Abstract
Recent preclinical and clinical evidence suggests that the stearoyl-CoA desaturase-1 (Scd1) enzyme plays a key role in the regulation of triglyceride (TG) biosynthesis and insulin sensitivity, and in vitro studies have found that antipsychotic medications up-regulate Scd1 mRNA expression. To investigate these effects in vivo, rats were treated with risperidone (1.5, 3, 6 mg/kg/d), paliperidone (1.5, 3, 6 mg/kg/d), olanzapine (2.5, 5, 10 mg/kg/d), quetiapine (5, 10, 20 mg/kg/d), haloperidol (1, 3 mg/kg/d) or vehicle through their drinking water for 40 d. Effects on liver Scd1 mRNA expression and an index of Scd1 activity (the plasma 18:1/18:0 ratio, ‘deaturation index’) were determined, as were postprandial plasma triglyceride (TG), glucose, insulin, and polyunsaturated fatty acid (PUFA) levels. All atypical antipsychotics increased the plasma 18:1/18:0 ratio, but not liver Scd1 mRNA expression, at doses found to also increase plasma TG levels. Among all rats (n=122), the plasma 18:1/18:0 ratio accounted for 56% of the variance in TG concentrations. The plasma 18:1/18:0 ratio was also positively associated with erythrocyte and heart membrane phospholipid 18:1n-9 composition. All antipsychotics except risperidone increased glucose levels at specific doses, and none of the antipsychotics significantly altered insulin levels. The plasma 18:1/18:0 ratio accounted for 20% of the variance in glucose levels. Plasma omega-3 and omega-6 PUFA levels were inversely correlated with the plasma 18:1/18:0 ratio and TG and glucose levels. These in vivo data demonstrate that different atypical antipsychotic medications increase the plasma 18:1/18:0 ratio in association with elevations in postprandial TG levels, and that concomitant elevations in PUFA biosynthesis oppose these effects.
Keywords: Stearoyl-CoA desaturase-1, oleic acid, desturation index, triglycerides, glucose, insulin, liver, erythrocytes, postprandial, rat
1. Introduction
Treatment-emergent hyperlipidemia, clinically significant weight gain, and insulin resistance are frequently observed in schizophrenic patients following chronic exposure to some atypical antipsychotic medications, and may increase risk for coronary heart disease and type 2 diabetes (Henderson, 2007; Meyer, 2001; Newcomer, 2007). The severity of hyperlipidemia and insulin resistance have been found to be greater following treatment with the atypical antipsychotics olanzapine and quetiapine compared with risperidone and typical antipsychotics (Garman et al., 2007; Meyer & Koro, 2004; Newcomer et al., 2002). Moreover, postprandial (non-fasting) triglyceride (TG) levels, which are a stronger predictor of cardiovascular risk (Eberly et al., 2003; Langsted et al., 2008; Bansal et al., 2008), are greater following treatment with olanzapine and quetiapine compared with risperidone and typical antipsychotics (Meyer et al., 2008; Smith et al., 2010). Furthermore, greater increases in glucose levels in response to a oral glucose bolus are observed in patients treated with olanzapine compared with risperidone (Smith et al., 2009). Despite this body of clinical evidence, however, the mechanisms mediating antipsychotic-induced elevations in TG synthesis and insulin resistance remain poorly understood.
Emerging evidence from rodent and clinical studies suggest that stearoyl-CoA desaturase-1 (SCD1, Δ9-desaturase) plays a central role in regulating TG biosynthesis and insulin sensitivity. SCD1 mediates oleic acid (18:1n-9) synthesis from stearic acid (18:0), and 18:1 is a substrate required for the de novo synthesis of phospholipids, cholesteryl esters, and TG (Paton & Ntambi, 2009). Mouse studies have demonstrated that Scd1 mutation is associated with reductions in Scd1 activity (liver and plasma 18:1/18:0 ratios) and deficits in TG biosynthesis (Attie et al., 2002; Miyazaki et al., 2000, 2001). Consistent with TG being stored in adipose tissue, Scd1 mutant mice also exhibit reduced adiposity independent of body weight gain, and are resistance to diet-induced obesity (Ntambi et al., 2002). Scd1 mutant mice also exhibit increased insulin sensitivity (Rahman et al., 2003). Similarly, pharmacological inhibition of the Scd1 enzyme reduces elevated TG and glucose levels in rodent disease models (Issandou et al., 2009; Uto et al., 2010). In human subjects, elevations in the plasma 18:1/18:0 ratio (‘desaturation index’), a validated index of SCD1 enzyme activity, is positively correlated with plasma TG levels and insulin resistance (Attie et al., 2002; Flowers & Ntambi, 2009; Mar-Heyming et al., 2008; Paillard et al., 2008; Warensjo et al., 2007). This body of evidence suggests that SCD1 expression and activity are required for TG biosynthesis and regulate insulin sensitivity.
