Abstract
Methamphetamine (MA) is an abused stimulant which can result in cognitive deficits and monoamine depletions. Animal models of neurotoxic MA exposure show reductions in dopamine, serotonin, and their associated transporters. MA abuse can result in long-term attention, working memory, and executive function deficits in humans and deficits in route-based egocentric learning, novel object recognition, and novel odor preference in rodents. MA has also been shown to affect brain-derived neurotrophic factor (BDNF) in humans and rodents. This experiment examined the effects of a MA binge dosing regimen (10 mg/kg × 4 at 2 h intervals, s.c.) in Sprague-Dawley rats on BDNF, tropomyosin receptor kinase B (TrkB), and tyrosine hydroxylase (TH) mRNA expression, and plasma corticosterone. Tissues were collected 1, 7, and 24 h following the last MA dose. Expression of BDNF and TrkB mRNA was analyzed using in situ hybridization with cRNA probes. Frontal, parietal, and entorhinal cortical BDNF mRNA expression were increased by MA exposure at all time-points. Increases in BDNF mRNA message were also seen in the hippocampal CA1, prefrontal cortex (PFC), piriform cortex, and locus coeruleus but only at specific times. TrkB mRNA expression was modified in several subregions of the hippocampus as well as in PFC and striatum. TH mRNA was increased at the 1 h time-point in the substantia nigra pars compacta with no differences noted at the other times. Corticosterone levels were increased at all three time-points. The findings suggest that BDNF and its receptor may be upregulated as a compensatory mechanism after MA exposure.
Keywords: Methamphetamine, brain-derived neurotrophic factor, BDNF, tropomyosin receptor kinase B, TrkB
Methamphetamine (MA) is an addictive psychostimulant widely abused in the United States with 52% of 50 year old and 11% of 18 year old high school graduates reporting use of at least one type of amphetamine in their lifetime (Johnson et al., 2009). With the percentage of substance abuse treatment admissions for primary MA abuse more than doubling between 1995 and 2005, and long-term abuse leading to health complications, understanding the mechanism of action and developing treatments for MA users is of increasing importance (Substance Abuse and Mental Health Services Administration, 2008).
Acutely, MA heightens attention, decreases fatigue, suppresses appetite, and increases anxiety (Meredith et al., 2005). It also causes sympathetic nervous system stimulation and increases cortisol (Fehm et al., 1984). Chronic MA abuse produces memory impairments, reductions in sustained attention, deficits in executive function, and neurochemical changes (Barr et al., 2006). Autopsy and imaging studies show that chronic MA users exhibit reductions in brain dopamine (DA) content, dopamine transporter (DAT) density (Wilson et al., 1996), brain serotonin (5-HT), tyrosine hydroxylase (TH) levels (Kish et al., 2009), serotonin reuptake transporter (SERT) density (Sekine et al., 2006), and in cases of extreme abuse, reductions in striatal VMAT2 (Kitamura et al., 2007). Binge MA exposure in rodent models do not fully mimic human abuse patterns of intake but importantly they do induce similar neurochemical changes in the brain. For example, rats show reductions in brain DA and 5-HT and their transporters (DAT and SERT) following exposure to 3–4 doses of MA (Chapman et al., 2001;Fukumura et al., 1998). Decreased activity of TH is also observed within 6 h following binge doses of MA that persist for at least 30 days post-exposure (Hotchkiss et al., 1979;Hotchkiss and Gibb, 1980). Deficits in cognitive function (egocentric navigation, novel object recognition, non-spatial recognition memory, fixed-route motor learning, and novel odor preference) have also been observed following binge doses of MA in rodents, with little or no effects on spatial learning (Belcher et al., 2005;Bisagno et al., 2002;Chapman et al., 2001;Daberkow et al., 2005;Friedman et al., 1998;He et al., 2006;Herring et al., 2008b;O'Dell et al., 2010;Schroder et al., 2003).
Neurotrophins may play a role in some of the effects of MA. In rodents alterations in basal levels of brain-derived neurotrophic factor (BDNF) decrease MA-induced neurotoxicity (Dluzen et al., 2002;Dluzen, 2004). In MA-dependent human abusers, plasma BDNF levels remain elevated after 30 or more days of abstinence (Kim et al., 2005). Through binding to its high affinity receptor tropomyosin receptor kinase B (TrkB), BDNF enhances neuronal growth, maintenance, and survival, is involved in neuronal plasticity and firing rates, and influences long-term potentiation and memory consolidation (Barbacid, 1995;Merlio et al., 1992;Thoenen, 1995). BDNF also modulates DA and 5-HT levels, contributes to the survival and maintenance of DA neurons, and can alter neurotransmitter synthesis, metabolism, and release (Guillin et al., 2001;Hyman et al., 1991;Lyons et al., 1999;Yurek and Seroogy, 2001;Altar et al., 1997).
The time-dependent effects of binge doses of MA on BDNF and TrkB expression have not been examined. The present experiment investigated the effects of a binge MA dosing regimen on BDNF and TrkB mRNA in brain regions important in the cognitive deficits associated with MA exposure. Given the catecholamine changes previously demonstrated following MA treatment (Herring et al., 2008b), mRNA levels of TH were included in selected regions. Plasma levels of corticosterone were determined as an additional marker of MA effects (Herring et al., 2008b). Corticosterone is known to affect levels of neurotrophins and their receptors (Schaaf et al., 1998;Schaaf et al., 2000;Smith et al., 1995).
2. Experimental Procedures
2.1 Animals
Adult male Sprague-Dawley CD IGS rats (250–275 g) were acquired from Charles River Laboratories, Raleigh, NC. Three days after arrival, rats were implanted with subcutaneous temperature transponders (IPTT-200, Biomedic Data Systems, Seaford, DE) under isoflurane anesthesia. Animals were initially housed 2 per cage and all procedures were in compliance with the Institutional Animal Care and Use Committee.
2.2 Methamphetamine administration
(+)-Methamphetamine-HCl (expressed as the freebase, provided by the National Institute on Drug Abuse, Rockville, MD, >95% pure) was administered subcutaneously in the dorsum in four doses of 10 mg/kg with 2 h intervals between doses. Control animals received saline (SAL; 3 ml/kg). During dosing, animals were maintained in 28 × 16 × 12 cm3 cages in a separate room from the colony at an ambient temperature of 23.8 ± 1°C. Body weights were obtained prior to treatment. Body temperatures were monitored at 30 min intervals beginning with the first injection and for 8 h thereafter. Animals were cooled in a shallow water bath if transponder readings reached 40.2°C to prevent severe hyperthermia (Herring et al., 2008a).
