Positron emission tomography (PET) imaging using the tracer [11C]DASB is a sensitive and noninvasive technique for measuring brain serotonin transporter (5-HTT) levels in vivo1 . Previously, Cannon et al. found that 5-HTT levels were increased in thalamus, striatum, insular and cingulate cortices in unmedicated, depressed subjects with major depressive disorder2 or bipolar disorder3. To explore this potential biomarker for mood disorders, we undertook a proof-of-principle genome-wide association study.
5-HTT levels, measured as [11C]DASB binding potential (BPND), were assessed in six brain regions-of-interest (ROIs). Healthy (n=22) and unmedicated participants diagnosed with bipolar disorder (n=16) or major depressive disorder (n=17), aged 18 to 48 years (Mean ± SD, 34 ± 9 yr) underwent PET scanning with [11C]DASB. Subject ascertainment, PET scanning, and analysis were described previously2-3. DNA was extracted from peripheral blood and genotyped on the Illumina Human-1 SNP array. Genotypes were called with BeadStudio v3.0. Data were filtered for minor allele frequencies < 1%, missing genotype rates > 8%, Hardy-Weinberg Equilibrium p-values <0.05, gender mismatch, and hidden relatedness. A total of 93,427 SNPs passed all quality control filters for all of the ROIs tested. Association analysis was performed by linear regression in PLINK4 (v1.02), with 3 covariates to control for ancestry. Genomic Control lambda values were all <1.05, indicating good control of confounding factors
Overall, 5 SNPs representing 5 different genes met our FDR<0.1 criterion for being declared of interest: rs6741892 in GALM, rs390704 near FRY, rs7161217 near TTLL5, rs583241 near CPLX4, and rs7095106 near CASC2. The strongest association was between thalamic [11C]DASB-BPND and a coding SNP in the gene GALM (rs6741892, p=4.67×10−8; FDR=0.004). This result meets the threshold of genome-wide significance under typical frequentist assumptions, but not if corrected for 6 ROIs. rs6741892 accounted for about 50% of the variance in [11C]DASB-BPND in thalamus. Carriers of the “TT” genotype showed the highest [11C]DASB-BPND.
Since the T-allele frequency differed by self-reported ancestry (10/13 in African-Americans, 3/13 in whites, and 0/7 in Hispanics), we also performed the analysis within each ancestry group, then combined the p-values by meta-analysis (META 5.3, http://userpage.fuberlin.de/~health/meta_e.htm), with similar results. We further confirmed this finding in a voxel-based analysis (Fig 1).
Figure 1.
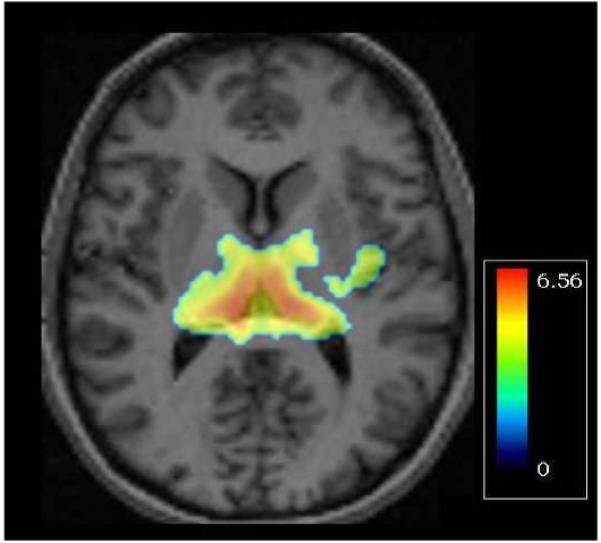
Map of t-values from voxel-wise analysis of rs6741892, overlaid on a sample axial MRI slice at the level of the medial thalamus (z=6 mm). Bilaterally, T-allele carriers (n=13) have greater serotonin-transporter binding potential than AA homozygotes (n=42). The color bar indicates the range of t-values displayed (max t = 6.56, df=53, p=2.3 × 10−8).