A number of in vitro studies have found that different antipsychotic medications up-regulate the expression of multiple lipogenic genes regulated by the sterol regulatory element-binding protein (SREBP) including SCD1 (Ferno et al., 2005; Lauressergues et al., 2010; Polymeropoulos et al., 2009; Raeder et al., 2006). Moreover, schizophrenic patients treated with olanzapine exhibit greater SCD1 mRNA expression in peripheral blood cells compared with drug-free patients (Vik-Mo et al., 2008). However, SCD1 mRNA expression and activity are regulated by myriad of dietary and hormonal factors not accounted for in prior studies, including glucose, polyunsaturated fatty acids (PUFA), insulin, and leptin. For example, both omega-3 and omega-6 PUFAs repress Scd1 expression at the level of transcription and mRNA stability (Landschulz et al., 1994; Ntambi, 1999; Sessler et al., 1996). Omega-3 PUFAs also reduce elevated TG levels in rodent disease models (Hassanali et al., 2010; Lu et al., 2011; Mustad et al., 2006) and in schizophrenic patients treated with atypical antipsychotic medications (Caniato et al., 2006; Peet et al., 2002). In previous studies we found that chronic treatment with different antipsychotic medications up-regulate PUFA biosynthesis in rats (McNamara et al., 2009a, 2011). Together, these findings suggest that antipsychotic effects on Scd1 expression and activity are modulated by PUFAs, and emphasize the importance of dietary PUFA intake.
The primary objective of the present study was to determine the effects of chronic exposure to different antipsychotic medications on liver Scd1 mRNA expression and activity in vivo, and to determine associations with postprandial TG, glucose, insulin and PUFA levels. Based on the evidence reviewed above, our specific prediction was that chronic exposure to atypical antipsychotic medications would up-regulate hepatic Scd1 mRNA expression and activity, and that these effects would be positively correlated with postprandial TG levels and inversely correlated with omega-3 PUFA levels.
2. Materials and methods
2.1. Animals and diet
Adult (P56) male Long-Evans hooded rats were purchased from Harlan-Farms Indianapolis, IN. Upon arrival, all rats were maintained on the same custom research diet (TD.04285, Harlan-TEKLAD, Madison, WI). This diet contained casein (vitamin-free) 200 g/kg, L-cystine 3 g/kg, sucrose 270 g/kg, dextrose monohydrate 99.5 g/kg, corn starch 200 g/kg, maltodextrin 60 g/kg, cellulose 50 g/kg, mineral mixture AIMN-93G-MX 35 g/kg, vitamin mixture AIN-93-VX 10 g/kg, choline bitartrate 2.5 g/kg, TBHQ (antioxidant) 0.02 g/kg). The diet contained 18:0 (9.4% of total fatty acids) and oleic acid (6.7% of total fatty acids). For complete diet lipid composition see Table 1 in McNamara et al. (2008). Rats were housed 2 per cage, and food and fluids were available ad libitum. Paired housing was selected to avoid confounding effects of single-housing stress on primary outcome measures (Perez et al., 1997). Rats were maintained under standard vivarium conditions on a 12:12 h light:dark cycle. Food (g/kg/d) and fluid (ml/kg/d) intake and body weight were routinely recorded. Rats were sacrificed by decapitation on P99-101 in a counter-balanced manner relative to the removal of food hoppers at 9:00 am. Trunk blood was collected into EDTA-coated tubes, plasma isolated by centrifugation, and erythrocytes washed 3x with 4°C 0.9% NaCl. Heart and liver samples were also collected. All samples were stored at −80°C deg. All experimental procedures were approved by the University of Institutional Animal Care and Use Committee, and adhere to the guidelines set by the National Institutes of Health.