2.3 Tissue collection
Animals were decapitated 1, 7, or 24 h after the last injections (N = 3–5/time point/group). Blood for corticosterone analysis was collected in 2% EDTA (0.05 ml/tube). Brains were removed, mounted on tissue-freezing medium (Ted Pella, Inc., Redding, CA), and frozen on dry ice.
2.4 Assessment of corticosterone
Blood was centrifuged (1399 RCF) for 25 min at 4°C, and then plasma was collected and stored at −80°C until assayed. Plasma was diluted 3:1 in assay buffer and assayed in duplicate for corticosterone with an EIA kit (Immunodiagnostic Systems Inc., Fountain Hills, AZ).
2.5 In situ hybridization
Frozen brains were serially sectioned on a cryostat (at 10 µm thickness) throughout the rostrocaudal aspects of the prefrontal cortex (PFC), striatum, hippocampus, ventral midbrain, and locus coeruleus (LC), thaw-mounted onto Superfrost Plus microslides (VWR, Batavia, IL), and stored at −20°C until hybridization. Semiadjacent sections from each area and time point were subsequently hybridized with 35S-labeled cRNA probes at a concentration of 1 × 106 cpm/50 µl for detection of BDNF and TrkB mRNAs, as well as for TH mRNA in the ventral midbrain and LC. Pretreatment, hybridization, and post-hybridization treatment of tissue sections were performed as described in detail previously (Numan, 1998;Numan et al., 2005;Seroogy and Herman, 1997). The BDNF cDNA plasmid (a gift from Christine Gall, University of California at Irvine) produced antisense RNA transcripts of 540 bases (Isackson et al., 1991). The TrkB plasmid resulted in antisense RNA transcripts of 200 bases (Dixon and McKinnon, 1994). The TH cDNA plasmid (provided by James Herman, University of Cincinnati) resulted in an antisense RNA transcript of 366 bases. The sections were incubated in hybridization solution at 60°C in a humidified chamber for 18–24 h. Following post-hybridization treatment, the slides were exposed to BioMax MR film (Kodak, Rochester, NY) for an appropriate amount of time for each region (TH = 1 day; BDNF = 10–15 days; TrkB = 8–17 days) for detection and localization of hybridization signal. The films were subsequently developed with GPX developer and fixer (Kodak).
2.6 Analysis
Semi-quantitative in situ hybridization analysis of the film autoradiographs was performed using Scion Image (NIH) software. The optical density (OD) of hybridization signal for each probe was obtained at each time point to compare densities of hybridization between MA- and SAL-treated animals. Anatomical delineations were determined according to a rat brain atlas (Paxinos and Watson, 1986). At least six measurements were taken from each region analyzed for each cRNA probe for each animal. A background control value was taken for each section (from an unlabeled region; e.g., corpus callosum) and the value was subtracted from the OD of the region examined to give the mean corrected grey level. The hybridization data were analyzed using t-tests at each time point and data for MA-treated animals are expressed as a percentage of controls. Plasma BDNF levels have been shown to be increased in humans following long-term MA exposure (Kim et al., 2005), in rodents after developmental MA exposure (Grace et al., 2008;Skelton et al., 2007), or after adult MDMA exposure (Hemmerle et al, submitted); hence increases were predicted and unidirectional t-tests were used for BDNF comparisons while two-tailed tests were used for all other comparisons. Body weight and corticosterone levels were analyzed using repeated measure analysis of variance (ANOVA, Proc Mixed, SAS 9.2, SAS Institute, Cary, NC). Body temperatures at 7 h and 24 h were analyzed similarly. Significance was set at p ≤ 0.05 and trends at p ≤ 0.10.
3. Results
3.1 Body Weight and Temperature
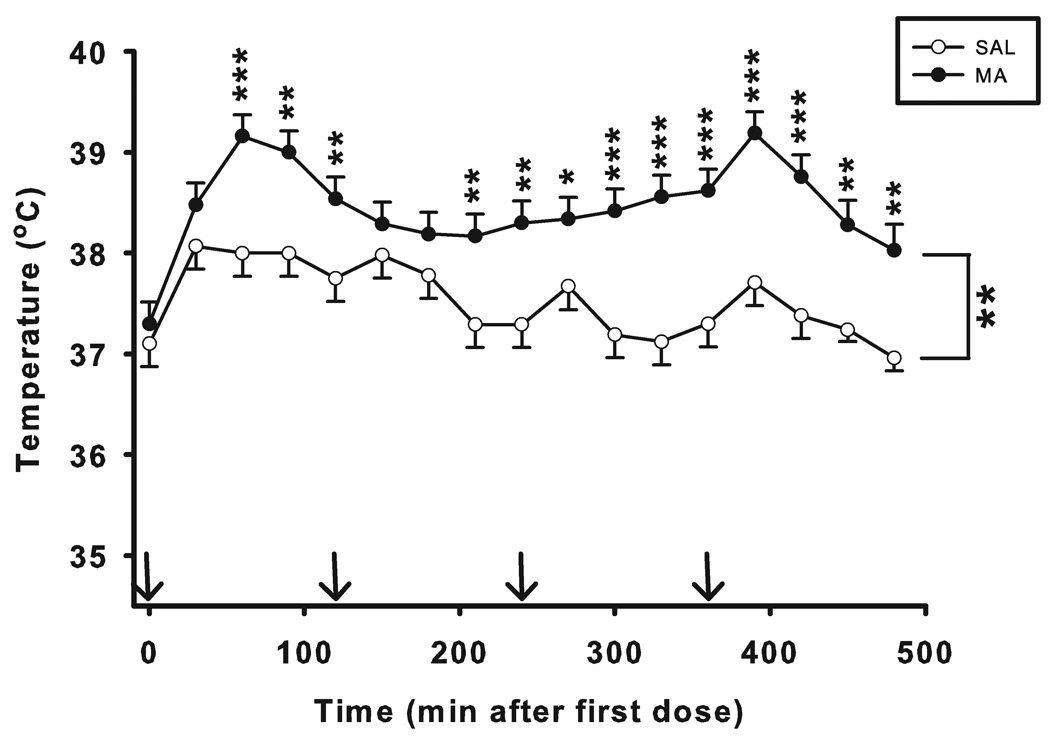
No differences in body weights were found between groups prior to treatment (SAL: 320.2 ± 2.6 g; MA: 324.1 ± 2.7 g). Two SAL animals had malfunctioning transponders and were not included in the temperature analysis. There were significant main effects of treatment (F(1, 51.5) = 29.13, p < 0.001) and time (F(16, 414) = 9.21, p < 0.001) on body temperature with MA-treated animals showing increased temperature compared with SAL controls (Fig. 1). A significant treatment × time interaction (F(16, 414) = 2.09, p < 0.01) was obtained such that animals treated with MA began showing hyperthermia 60 min following the first dose (p < 0.001) and this continued for the remainder of the monitoring period (p < 0.05 to 0.001) with the exception of the 150 and 180 min time points, where there were no significant differences in temperature (initial N = 16/MA, 14/SAL; last 2 time points N = 10/MA, 9/SAL).
