsychotic medications. Indeed, only risperidone and paliperidone significantly up-regulated Fads2 mRNA expression, and no antipsychotic up-regulated Fads1 mRNA expression. These different results may be due in part to multiple physiological factors that regulate Fads1 and Fads2 mRNA expression and activity, including insulin/glucose (Brenner, 2003), gonadal hormones (Childs et al., 2010; McNamara et al., 2009a), and long-chain PUFAs (Igarashi et al., 2007), not represented in vitro. Additional studies will be required to determine whether these physiological factors mediate or mitigate antipsychotic effects on Fads2 mRNA expression in vivo.
Despite differential effects of antipsychotics on indices of PUFA biosynthesis, all antipsychotic medications increased erythrocyte DHA and/or AA composition at certain doses. Importantly, erythrocyte membrane AA and DHA composition is regulated by not only liver biosynthesis but also by circulating PLA2 activity and lipid peroxidation. Regardless of the mechanism, the present data suggest that increasing erythrocyte PUFA composition is common to both typical and atypical antipsychotic medications. It is notable therefore that we previously found that chronic treatment with the antidepressant fluoxetine did not significantly alter erythrocyte DHA or AA composition (McNamara et al., 2010b), and chronic treatment with the mood-stabilizer lithium increased erythrocyte AA, but not DHA, composition (McNamara et al., 2008). Together, these data suggest that increasing both erythrocyte DHA and AA composition may be a mechanism specific to antipsychotic medications. These data may take on additional significance in view of a prior prospective longitudinal study finding that chronic exposure to risperidone or olanzapine also increased erythrocyte DHA and AA composition in medication-naïve first-episode psychotic patients (Evans et al., 2003).
Antipsychotic medications may have adverse effects on cardiac function, as evidenced by increased heart rate variability and QTc prolongation, which may increase risk for cardiac arrhythmias (Czekalla et al., 2001; Silke et al., 2002). Prior preclinical evidence indicates that n-3 fatty acids are protective against cardiac arrhythmias (Billman et al., 1999; Ninio et al., 2005), and clinical studies have found that greater n-3 fatty acid intake is associated with reduced rates of sudden cardiac mortality (Harris, 2008). Additionally, we found that erythrocyte and heart DHA compositions were positively correlated, a finding consistent with a prior human cardiac biopsy study (Harris et al., 2004). Importantly, cardiac biopsy studies have found that low heart AA and DHA composition is associated with increased mortality in patients with a history of coronary heart disease (Chattipakorn et al., 2009). In the present study, we found that risperidone and paliperidone robustly increased heart DHA and AA compositions compared with olanzapine, quetiapine, and haloperidol. The effect may represent one mechanism accounting for the reduced relative risk of cardiovascular disease in patients treated with risperidone compared with other antipsychotic medications (Daumit et al., 2008). It may also be relevant that adjunctive treatment with long-chain n-3 fatty acids reduce elevated triglyceride levels, an independent risk factor for coronary heart disease, in schizophrenic patients treated with clozapine (Caniato et al., 2006).
In conclusion, the present preclinical data demonstrate that chronic treatment with risperidone or it principle metabolite paliperidone uniquely and preferentially up-regulate liver Fads2 mRNA expression and associated plasma indices of n-3 and n-6 fatty acid biosynthesis. These findings confirm and extend our previous report (McNamara et al., 2009b) by demonstrating that the mechanism mediating augmentation of n-3 fatty acid biosynthesis by risperidone involves up-regulation of liver Fads2 mRNA expression. Although the clinical relevance of this mechanism remains to be determined, it is notable that adjunctive treatment with long-chain n-3 fatty acids were found to accelerate treatment response and improved tolerability in first-episode psychotic patients treated with atypical antipsychotic medications (Berger et al., 2007).