2.2. Drug administration
On P60, rats (n=122) were randomly assigned to receive drug vehicle (0.1 M acetic acid diluted in deionized water), risperidone (1.5, 3, 6 mg/kg/d; supplied by Ortho-McNeil Janssen Scientific Affairs LLC), paliperidone (1.5, 3, 6 mg/kg/d, supplied by Ortho-McNeil Janssen Scientific Affairs LLC), olanzapine (2.5, 5, 10 mg/kg/d, supplied by Eli Lilly and Company), quetiapine (5, 10, 20 mg/kg/d, supplied by AstraZeneca Pharmaceuticals), or haloperidol (1, 3 mg/kg/d, Sigma-Aldrich Chemicals) through their drinking water (n=8/drug dose). Doses of risperidone, olanzapine, and haloperidol were selected based on prior studies finding production of therapeutically-relevant plasma concentrations in rats following chronic oral administration (Andersson et al., 2002; McNamara et al., 2009a; Terry et al., 2005). Doses of quetiapine were selected based on prior behavioral and neurochemical studies finding significant effects within this dose range (Migler et al., 1993; Tarazi et al., 2002), and to avoid sedative effects observed at higher doses (≥40 mg/kg, Betz et al., 2005) which could impair food and fluid intake. Drugs were administered through the rat’s drinking water to avoid daily injection stress and surgical implantation of mini-pumps, to mimic oral administration in human patients, and to permit maintenance of drug dose in accordance with age-related increases in body weight and fluid intake. For three days prior to drug delivery, 24 h water consumption was determined for each cage using bottle weights (1 g water = 1 ml water), and ml water intake/mean kg body weight calculated. All drugs were dissolved and diluted in 0.1 M acetic acid to prepare a stock solution (stored at 4 deg) which was added to tap water at a volume required to deliver the targeted daily dose. To maintain intake of the targeted daily dose, drug concentrations were adjusted to mean daily fluid intake and mean body weight (ml/kg/day) every 3 days. Red opaque drinking bottles were used to protect drug from light degradation. Rats were maintained on their respective drug and dose until being sacrificed on P99-101 (39–41 days of treatment). Dehydration was routinely monitored over the course of the study using the skin tenting method.
2.3. Fatty acid composition
The gas chromatography procedure used to determine plasma, erythrocyte, and heart fatty acid composition has been described in detail previously (McNamara et al., 2009a). Briefly, total fatty acid composition was determined with a Shimadzu GC-2014 (Shimadzu Scientific Instruments Inc., Columbia MD). Analysis of fatty acid methyl esters was based on area under the curve calculated with Shimadzu Class VP 4.3 software. Fatty acid identification was based on retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). All analyses were performed by a technician blinded to treatment. We focused our primary analysis on the principle substrate and product of Scd1, stearic acid (18:0) and oleic acid (18:1n-9), respectively. The plasma 18:1/18:0 ratio (‘desaturation index’) was calculated as an index of liver Scd1 activity (Attie et al., 2002). Principle plasma PUFAs, including eicosapentaenoic acid (EPA, 20:5n-3), docosahexaenoic acid (DHA, 22:6n-3), linoleic acid (18:2n-6), and arachidonic acid (20:4n-6), were also investigated.
2.4. Plasma TG, glucose, and insulin
Postprandial (non-fasting) plasma TG (GPO-PAP, RANDOX Laboratories Ltd., Antrim UK), glucose (GOD-POD, Genzyme Diagnostics P.E.I. Inc., Charlottetown, PE, Canada), and insulin (ELISA, Linco Research, St. Charles MI, USA) concentrations (mg/dL) were determined using commercially available kits according to the manufacturer’s instructions. All analyses were performed by a technician blinded to treatment.
2.5. Liver Scd1 mRNA expression
Frozen liver was homogenized (BioLogics Model 300 V/T ultrasonic homogenizer, Manassas, VA) in Tri Reagent, and total RNA isolated and eluted according to the manufacturer’s instructions (RNeasy Lipid Tissue Mini Kit, Qiagen, Valencia, CA). RNA was quantified using a Nanodrop instrument (Nanodrop Instruments, Wilmington, DE). RNA quality was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). cDNA was prepared from total RNA using a high-capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). mRNA levels of stearoyl-CoA desaturase (Scd1, Rn00594894_m1) were measured in triplicate with real-time quantitative PCR using an ABI 7500 Real Time PCR System (Applied Biosystems, Foster City, CA). Data were analyzed by comparing the difference between target (Scd1) and endogenous control (GAPDH, Rn99999916_s1) cycle thresholds using the comparative Ct method (Livak et al., 2001).