Figure 1. Body temperature of animals (mean ± SEM).
No differences in initial temperatures were observed (Time = 0); MA produced significant increases in body temperature. Arrows denote injection times. Starting N was SAL = 14; MA = 16. Final N was SAL = 9; MA = 10. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
3.2 Corticosterone Levels
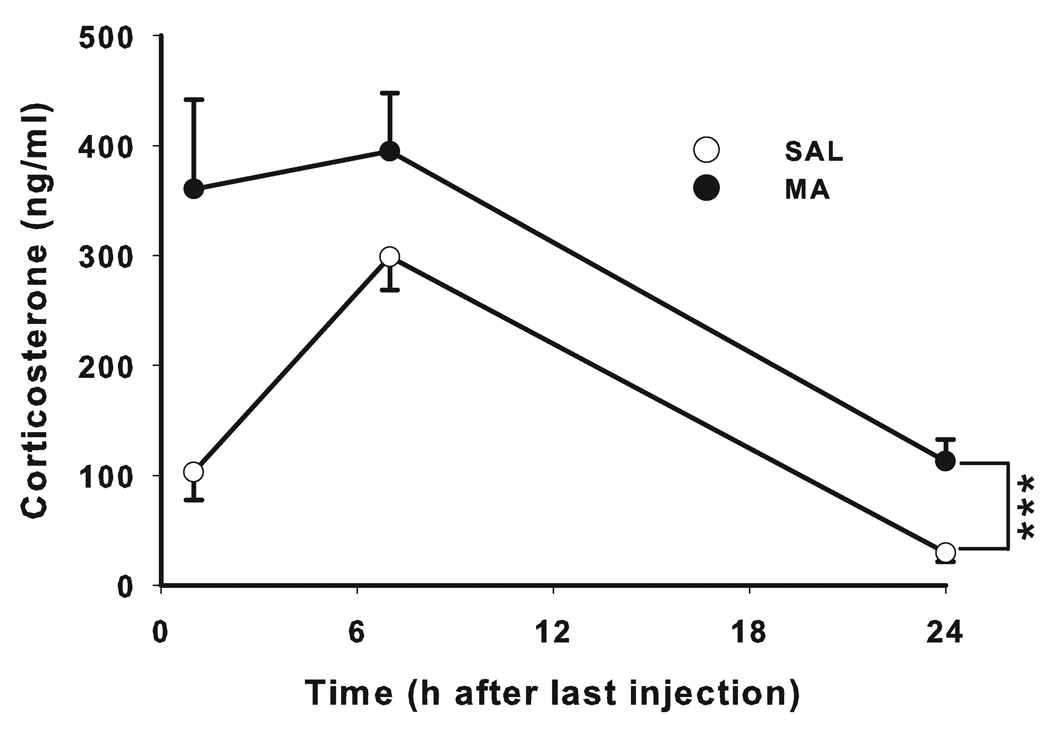
Corticosterone levels were increased in MA-treated animals across all time points compared to SAL-treated animals (F(1,23) = 17.57, p < 0.001) (Fig. 2). Time was also significant (F(2,23) = 19.75, p < 0.001), whereas only a trend was observed for the treatment × time interaction (F(2,23) = 2.58, p < 0.10).
Figure 2. Plasma corticosterone levels (mean ± SEM).
CORT levels were increased following MA exposure up to 24 h after the first dose compared with control animals. N at 1 h was SAL = 16, MA = 16; N at 7 and 24 h was SAL = 10; MA = 10. ***p ≤ 0.001.
3.3 Gene Expression
3.3.1 BDNF mRNA
Results are shown in Table 1. MA-treated animals had significantly increased BDNF mRNA expression in the entorhinal cortex (1 h: t(8) = 3.73, p < 0.01; N = 5/group; 7 h: t(7) = 2.44, p < 0.05; N = 4/MA, 5/SAL; 24 h: t(8) = 2.68, p < 0.01; N = 5/group) and the parietal cortex (1 h: t(4) = 14.42, p < 0.001; N = 3/group; 7 h: t(6) = 7.80, p < 0.001; N = 3/MA, 5/SAL; 24 h: t(8) = 3.10, p < 0.01; N = 5/group) relative to SAL-treated controls. In the anterior cingulate cortex (Acc) elevated BDNF mRNA expression in MA-treated rats was observed at 1 h, but not 7 or 24 h compared with controls (t(4) = 2.29, p < 0.05; N = 3/group).
Table 1.