Nominally-significant associations were also observed between rs6741892 and [11C]DASB-BPND in dorsal cingulate cortex (DCC; p=4.03×10−2) and insula (p=1.14×10−3). No SNPs near the gene encoding the 5-HTT (SLC6A4) showed significant association in this sample, consistent with one prior study5, but we may have missed a true association due to lack of power. Rs6741892 was also individually genotyped using a modification of the 5′ nuclease (Taqman) assay, with similar results.
Consistent with our prior findings2-3, we found a significant main effect of diagnosis on [11C]DASB-BPND measured in thalamus (F (2, 52) =7.07, p<0.002) due to significantly higher thalamic [11C]DASB-BPND in participants with mood disorders. However, the overall association results were not dependent on diagnosis: rs6741892 was also associated with [11C]DASB-BPND in healthy participants.
Supportive evidence of association with rs6741892 was obtained from an independent replication sample of 51 European-ancestry subjects (16 females aged 43±20 yr, 35 males aged 34±18 yr) ascertained in Denmark, using TaqMan. Imaging and analysis were as described6. Significant associations were observed between rs6741892 and [11C]DASB-BPND in DCC (p=3.5×10−3) and insula (p=4.9×10−3), but not in thalamus, although [11C]DASB-BPND is highly correlated across subcortical structures7. Combined analysis with META 5.3 supported association between rs6741892 and [11C]DASB-BPND in all 3 regions of interest (DCC, p=9.18×10−4; insula, p=3.25×10−5; and thalamus, p=4.6×10−6).
GALM encodes galactose mutarotase, which catalyzes the conversion of beta-D-galactose to alpha-D-galactose8, important in carbohydrate metabolism and the production of complex oligosaccharides. Galactose mutarotase might affect regional neurophysiology, leading to local increases in serotonin release and in membrane trafficking of 5-HTT9, thereby increasing [11C]DASB-BPND. Galactose mutarotase may also play a role in N-glycosylation, which is important for surface expression of 5-HTT10.
To our knowledge, this is the first genome-wide association study of brain 5-HTT. These preliminary results suggest that neuroimaging phenotypes could represent informative targets for a GWAS, even in relatively small samples. Further studies are needed to confirm these findings and determine the underlying biological mechanisms.
Acknowledgments
Funded by the NIMH Intramural Research Program.
Footnotes
The authors report no conflicts of interest.
References
- 1.Ichimiya T, Suhara T, Sudo Y, Okubo Y, Nakayama K, Nankai M, et al. Biological Psychiatry. 2002;51(9):715–722. doi: 10.1016/s0006-3223(01)01351-8. [DOI] [PubMed] [Google Scholar]
- 2.Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, et al. Biol Psychiatry. 2007;15(62):870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Cannon DM, Ichise M, Fromm SJ, Nugent AC, Rollis D, Gandhi SK, et al. Biol Psychiatry. 2006;60(3):207–217. doi: 10.1016/j.biopsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. American journal of human genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, et al. Synapse. 2003;48(4):184–188. doi: 10.1002/syn.10204. [DOI] [PubMed] [Google Scholar]
- 6.Kalbitzer J, Frokjaer VG, Erritzoe D, Svarer C, Cumming P, Nielsen FA, et al. Neuroimage. 2009;45(2):280–285. doi: 10.1016/j.neuroimage.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Erritzoe D, Holst K, Frokjaer VG, Licht CL, Kalbitzer J, Nielsen FA, et al. J Neurosci. 2010;30(9):3391–3397. doi: 10.1523/JNEUROSCI.2852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey PA. Faseb J. 1996;10(4):461–470. [PubMed] [Google Scholar]
- 9.Blakely RD, Ramamoorthy S, Schroeter S, Qian Y, Apparsundaram S, Galli A, et al. Biol Psychiatry. 1998;44(3):169–178. doi: 10.1016/s0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 10.Blakely RD, De Felice LJ, Hartzell HC. J Exp Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]