2.6. Statistical analysis
Within-drug differences in plasma fatty acid composition and metabolic markers were evaluated with a one-way ANOVA (vehicle vs. drug doses), and pairwise comparisons made with unpaired t-tests (2-tail, α=0.05). Homogeneity of variance was confirmed using Bartlett’s test. Parametric correlation analyses were used to determine the relationship between plasma fatty acid composition and other plasma analytes (2-tail, α=0.05). Analyses were performed with GB-STAT (V.10, Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Food/fluid intake and body weight
Food/fluid intake and body weight are illustrated in Supplemental Fig. 1. Briefly, for food intake (g/kg/d), the main effect was not significant for risperidone, F(3,16)=1.8, p=0.19, paliperidone, F(3,16)=2.3, p=0.13, olanzapine, F(3,16)=1.5, p=0.25, quetiapine, F(3,16)=1.1, p=0.37, or haloperidol, F(2,14)=1.8, p=0.21. For fluid intake (ml/kg/d), the main effect was significant for olanzapine, F(3,16)=47.6, p≤0.0001, quetiapine, F(3,16)=3.9, p=0.04, and haloperidol, F(2,14)=30, p≤0.0001, but not risperidone, F(3,16)=1.2, p=0.33, or paliperidone, F(3,16)=0.5, p=0.69. For endpoint body weight (kg), the main effect was significant for paliperidone, F(3,33)=3.0, p=0.04, and olanzapine, F(3,33)=3.5, p=0.03, but not for risperidone, F(3,33)=0.7, p=0.57, quetiapine, F(3,33)=1.2, p=0.31, or haloperidol, F(2,25)=1.4, p=0.26.
3.2. Plasma 18:1/18:0 ratio, TG, glucose and insulin levels
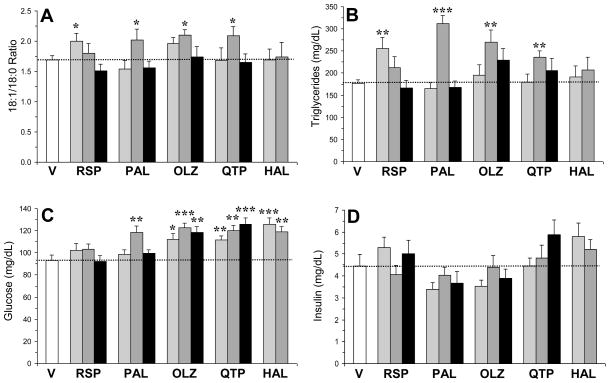
For the plasma 18:1/18:0 ratio (Fig. 1A), the main effect was significant for risperidone, F(3,33)=2.8, p=0.04, paliperidone, F(3,33)=4.8, p=0.007, and quetiapine, F(3,33)=3.5, p=0.02, but not for olanzapine, F(3,33)=2.1, p=0.1, or haloperidol, F(2,25)=0.03, p=0.97. For plasma TG concentrations (Fig. 1B), the main effect was significant for risperidone, F(3,33)=4.2, p=0.01, paliperidone, F(3,33)=23.9, p≤0.0001, olanzapine, F(3,33)=3.5, p=0.03, quetiapine, F(3,33)=2.8, p=0.04, but not for haloperidol, F(2,25)=0.5, p=0.59. For plasma glucose concentrations (Fig. 1C), the main effect was significant for paliperidone, F(3,33)=5.7, p=0.003, olanzapine, F(3,33)=7.6, p=0.0006, quetiapine, F(3,33)=9.3, p=0.0002, and haloperidol, F(2,25)=11.8, p=0.0004, but not for risperidone, F(3,33)=1.2, p=0.33. For plasma insulin concentrations (Fig. 1D), the main effect was not significant for risperidone, F(3,33)=0.9, p=0.45, paliperidone, F(3,33)=1.1, p=0.38, olanzapine, F(3,33)=0.95, p=0.43, quetiapine, F(3,33)=1.5, p=0.24, or haloperidol, F(2,25)=1.2, p=0.31.
Fig. 1.