BDNF mRNA expression percent change in MA-treated animals compared to controls (mean ± SEM)
| Brain Region | 1 h | 7 h | 24 h |
|---|---|---|---|
| FC | 228.9 ± 2.3 *** | 137.25 ± 14.9 * | 127.9 ± 2.1 *** |
| Acc | 126.7 ± 10.2 | 112.3 ± 7.9 | 121.2 ± 17.5 |
| PFC | 151.7 ± 7.1 *** | 112.3 ± 7.9 | 148.3 ± 10.8 * |
| Parietal Ctx | 177.1 ± 4.6 *** | 190.8 ± 12.1 *** | 116.5 ± 1.2 ** |
| Piriform Ctx | N.A.D. | 109.9 ± 7.2 | 135.8 ± 4.9 ** |
| Entorhinal Ctx | 140.5 ± 9.1 ** | 123.3 ± 6.9 * | 144.9 ± 14.6 ** |
| CA1 | 173.0 ± 14.4 * | 145.5 ± 39.0 | 125.3 ± 8.7 * |
| CA3 | 138.2 ± 14.9 | 101.5 ± 8.9 | 108.1 ± 4.7 ʈ |
| DG | 128.4 ± 16.6 | 117.9 ± 12.6 | 97.3 ± 4.0 |
| LC | 132.2 ± 9.9 ** | 120.5 ± 7.3 ʈ | 112.1 ± 13.9 |
| VTA | 99.5 ± 0.9 | 101.9 ± 1.4 | 106.4 ± 6.9 |
| SNpc | 89.7 ± 6.8 | 83.5 ± 5.4 | 103.1 ± 16.9 |
Significance denoted as difference between MA-treated groups and SAL-treated groups within each time point.
p ≤ 0.1;
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001.
N.A.D. = No Available Data
In the PFC, BDNF mRNA was increased 1 h and 24 h post MA treatment (1 h: t(4) = 6.72, p < 0.001; 24 h: t(4) = 2.46, p < 0.05; N = 3/group) compared to controls, but no change was seen at 7 h. In the frontal cortex (FC), MA treatment increased BDNF mRNA expression at all three time points (1 h: t(4) = 15.35, p < 0.001; N = 3/group; 7 h: t(6) = 2.39, p < 0.05; N = 3/MA, 5/SAL; 24 h: t(8) = 7.01, p < 0.001; N = 5/group).
Increased BDNF mRNA expression was also seen in the CA1 region of the hippocampus (1 h (t(5) = 2.16, p < 0.05; N = 4/MA, 3/SAL) and 24 h (t(6) = 2.02, p < 0.05; N = 3/MA, 5/SAL) following MA treatment but no change at 7 h. In the hippocampal CA3 region, a trend was seen at 24 h for increased BDNF mRNA in MA-treated animals compared to SAL-treated animals (t(6) = 1.57, p = 0.08, N = 3/MA, 5/SAL) with no differences at the 1 (N = 4/MA, 3/SAL) or 7 h (N = 4/MA, 5/SAL) time points. No differences were observed in BDNF mRNA in the stratum granulosum of the dentate gyrus (DG).
For BDNF mRNA in the locus coeruleus (LC), expression significantly increased 1 h post MA treatment (t(7) = 2.73, p < 0.01; N = 4/MA, 5/SAL), with a trend towards increased expression at 7 h (t(6) = 1.72, p = 0.07; N = 4/group), but no difference from SAL was observed at 24 h. Freezer malfunction caused loss of tissue for the 1 h time point for the piriform cortex. No alteration in BDNF mRNA expression in the piriform cortex was seen 7 h post MA treatment, however hybridization was significantly increased 24 h later (t(8) = 3.79, p < 0.01; N = 5/group) compared to controls.
No significant difference in BDNF mRNA was seen in the ventral tegmental area (VTA) or substantia nigra pars compacta (SNpc) following MA exposure.
3.3.2 TrkB mRNA
Results are shown in Table 2. In the parietal cortex, TrkB mRNA levels were elevated 7 h (t(6) = 2.42, p < 0.05; N = 5/group) post-treatment but were not different from controls at 24 h. A trend toward increased TrkB expression in the FC was present at 7 h (t(6) = 2.05, p = 0.09; N = 3/MA, 5/SAL) in MA-treated animals, with no difference from SAL-treated animals observed at 24 h. The 1 h data were not available from the same freezer malfunction.
Table 2.
TrkB mRNA expression percent change in MA-treated animals compared to controls (mean ± SEM)
| Brain Region | 1 h | 7 h | 24 h |
|---|---|---|---|
| FC | N.A.D. | 109.6 ± 3.8ʈ | 100.8 ± 1.3 |
| Acc | 103.1 ± 2.5 | 95.7 ± 3.7 | 103.6 ± 11.0 |
| PFC | 120.9 ± 2.1** | 107.2 ± 3.9 | 106.9 ± 4.1 |
| Parietal Ctx | N.A.D. | 111.2 ± 2.6* | 94.6 ± 3.7 |
| Piriform Ctx | N.A.D. | 104.0 ± 1.6 | 106.1 ± 3.7 |
| Entorhinal Ctx | 97.63 ± 2.5 | 89.7 ± 4.2 | 110.5 ± 4.3 |
| CA1 | 101.7 ± 2.8 | 101.7 ± 6.4 | 102.3 ± 0.7** |
| CA3 | 97.6 ± 6.3 | 103.6 ± 7.1 | 96.6 ± 1.9ʈ |
| DG | 96.1 ± 4.8 | 85.5 ± 12.2 | 105.3 ± 0.6*** |
| VTA | 98.9 ± 2.3 | 97.8 ± 3.0 | 104.8 ± 2.7 |
| SNpc | 104.7 ± 2.5 | 97.7 ± 3.0 | N.A.D. |
| Striatum | 130.2 ± 2.7* | 144.6 ± 11.9* | 104.8 ± 5.2 |
Significance denoted as difference between MA-treated groups and SAL-treated groups within each time point.
p ≤ 0.1;
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001.
N.A.D. = No Available Data
TrkB mRNA hybridization in the PFC was increased at the 1 h time point (t(4) = 6.13, p < 0.01; N = 3/group), but not at 7 or 24 h in MA-treated animals relative to controls (Fig. 10A). In the striatum, MA increased TrkB expression 1 h (t(4) = 4.02, p < 0.05; N = 3/group) and 7 h (t(4) = 2.95, p < 0.05; N = 3/group) post-treatment, but no difference was observed at 24 h.
In the CA1 and DG, TrkB expression was significantly increased in MA-treated rats at 24 h (CA1: t(6) = 3.62, p < 0.01; DG: t(6) = 5.56, p < 0.001; N = 3/MA, 5/SAL) but was not different at 1 h or 7 h. A trend at 24 h for decreased TrkB expression in the CA3 region was observed in MA-treated animals compared with SAL controls (t(6) = 2.04, p = 0.09; N = 3/MA, 5/SAL).