Supplementary Material
Acknowledgments
This work was supported in part by an investigator-initiated research grant from Ortho-McNeil Janssen Scientific Affairs LLC (R076477SCH0004) and NIH grants MH073704 (to R.K.M.), DK59630 (to P.T.), and DA017399 (to J.W.L.). The authors thank Ortho-McNeil Janssen Scientific Affairs for providing the risperidone and paliperidone, AstraZeneca Pharmaceuticals for providing the quetiapine, and Eli-Lilly and Company for providing the olanzapine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson C, Hamer RM, Lawler CP, Mailman RB, Lieberman JA. Striatal volume changes in the rat following long-term administration of typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2002;27:143–151. doi: 10.1016/S0893-133X(02)00287-7. [DOI] [PubMed] [Google Scholar]
- Berger GE, Proffitt TM, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry. 2007;68:1867–1875. doi: 10.4088/jcp.v68n1206. [DOI] [PubMed] [Google Scholar]
- Betz A, Ishiwari K, Wisniecki A, Huyn N, Salamone JD. Quetiapine (Seroquel) shows a pattern of behavioral effects similar to the atypical antipsychotics clozapine and olanzapine: studies with tremulous jaw movements in rats. Psychopharmacology (Berl) 2005;179:383–392. doi: 10.1007/s00213-004-2046-9. [DOI] [PubMed] [Google Scholar]
- Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- Brenner RR. Hormonal modulation of delta6 and delta5 desaturases: case of diabetes. Prostaglandins Leukot Essent Fatty Acids. 2003;68:151–162. doi: 10.1016/s0952-3278(02)00265-x. [DOI] [PubMed] [Google Scholar]
- Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Caniato RN, Alvarenga ME, Garcia-Alcaraz MA. Effect of omega-3 fatty acids on the lipid profile of patients taking clozapine. Aust N Z J Psychiatry. 2006;40:691–697. doi: 10.1080/j.1440-1614.2006.01869.x. [DOI] [PubMed] [Google Scholar]
- Chattipakorn N, Settakorn J, Petsophonsakul P, Suwannahoi P, Mahakranukrauh P, Srichairatanakool S, Chattipakorn SC. Cardiac mortality is associated with low levels of omega-3 and omega-6 fatty acids in the heart of cadavers with a history of coronary heart disease. Nutr Res. 2009;29:696–704. doi: 10.1016/j.nutres.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. The polyunsaturated fatty acid composition of hepatic and plasma lipids differ by both sex and dietary fat intake in rats. J Nutr. 2010;140:245–250. doi: 10.3945/jn.109.115691. [DOI] [PubMed] [Google Scholar]
- Cho HP, Nakamura MT, Clarke SD. Cloning, expression, and nutritional regulation of the mammalian delta-6 desaturase. J Biol Chem. 1999a;274:471–477. doi: 10.1074/jbc.274.1.471. [DOI] [PubMed] [Google Scholar]
- Cho HP, Nakamura M, Clarke SD. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J Biol Chem. 1999b;274:37335–37339. doi: 10.1074/jbc.274.52.37335. [DOI] [PubMed] [Google Scholar]
- Czekalla J, Kollack-Walker S, Beasley CM., Jr Cardiac safety parameters of olanzapine: comparison with other atypical and typical antipsychotics. J Clin Psychiatry. 2001;62 (Suppl 2):35–40. [PubMed] [Google Scholar]
- Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, Rosenheck R, Davis SM, Hsiao JK, Stroup TS, Lieberman JA. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105:175–187. doi: 10.1016/j.schres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fatty Acids. 2003;69:393–399. doi: 10.1016/j.plefa.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Fernø J, Raeder MB, Vik-Mo AO, Skrede S, Glambek M, Tronstad KJ, Breilid H, Løvlie R, Berge RK, Stansberg C, Steen VM. Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: a novel mechanism of action? Pharmacogenomics J. 2005;5:298–304. doi: 10.1038/sj.tpj.6500323. [DOI] [PubMed] [Google Scholar]
- Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res. 2010;49:186–199. doi: 10.1016/j.plipres.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Harmon SD, Kaduce TL, Manuel TD, Spector AA. Effect of the delta6-desaturase inhibitor SC-26196 on PUFA metabolism in human cells. Lipids. 20003;38:469–476. doi: 10.1007/s11745-003-1086-9. [DOI] [PubMed] [Google Scholar]
- Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997–2002. doi: 10.1093/ajcn/87.6.1997S. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- Innis SM. Dietary omega-3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, McEvoy J, Baillie RA, Lee D, Yao JK, Doraiswamy PM, Krishnan KR. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol Psychiatry. 2007;12:934–945. doi: 10.1038/sj.mp.4002000. [DOI] [PubMed] [Google Scholar]
- Levant B, Crane JF, Carlson SE. Sub-chronic antipsychotic drug treatment does not alter brain phospholipid fatty acid composition in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:728–732. doi: 10.1016/j.pnpbp.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Takashima N, Matsumata M, Ikegami S, Kontani M, Hara Y, Kawashima H, Owada Y, Kiso Y, Yoshikawa T, Inokuchi K, Osumi N. Arachidonic acid drives postnatal neurogenesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS One. 2009;4(4):e5085. doi: 10.1371/journal.pone.0005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt A, Stöhr H, White K, Weber BH. cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics. 2000;66:175–183. doi: 10.1006/geno.2000.6196. [DOI] [PubMed] [Google Scholar]
- Martinelli N, Girelli D, Malerba G, Guarini P, Illig T, Trabetti E, Sandri M, Friso S, Pizzolo F, Schaeffer L, Heinrich J, Pignatti PF, Corrocher R, Olivieri O. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am J Clin Nutr. 2008;88:941–949. doi: 10.1093/ajcn/88.4.941. [DOI] [PubMed] [Google Scholar]
- Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Yoshikawa T, Hasty AH, Tamura Y, Osuga J, Okazaki H, Iizuka Y, Takahashi A, Sone H, Gotoda T, Ishibashi S, Yamada N. Dual regulation of mouse delta(5)- and delta(6)-desaturase gene expression by SREBP-1 and PPARalpha. J Lipid Res. 2002;43:107–114. [PubMed] [Google Scholar]
- McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostogland Leukotrienes Essential Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Ostrander M, Abplanalp W, Richtand NM, Benoit S, Clegg D. Modulation of phosphoinositide-protein kinase C signal transduction by omega-3 fatty acids: Implications for the pathophysiology and treatment of recurrent neuropsychiatric illness. Prostogland Leukotrienes Essential Fatty Acids. 2006;75:237–257. doi: 10.1016/j.plefa.2006.07.009. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Sullivan J, Richtand NM. Omega-3 fatty acid deficiency augments the development of behavioral sensitization in adult mice: Prevention by chronic lithium treatment. J Psychiatric Res. 2008;42:458–468. doi: 10.1016/j.jpsychires.2007.05.009. [DOI] [PubMed] [Google Scholar]
- McNamara RK. Modulation of polyunsaturated fatty acid biosynthesis by antipsychotic medications: Implications for the pathophysiology and treatment of schizophrenia. Clinical Lipidology. 2009;4:809–820. [Google Scholar]
- McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P. Gender differences in rat erythrocyte and brain docosahexaenoic acid composition: Role of ovarian hormones and dietary omega-3 fatty acid composition. Psychoneuroendocrinology. 2009a;34:532–539. doi: 10.1016/j.psyneuen.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Jandacek R, Rider T, Tso P. Chronic risperidone treatment preferentially increases rat erythrocyte and prefrontal cortex omega-3 fatty acid composition: Evidence for augmented biosynthesis. Schizophr Res. 2009b;107:150–157. doi: 10.1016/j.schres.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Rider T, Jandacek R, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: Relationship with central serotonin turnover. Prostogland Leukotrienes Essential Fatty Acids. 