Effects of chronic treatment with drug vehicle (V)(n=10), risperidone (RSP)(1.5, 3, 6 mg/kg/d), paliperidone (PAL)(1.5, 3, 6 mg/kg/d), olanzapine (OLZ)(2.5, 5, 10 mg/kg/d), quetiapine (QTP)(5, 10, 20 mg/kg/d), or haloperidol (HAL)(1, 3 mg/kg/d)(n=8/drug dose) on the plasma 18:1/18:0 ratio (A) and plasma TG (B) glucose (C), and insulin (D) concentrations. Values are group mean ± S.E.M. *p≤0.05, **p≤0.01, ***p≤0.0001 vs. Vehicle.
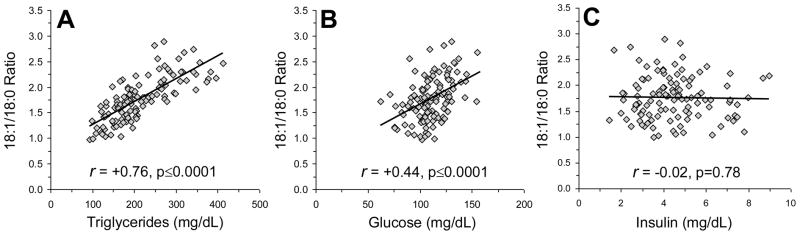
Among all rats (n=122), the plasma 18:1/18:0 ratio was positively correlated with postprandial plasma TG (r = +0.76, p≤0.0001)(Fig. 2A) and glucose (r = +0.44, p≤0.0001)(Fig. 2B) levels, but not with plasma insulin levels (r = −0.02, p=0.78)(Fig. 2C). The plasma 18:1/18:0 ratio accounted for 56% of the variance in TG concentrations and 20% of the variance in glucose concentrations. Plasma TG concentrations were positively correlated with plasma 18:1 composition (r = +0.75, p 0.0001), and inversely correlated with plasma 18:0 composition (r = −0.34, p=0.0002). Similarly, plasma glucose concentrations were positively correlated with plasma 18:1 composition (r = +0.40, p≤0.0001), and inversely correlated with plasma 18:0 composition (r = −0.45, p≤0.0001). TG concentrations were positively correlated with glucose concentrations (r = +0.56, p≤0.0001), but not insulin concentrations (r = +0.18, p=0.06). Endpoint body weight was not significantly correlated with the plasma 18:1/18:0 ratio (r = +0.03, p=0.71), TG (r = +0.02, p=0.85), glucose (r = −0.02, p=0.80), or insulin (r = +0.10, p=0.28). Baseline to endpoint weight gain was not significantly correlated with the plasma 18:1/18:0 ratio (r = +0.00, p=0.95), TG (r = +0.04, p=0.69), glucose (r = −0.00, p=0.94), or insulin (r = +0.05, p=0.57).
Fig. 2.
Relationships between the plasma 18:1/18:0 ratio and postprandial TG (A), glucose (B), and insulin (C) concentrations among all rats (n=122). Pearson correlation coefficients and associated p-values (two-tailed) are presented.
3.3. Plasma and membrane phospholipid 18:1n-9 composition
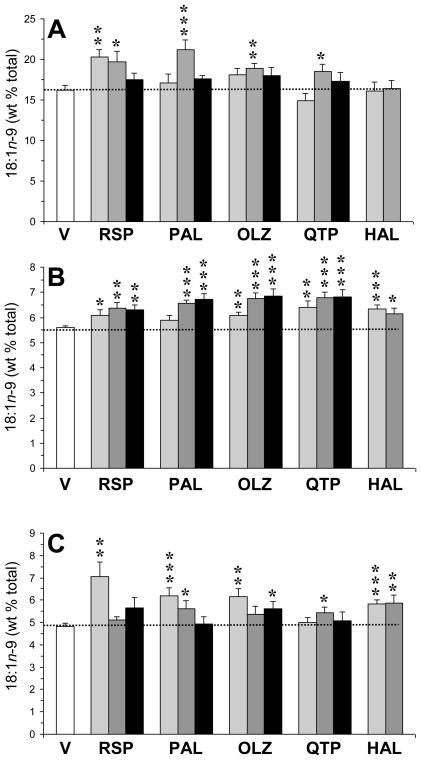
For plasma oleic acid (18:1n-9) composition (Fig. 3A), the main effect was significant for risperidone, F(3,33)=4.4, p=0.01, paliperidone, F(3,33)=6.4, p=0.002, olanzapine, F(3,33)=3.2, p=0.04, quetiapine, F(3,33)=2.9, p=0.04, but not for haloperidol, F(2,25)=0.01, p=0.98. Among all rats (n=122), plasma 18:1 composition was inversely correlated with plasma 18:0 composition (r = −0.34, p=0.0002). For erythrocyte 18:1n-9 composition (Fig. 3B), the main effect was significant for risperidone, F(3,33)=3.9, p=0.02, paliperidone, F(3,33)=11.7, p≤0.0001, olanzapine, F(3,33)=10.8, p≤0.0001, quetiapine, F(3,33)=7.4, p=0.0008, and haloperidol, F(2,25)=7.2, p=0.004. For heart 18:1n-9 composition (Fig. 3C), the main effect was significant for risperidone, F(3,33)=6.3, p=0.002, paliperidone, F(3,33)=4.7, p=0.009, olanzapine, F(3,33)=3.7, p=0.02, and haloperidol, F(2,25)=9.5, p=0.001, but not quetiapine, F(3,33)=1.0, p=0.38. Plasma 18:1n-9 composition was positively correlated with erythrocyte (r = +0.24, p=0.009) and heart (r = +0.30, p=0.0008) 18:1n-9 composition. Similarly, the plasma 18:1/18:0 ratio was positively correlated with erythrocyte (r = +0.19, p=0.04) and heart (r = +0.23, p=0.01) 18:1n-9 composition.