No alterations in TrkB mRNA expression were found in the Acc, LC, SNpc, VTA, piriform or entorhinal cortices from MA treatment.
3.3.3 TH mRNA
Results are shown in Table 3. TH mRNA expression in the SNpc was increased at 1 h in MA-treated animals compared with SAL-treated animals (t(8) = 2.36, p < 0.05; N = 5/group) with no other alterations seen. No TH expression changes were observed in MA-treated animals in the VTA or LC.
Table 3.
TH mRNA expression percent change in MA-treated animals compared to controls (mean ± SEM)
| Brain Region | 1 h | 7 h | 24 h |
|---|---|---|---|
| LC | 97.2 ± 8.1 | 85.7 ± 6.6 | 91.6 ± 5.6 |
| VTA | 98.2 ± 2.6 | 101.3 ± 1.8 | 103.4 ± 3.4 |
| SNpc | 129.0 ± 12.1 * | 96.5 ± 18.6 | 104.9 ± 12.9 |
Significance denoted as difference between MA-treated groups and SAL-treated groups within each time point.
p ≤ 0.05.
N.A.D. = No Available Data
4. Discussion
Both the temperature and corticosterone increases seen in this experiment are congruent with previous studies and are indicative of the neurotoxic effects of MA using a binge exposure model in adult animals (Herring et al., 2008b;Szumlinski et al., 2001). The results demonstrate that binge MA treatment causes increases in brain BDNF mRNA expression. The data support MA regulation of BDNF expression and that such effects are likely independent of corticosterone, as previously protracted corticosterone increases have been linked with decreased BDNF protein and mRNA expression levels (Schaaf et al., 1998;Schaaf et al., 2000;Smith et al., 1995) whereas increases were seen here. While TrkB also exhibited increases in transcript levels following MA exposure, affected regions were less widespread and no expression alterations were seen across all time points in any brain region. TrkB expression was uniquely decreased in the hippocampal DG. BDNF, but not TrkB, mRNA levels were affected by MA treatment in the entorhinal and piriform cortices, Acc, and LC. These results are indicative of differential MA-induced regulation of TrkB and BDNF.
It is currently unknown what the functional effects of BDNF and TrkB mRNA increases are following MA exposure. Neurotrophins, including BDNF, are synthesized as precursor proteins prior to being cleaved into their mature form (Edwards et al., 1988). When secreted, proBDNF binds with high affinity to the proapoptotic receptor p75NTR (Lee et al., 2001), unlike BDNF that binds with weak affinity. It is not possible to determine if the BDNF expression increases seen herein were cleaved into the mature BDNF or not based on the current data. However, levels of proBDNF are usually small compared to BDNF (Yang et al., 2009), and prior studies have shown a neuroprotective effect of increased BDNF following a neurotoxic insult (Canals et al., 2001;Martinez-Serrano and Bjorklund, 1996;Perez-Navarro et al., 2000), not unlike the pattern of effects seen here.
The data are consistent with previous studies showing BDNF and TrkB increases following other types of CNS injury such as seizures, ischemia, excitotoxicity, traumatic injury, and hypoglycemia in multiple brain regions, including the hippocampus (Gall, 1993;Hicks et al., 1999a;Madinier et al., 2009;Merlio et al., 1993;Metsis et al., 1993;Nibuya et al., 1995;Canals et al., 1998;Canals et al., 2001;Kokaia et al., 1998;McAllister et al., 1999;Rocamora et al., 1996). The hippocampus is known to be vulnerable to injury and to exhibit BDNF and TrkB expression increases following damage (Hicks et al., 1997;Hicks et al., 1998;Hicks et al., 1999b;Merlio et al., 1993;Metsis et al., 1993;Nibuya et al., 1995). Therefore, mechanisms behind the increases seen here after MA treatment may be similar. The TrkB decreases seen in the hippocampal DG stand out as an exception to the general pattern of injury-induced increases (Merlio et al., 1993;Nibuya et al., 1995). This decrease was seen only at the 24 h time period but may reflect a longer-term biphasic response to the drug. Future experiments should assess TrkB at longer intervals to determine if this downward shift persists.
Other brain regions exhibiting MA-induced changes in BDNF or TrkB expression are also involved in behavioral abnormalities caused by MA. Chronic MA use in humans is associated with impairments in working memory, attention, and executive function; behaviors mediated to a large extent by the FC, PFC, and Acc (Barr et al., 2006;Dalley et al., 2004). In rats, MA exposure causes deficits in novel object recognition (Belcher et al., 2005), a behavior mediated through the piriform cortex (Broad et al., 2002) and hippocampus (Broadbent et al., 2010), as well as causing deficits in egocentric navigation (Herring et al., 2008a). Egocentric learning is a navigational task that is distinct from spatial learning and relies on the integration of local and self-movement cues (Byrne, 1982;Cook and Kesner, 1988). Both the entorhinal (Moser et al., 2008) and parietal cortices (Butters et al., 1972;Sato et al., 2006) contain cell bodies critical for this type of navigation and both show BDNF mRNA increases for at least 24 h following the MA treatment.
The striatum, where TrkB increases following MA treatment were seen, is also implicated in egocentric navigation and fixed-route motor learning (Chapman et al., 2001;Cook and Kesner, 1988;Packard and Knowlton, 2002). Striatal TrkB mRNA increases have previously been shown to occur after excitotoxic injury from glutamate receptor agonists (Canals et al., 1999). With virtually no BDNF mRNA found within the striatum, the substantial levels of BDNF protein found in striatal neurons are thought to be trafficked via cortical and other afferents to this region in an activity-dependent manner (Altar et al., 1997). Cortical BDNF upregulation seen following striatal excitotoxic injury can be blocked by exogenous administration of BDNF in striatal neurons, suggesting that cortical BDNF may be an endogenous protective response to striatal insult (Canals et al., 2001).
BDNF expression in the LC was increased 1 h post-treatment. Noradrenergic neurons in the LC act as a neuroprotective agent against nigrostriatal DA neuronal toxicity following exposure to MA (5 mg/kg × 3, 2 h apart) and other DA neurotoxins (Fornai et al., 1995;Fornai et al., 1998;Weinshenker et al., 2008), and this may have occurred here, although it was beyond the scope of this experiment to test this idea.