2010a;83:185–191. doi: 10.1016/j.plefa.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Rider T, Jandacek R, Tso P. Effect of chronic fluoxetine treatment on male and female rat erythrocyte and prefrontal cortex fatty acid composition. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010b;34:1317–1321. doi: 10.1016/j.pnpbp.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migler BM, Warawa EJ, Malick JB. Seroquel: behavioral effects in conventional and novel tests for atypical antipsychotic drugs. Psychopharmacology (Berl) 1993;112:299–307. doi: 10.1007/BF02244925. [DOI] [PubMed] [Google Scholar]
- Myers CS, Contreras MA, Chang MC, Rapoport SI, Appel NM. Haloperidol down-regulates phospholipase A(2) signaling in rat basal ganglia circuits. Brain Res. 2001;896:96–101. doi: 10.1016/s0006-8993(01)02014-5. [DOI] [PubMed] [Google Scholar]
- Ninio DM, Murphy KJ, Howe PR, Saint DA. Dietary fish oil protects against stretch-induced vulnerability to atrial fibrillation in a rabbit model. J Cardiovasc Electrophysiol. 2005;16:1189–1194. doi: 10.1111/j.1540-8167.2005.50007.x. [DOI] [PubMed] [Google Scholar]
- Obukowicz MG, Welsch DJ, Salsgiver WJ, Martin-Berger CL, Chinn KS, Duffin KL, Raz A, Needleman P. Novel, selective delta6 or delta5 fatty acid desaturase inhibitors as antiinflammatory agents in mice. J Pharmacol Exp Ther. 1998;287:157–166. [PubMed] [Google Scholar]
- Parikh V, Khan MM, Mahadik SP. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2003;37:43–51. doi: 10.1016/s0022-3956(02)00048-1. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Licamele L, Volpi S, Mack K, Mitkus SN, Carstea ED, Getoor L, Thompson A, Lavedan C. Common effect of antipsychotics on the biosynthesis and regulation of fatty acids and cholesterol supports a key role of lipid homeostasis in schizophrenia. Schizophr Res. 2009;108:134–142. doi: 10.1016/j.schres.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chang L, Klaff J, Balbo A, Rapoport SI. Imaging brain phospholipase A2 activation in awake rats in response to the 5-HT2A/2C agonist (+/−)2,5-dimethoxy-4-iodophenyl-2-aminopropane (DOI) Neuropsychopharmacology. 2003;28:244–252. doi: 10.1038/sj.npp.1300022. [DOI] [PubMed] [Google Scholar]
- Raeder MB, Fernø J, Vik-Mo AO, Steen VM. SREBP activation by antipsychotic- and antidepressant-drugs in cultured human liver cells: relevance for metabolic side-effects? Mol Cell Biochem. 2006;289:167–173. doi: 10.1007/s11010-006-9160-4. [DOI] [PubMed] [Google Scholar]
- Ryan AS, Astwood JD, Gautier S, Kuratko CN, Nelson EB, Salem N., Jr Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids. 2010;82:305–314. doi: 10.1016/j.plefa.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- Schaeffer L, Gohlke H, Müller M, Heid IM, Palmer LJ, Kompauer I, Demmelmair H, Illig T, Koletzko B, Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- Silke B, Campbell C, King DJ. The potential cardiotoxicity of antipsychotic drugs as assessed by heart rate variability. J Psychopharmacol. 2002;16:355–360. doi: 10.1177/026988110201600410. [DOI] [PubMed] [Google Scholar]
- Stoffel W, Holz B, Jenke B, Binczek E, Günter RH, Kiss C, Karakesisoglou I, Thevis M, Weber AA, Arnhold S, Addicks K. Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. EMBO J. 2008;27:2281–2292. doi: 10.1038/emboj.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on serotonin 1A, 2A and 2C receptors in rat forebrain regions. Psychopharmacology (Berl) 2002;161:263–270. doi: 10.1007/s00213-002-1016-3. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Mahadik SP, Warsi S, Davis LW, Waller JL. Chronic exposure to typical or atypical antipsychotics in rodents: temporal effects on central alpha7 nicotinic acetylcholine receptors. Neuroscience. 2005;136:519–529. doi: 10.1016/j.neuroscience.2005.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.