Fig. 3.
Effects of chronic treatment with drug vehicle (V)(n=10), risperidone (RSP)(1.5, 3, 6 mg/kg/d), paliperidone (PAL)(1.5, 3, 6 mg/kg/d), olanzapine (OLZ)(2.5, 5, 10 mg/kg/d), quetiapine (QTP)(5, 10, 20 mg/kg/d), or haloperidol (HAL)(1, 3 mg/kg/d)(n=8/drug dose) on the oleic acid (18:1n-9) composition in plasma (A), erythrocytes (B), and heart (C). Values are group mean ± S.E.M. *p≤0.05, **p≤0.01, ***p≤0.0001 vs. Vehicle.
3.4. Liver Scd1 mRNA expression
Liver Scd1 mRNA expression was determined in controls and individual dose groups exhibiting the greatest elevation in the plasma 18:1/18:0 ratio (risperidone: 1.5 mg/kg; paliperidone: 3 mg/kg; olanzapine: 2.5 mg/kg; quetiapine: 10 mg/kg; haloperidol: 1 mg/kg). The main effect of treatment was not significant, F(5,47)=1.6, p=0.18, and rats treated with risperidone (p=0.63), paliperidone (p=0.52), olanzapine (p=0.81), quetiapine (p=0.69) and haloperidol (p=0.07) did not exhibit significant alterations in liver Scd1 mRNA expression relative to controls. Liver Scd1 mRNA expression was not correlated with the plasma 18:1/18:0 ratio (r = −0.11, p=0.44).
3.5. Relationship with plasma PUFAs
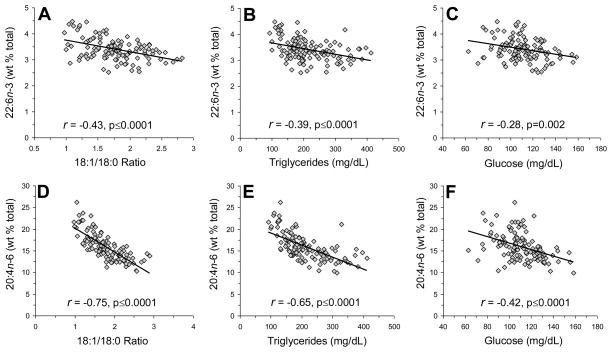
Among all rats (n=122), plasma DHA (22:6n-3) composition was inversely correlated with the plasma 18:1/18:0 ratio (r = −0.43, p≤0.0001)(Fig. 4A), TG (r = −0.39, p≤0.0001)(Fig. 4B), and glucose (r = −0.28, p=0.002)(Fig. 4C), but not insulin (r = −0.06, p=0.54). Plasma EPA composition was inversely correlated with TG (r = −0.37, p≤0.0001) and glucose (r = −0.23, p=0.01), but not with the plasma 18:1/18:0 ratio (r = −0.04, p=0.69) or insulin (r = −0.03, p=0.78) (see Supplemental Fig. 2). Plasma AA (20:4n-6) composition was inversely correlated with the plasma 18:1/18:0 ratio (r = −0.75, p≤0.0001)(Fig. 4D), TG (r = −0.65, p≤0.0001)(Fig. 4E) and glucose (r = −0.42, p≤0.0001)(Fig. 4F), but not insulin (r = −0.01, p=0.92). Plasma linoleic acid (18:2n-6) composition was inversely correlated with the plasma 18:1/18:0 ratio (r = −0.24, p=0.008), TG (r = −0.48, p≤0.0001), glucose (r = −0.19, p=0.04), and insulin (r = −0.33, p=0.0001). Plasma DHA (r = −0.82, p=0.01) and AA (r = −0.79, p=0.02) compositions were inversely correlated with liver Scd1 mRNA expression in controls, but not in individual treatment groups.