Previously it has been found that BDNF mRNA in the ventral midbrain (VTA/SNpc) increases 24 h following binge MA exposure in conjunction with 3 weeks of MA preconditioning (Cadet et al., (2009)), but not following binge-only MA treatment. This is consistent with the present data showing no change in BDNF or TrkB mRNA levels in either the VTA or SNpc, which are present within the DA cells in this region (Numan and Seroogy, 1999;Seroogy et al., 1994). The SNpc was the only region that showed increased TH expression levels 1 h following MA treatment, indicative of increased DA content. An acute (15 mg/kg) dose of MA has also been shown to increase TH activity in the striatum 1 h after exposure and this area receives abundant projections from the SNpc (Haughey et al., 1999). These data are in contrast to the large TH reductions seen at longer intervals after binge dosing regimens of MA (Cappon et al., 2000;Krasnova and Cadet, 2009). TH mRNA expression has previously been shown to be decreased in the SNpc 13 days following binge MA exposure (Chapman et al., 2001). TH protein reductions typically occur 12–48 h after treatment, but these are different than for TH mRNA (Cappon et al., 2000).
The dense DA projections to the striatum and other related areas, such as the PFC, are highly sensitive to MA and have been hypothesized to mediate the DA-dependent cognitive deficits seen in MA users (i.e., they lack deficits in non-dopaminergic spatial learning processes) (Barr et al., 2006). Increases in DA release are a common characteristic of drugs of abuse, and cocaine, amphetamine, and morphine have all shown to increase BDNF expression (Le Foll et al., 2005;Meredith et al., 2002) suggesting that a DA-mediated mechanism may be involved in regulation of BDNF expression following exposure to such drugs (Guillin et al., 2001;Le Foll et al., 2005). This is in line with evidence that the BDNF/TrkB pathway can modulate MA-dependent DA release and is involved in the induction of DA-related behavior (Blochl and Sirrenberg, 1996;Guillin et al., 2001;Narita et al., 2003).
MA-induced excitotoxic damage is another potential mechanism for BDNF and TrkB expression increases. Other models of excitotoxic neuronal damage also show BDNF and TrkB mRNA increases (Canals et al., 1998;Canals et al., 1999;Dong et al., 2006;Martinez-Serrano and Bjorklund, 1996;Perez-Navarro et al., 2000), and c-fos and BDNF expression increases have been shown to be co-localized in hippocampal neurons following excitotoxic damage (Dong et al., 2006). These expression alterations have differential regulation as compared to other neurotrophins and their receptors (Canals et al., 1999;Canals et al., 2001), implying a specific role for the BDNF/TrkB pathway following exposure to excitotoxins. The BDNF increases seen following excitotoxic neuronal injury appears to be neuroprotective (Canals et al., 2001;Martinez-Serrano and Bjorklund, 1996;Perez-Navarro et al., 2000), potentially having similar involvement following neurotoxic MA exposure. In vitro experiments in primary cortical neurons have shown that 12 h pretreatment with recombinant BDNF provides protection from MA-induced cell death (Matsuzaki et al., 2004). In addition, mice that over- or under-express BDNF mRNA exhibit decreased striatal DA loss compared to wild-type mice following acute MA treatment (Dluzen et al., 2002;Dluzen, 2004;Joyce et al., 2004), although another study found that intrastriatal administration of BDNF 24 h prior to acute MA treatment was ineffective against striatal DA loss (Cass et al., 2006). Continued work is needed to elaborate on the potential neuroprotective effects of BDNF pret on neurotoxic MA exposure as well as to understand the role that increases in endogenous BDNF have on the brain following MA-induced insult. Furthermore, studies are needed to determine how long following the injury this response remains if, in fact, BDNF and/or TrkB exert neuroprotective effects in response to MA exposure.
In conclusion, the present data demonstrate that a binge/neurotoxic treatment regimen of MA has selective effects on BDNF, TrkB, and TH mRNA expression in different brain regions up to 24 h following exposure. Most of the brain regions affected showed BDNF and TrkB increases. These are the same regions associated with several of the CNS dysfunctions observed in chronic MA users and in rats given neurotoxic MA doses. The relationship between neurotrophic changes and the behavioral consequences of MA-induced neurotoxicity may provide insight into future ways to ameliorate such deficits.
Acknowledgements
Support by NIH grants R01 DA006733, T32 ES7052, R01 NS39128, and a Scottish Rite Schizophrenia Fellowship (NRH).
Abbreviations
- Acc
anterior cingulate cortex
- BDNF
brain-derived neurotrophic factor
- DA
dopamine
- FC
frontal cortex
- LC
locus coeruleus
- MA
(+)-methamphetamine
- PFC
prefrontal cortex
- 5-HT
serotonin
- SNpc
substantia nigra pars compacta
- TrkB
tropomyosin receptor kinase B
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, O'Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res. 2002;940:95–101. doi: 10.1016/s0006-8993(02)02599-4. [DOI] [PubMed] [Google Scholar]
- Blochl A, Sirrenberg C. Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via Trk and p75Lntr receptors. J Biol Chem. 1996;271:21100–21107. doi: 10.1074/jbc.271.35.21100. [DOI] [PubMed] [Google Scholar]
- Broad KD, Mimmack ML, Keverne EB, Kendrick KM. Increased BDNF and trk-B mRNA expression in cortical and limbic regions following formation of a social recognition memory. Eur J Neurosci. 2002;16:2166–2174. doi: 10.1046/j.1460-9568.2002.02311.x. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters N, Soeldner C, Fedio P. Comparison of parietal and frontal lobe spatial deficits in man: Extrapersonal vs personal (egocentric) space. Perceptual & Motor Skills. 1972;34:27–34. doi: 10.2466/pms.1972.34.1.27. [DOI] [PubMed] [Google Scholar]
- Byrne RW. Normality and pathology in cognitive functions. London: Academic; 1982. Geographical knowledge and orientation; pp. 239–264. [Google Scholar]
- Canals JM, Checa N, Marco S, Akerud P, Michels A, Perez-Navarro E, Tolosa E, Arenas E, Alberch J. Expression of brain-derived neurotrophic factor in cortical neurons is regulated by striatal target area. J Neurosci. 2001;21:117–124. doi: 10.1523/JNEUROSCI.21-01-00117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals JM, Checa N, Marco S, Michels A, Perez-Navarro E, Alberch J. The neurotrophin receptors trkA, trkB and trkC are differentially regulated after excitotoxic lesion in rat striatum. Brain Res Mol Brain Res. 1999;69:242–248. doi: 10.1016/s0169-328x(99)00130-8. [DOI] [PubMed] [Google Scholar]
- Canals JM, Marco S, Checa N, Michels A, Perez-Navarro E, Arenas E, Alberch J. Differential regulation of the expression of nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 after excitotoxicity in a rat model of Huntington's disease. Neurobiol Dis. 1998;5:357–364. doi: 10.1006/nbdi.1998.0211. [DOI] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res. 2000;863:106–111. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Cass WA, Peters LE, Harned ME, Seroogy KB. Protection by GDNF and other trophic factors against the dopamine-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:272–281. doi: 10.1196/annals.1369.024. [DOI] [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- Cook D, Kesner RP. Caudate nucleus and memory for egocentric localization. Behav Neural Biol. 1988;49:332–343. doi: 10.1016/s0163-1047(88)90338-x. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dixon JE, McKinnon D. Expression of the trk gene family of neurotrophin receptors in prevertebral sympathetic ganglia. Brain Res Dev Brain Res. 1994;77:177–182. doi: 10.1016/0165-3806(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Dluzen DE. The effect of gender and the neurotrophin, BDNF, upon methamphetamine-induced neurotoxicity of the nigrostriatal dopaminergic system in mice. Neurosci Lett. 2004;359:135–138. doi: 10.1016/j.neulet.2004.01.061. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Anderson LI, McDermott JL, Kucera J, Walro JM. Striatal dopamine output is compromised within +/− BDNF mice. Synapse. 2002;43:112–117. doi: 10.1002/syn.10027. [DOI] [PubMed] [Google Scholar]
- Dong M, Wu Y, Fan Y, Xu M, Zhang J. c-fos modulates brain-derived neurotrophic factor mRNA expression in mouse hippocampal CA3 and dentate gyrus neurons. Neurosci Lett. 2006;400:177–180. doi: 10.1016/j.neulet.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Selby MJ, Garcia PD, Rutter WJ. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J Biol Chem. 1988;263:6810–6815. [PubMed] [Google Scholar]
- Fehm HL, Holl R, Steiner K, Klein E, Voigt KH. Evidence for ACTH-unrelated mechanisms in the regulation of cortisol secretion in man. Klin Wochenschr. 1984;62:19–24. doi: 10.1007/BF01725188. [DOI] [PubMed] [Google Scholar]
- Fornai F, Alessandri MG, Torracca MT, Bassi L, Scalori V, Corsini GU. Noradrenergic modulation of methamphetamine-induced striatal dopamine depletion. Ann N Y Acad Sci. 1998;844:166–177. [PubMed] [Google Scholar]
- Fornai F, Bassi L, Torracca MT, Scalori V, Corsini GU. Norepinephrine loss exacerbates methamphetamine-induced striatal dopamine depletion in mice. Eur J Pharmacol. 1995;283:99–102. doi: 10.1016/0014-2999(95)00313-a. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Castaneda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Fukumura M, Cappon GD, Broening HW, Vorhees CV. Methamphetamine-induced dopamine and serotonin reductions in neostriatum are not gender specific in rats with comparable hyperthermic responses. Neurotoxicol Teratol. 1998;20:441–448. doi: 10.1016/s0892-0362(97)00094-9. [DOI] [PubMed] [Google Scholar]
- Gall CM. Seizure-induced changes in neurotrophin expression: implications for epilepsy. Exp Neurol. 1993;124:150–166. doi: 10.1006/exnr.1993.1186. [DOI] [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Herring NR, Skelton MR, McCrea AE, Vorhees CV, Williams MT. (+)-Methamphetamine increases corticosterone in plasma and BDNF in brain more than forced swim or isolation in neonatal rats. Synapse. 2008;62:110–121. doi: 10.1002/syn.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Hanson GR. Differential regional effects of methamphetamine on the activities of tryptophan and tyrosine hydroxylase. J Neurochem. 1999;72:661–668. doi: 10.1046/j.1471-4159.1999.0720661.x. [DOI] [PubMed] [Google Scholar]
- He J, Yang Y, Yu Y, Li X, Li XM. The effects of chronic administration of quetiapine on the methamphetamine-induced recognition memory impairment and dopaminergic terminal deficit in rats. Behav Brain Res. 2006;172:39–45. doi: 10.1016/j.bbr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Effect of +-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 2008a;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Tang PH, Skelton MR, Lucot JP, Gudelsky GA, Vorhees CV, Williams MT. Comparison of time-dependent effects of (+)-methamphetamine or forced swim on monoamines, corticosterone, glucose, creatine, and creatinine in rats. BMC Neurosci. 2008b;9:49. doi: 10.1186/1471-2202-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RR, Li C, Zhang L, Dhillon HS, Prasad MR, Seroogy KB. Alterations in BDNF and trkB mRNA levels in the cerebral cortex following experimental brain trauma in rats. J Neurotrauma. 1999a;16:501–510. doi: 10.1089/neu.1999.16.501. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Martin VB, Zhang L, Seroogy KB. Mild experimental brain injury differentially alters the expression of neurotrophin and neurotrophin receptor mRNAs in the hippocampus. Exp Neurol. 1999b;160:469–478. doi: 10.1006/exnr.1999.7216. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Numan S, Dhillon HS, Prasad MR, Seroogy KB. Alterations in BDNF and NT-3 mRNAs in rat hippocampus after experimental brain trauma. Brain Res Mol Brain Res. 1997;48:401–406. doi: 10.1016/s0169-328x(97)00158-7. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Zhang L, Dhillon HS, Prasad MR, Seroogy KB. Expression of trkB mRNA is altered in rat hippocampus after experimental brain trauma. Brain Res Mol Brain Res. 1998;59:264–268. doi: 10.1016/s0169-328x(98)00158-2. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Hotchkiss AJ, Morgan ME, Gibb JW. The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sci. 1979;25:1373–1378. doi: 10.1016/0024-3205(79)90414-4. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Johnson LD, O'Malley PM, Bachman JG, Schulenberg JE. Volume II: College students and adults ages 19–50. Bethesda, MD: National Institute of Drug Abuse; 2009. Monitoring the future national survey results on drug use, 1975–2008. (NIH Publication No. 09-7403). [Google Scholar]
- Joyce JN, Renish L, Osredkar T, Walro JM, Kucera J, Dluzen DE. Methamphetamine-induced loss of striatal dopamine innervation in BDNF heterozygote mice does not further reduce D3 receptor concentrations. Synapse. 2004;52:11–19. doi: 10.1002/syn.10309. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Roh S, Kim Y, Yoon SJ, Lee HK, Han CS, Kim YK. High concentrations of plasma brain-derived neurotrophic factor in methamphetamine users. Neurosci Lett. 2005;388:112–115. doi: 10.1016/j.neulet.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Fitzmaurice PS, Boileau I, Schmunk GA, Ang LC, Furukawa Y, Chang LJ, Wickham DJ, Sherwin A, Tong J. Brain serotonin transporter in human methamphetamine users. Psychopharmacology (Berl) 2009;202:649–661. doi: 10.1007/s00213-008-1346-x. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Tokunaga I, Gotohda T, Kubo S. Immunohistochemical investigation of dopaminergic terminal markers and caspase-3 activation in the striatum of human methamphetamine users. Int J Legal Med. 2007;121:163–168. doi: 10.1007/s00414-006-0087-9. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Andsberg G, Yan Q, Lindvall O. Rapid alterations of BDNF protein levels in the rat brain after focal ischemia: evidence for increased synthesis and anterograde axonal transport. Exp Neurol. 1998;154:289–301. doi: 10.1006/exnr.1998.6888. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madinier A, Bertrand N, Mossiat C, Prigent-Tessier A, Beley A, Marie C, Garnier P. Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS One. 2009;4:e8101. doi: 10.1371/journal.pone.0008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Serrano A, Bjorklund A. Protection of the neostriatum against excitotoxic damage by neurotrophin-producing, genetically modified neural stem cells. J Neurosci. 1996;16:4604–4616. doi: 10.1523/JNEUROSCI.16-15-04604.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H, Namikawa K, Kiyama H, Mori N, Sato K. Brain-derived neurotrophic factor rescues neuronal death induced by methamphetamine. Biol Psychiatry. 2004;55:52–60. doi: 10.1016/s0006-3223(03)00785-6. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res. 2002;949:218–227. doi: 10.1016/s0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- Merlio JP, Ernfors P, Jaber M, Persson H. Molecular cloning of rat trkC and distribution of cells expressing messenger RNAs for members of the trk family in the rat central nervous system. Neuroscience. 1992;51:513–532. doi: 10.1016/0306-4522(92)90292-a. [DOI] [PubMed] [Google Scholar]
- Merlio JP, Ernfors P, Kokaia Z, Middlemas DS, Bengzon J, Kokaia M, Smith ML, Siesjo BK, Hunter T, Lindvall O. Increased production of the TrkB protein tyrosine kinase receptor after brain insults. Neuron. 1993;10:151–164. doi: 10.1016/0896-6273(93)90307-d. [DOI] [PubMed] [Google Scholar]
- Metsis M, Timmusk T, Arenas E, Persson H. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proc Natl Acad Sci U S A. 1993;90:8802–8806. doi: 10.1073/pnas.90.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine-related behaviors induced by methamphetamine. Neuroscience. 2003;119:767–775. doi: 10.1016/s0306-4522(03)00099-x. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan S. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. 1998 doi: 10.1523/JNEUROSCI.18-24-10700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan S, Gall CM, Seroogy KB. Developmental expression of neurotrophins and their receptors in postnatal rat ventral midbrain. J Mol Neurosci. 2005;27:245–260. doi: 10.1385/JMN:27:2:245. [DOI] [PubMed] [Google Scholar]
- Numan S, Seroogy KB. Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: a double-label in situ hybridization study. J Comp Neurol. 1999;403:295–308. doi: 10.1002/(sici)1096-9861(19990118)403:3<295::aid-cne2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- O'Dell SJ, Feinberg LM, Marshall JF. A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press, Inc; 1986. [Google Scholar]
- Perez-Navarro E, Canudas AM, Akerund P, Alberch J, Arenas E. Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 prevent the death of striatal projection neurons in a rodent model of Huntington's disease. J Neurochem. 2000;75:2190–2199. doi: 10.1046/j.1471-4159.2000.0752190.x. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Welker E, Pascual M, Soriano E. Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Sakata H, Tanaka YL, Taira M. Navigation-associated medial parietal neurons in monkeys. Proc Natl Acad Sci U S A. 2006;103:17001–17006. doi: 10.1073/pnas.0604277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf MJ, de Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, de JJ, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- Schroder N, O'Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Herman JP. In situ hybridization approaches to the study of the nervous system. In: Turner AJ, Bachelard HS, editors. Neurochemistry: A Practical Approach. Oxford, U.K: Oxford University Press; 1997. pp. 121–150. [Google Scholar]
- Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration OoAS. Rockville, MD: 2008. The DASIS report: Primary methampetamine/amphetamine admissions to substance abuse treatment: 2005. [Google Scholar]
- Szumlinski KK, Haskew RE, Balogun MY, Maisonneuve IM, Glick SD. Iboga compounds reverse the behavioural disinhibiting and corticosterone effects of acute methamphetamine: Implications for their antiaddictive properties. Pharmacol Biochem Behav. 2001;69:485–491. doi: 10.1016/s0091-3057(01)00564-0. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Ferrucci M, Busceti CL, Biagioni F, Lazzeri G, Liles LC, Lenzi P, Pasquali L, Murri L, Paparelli A, Fornai F. Genetic or pharmacological blockade of noradrenaline synthesis enhances the neurochemical, behavioral, and neurotoxic effects of methamphetamine. J Neurochem. 2008;105:471–483. doi: 10.1111/j.1471-4159.2007.05145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurek DM, Seroogy KB. Neurobiology of the Neurotrophins. Johnson City, TN: F.P. Graham Publishing; 2001. Neurotrophic factor protection of dopaminergic neurons; pp. 355–397. [Google Scholar]