Fig. 4.
Relationships between plasma docosahexaenoic acid (DHA, 22:6n-3)(A–C) and arachidonic acid (AA, 20:4n-6)(D–F) compositions and the plasma 18:1/18:0 ratio (A,D), plasma TG concentrations (mg/dL)(B,E), and plasma glucose concentrations (mg/dL)(C,F) among all rats (n=122). Pearson correlation coefficient and associated p-values (two-tailed) are presented.
4. Discussion
The main finding of this study is that chronic exposure to atypical antipsychotic medications, but not the typical antipsychotic haloperidol, significantly increased both postprandial TG levels and an index of Scd1 activity (plasma 18:1/18:0 ratio) at specific doses independent of changes in liver Scd1 mRNA expression. Among all rats, the plasma 18:1/18:0 ratio was positively correlated with postprandial TG levels and erythrocyte and heart membrane phospholipid 18:1n-9 composition. Chronic exposure to all antipsychotic medications except risperidone increased postprandial glucose levels at specific doses, and glucose levels and the plasma 18:1/18:0 ratio were positively correlated among all rats. Elevated glucose levels were not associated with compensatory elevations in insulin concentrations, a finding consistent with antipsychotic-induced impairment of glucose-stimulated pancreatic β-cell insulin secretion (Best et al., 2005; Sasaki et al., 2006). Elevations in postprandial TG and glucose levels and the 18:1/18:0 ratio could not be attributed to differences in dietary intake of 18:1n-9 or 18:0, and were not associated with body weight gain. Among all rats, plasma PUFA compositions were inversely correlated with the plasma 18:1/18:0 ratio, TG, and glucose levels. These preclinical findings demonstrate that elevations in postprandial TG and glucose levels following chronic treatment with atypical antipsychotics are positively correlated with Scd1 activity in vivo, and that these effects are modulated by concomitant changes in PUFA biosynthesis.
This study has three notable limitations. First, rats treated with higher doses of olanzapine and quetiapine, and both doses of haloperidol, exhibited significant reductions in fluid intake which may have compromised drug intake. However, because drug concentrations were adjusted every 3 days to daily fluid intake and body weight (ml/kg/day), reductions in fluid intake should not have substantially altered daily drug intake. Nevertheless, in the absence of plasma drug concentration data it remains possible that greater changes in primary outcome measures may have been observed using a different mode of administration. Moreover, while severe dehydration was not observed in rats exhibiting decreased fluid intake, milder dehydration may have influenced the results. Second, only male rats were employed, precluding evaluation of potential gender differences. However, male rats were selected to obviate potential interactions with ovarian hormones previously found to influence the biosynthesis of PUFAs (Childs et al., 2010; McNamara et al., 2009b), which regulate Scd1 expression/activity (Landschulz et al., 1994; Ntambi, 1999; Sessler et al., 1996), and TG synthesis (Hassanali et al., 2010; Lu et al., 2011; Mustad et al., 2006). Third, we did not directly evaluate Scd1 enzyme activity, and used the plasma 18:1/18:0 ratio as a surrogate measure. However, the plasma 18:1/18:0 ratio is a validated measure of Scd1 activity (Attie et al., 2002), and the pattern of changes in plasma fatty acids observed in the present study, i.e., reductions in the short-chain precursor (18:0) and reciprocal elevations in the long-chain product (18:1), are consistent with elevated liver Scd1 activity (Attie et al., 2002; Issandou et al., 2009; Miyazaki et al., 2001).
In the present study, elevations in the plasma 18:1/18:0 ratio following atypical antipsychotic exposure were not associated with corresponding elevations in hepatic Scd1 mRNA expression. This in vivo finding is not consistent with prior in vitro studies finding that SCD1 mRNA expression is up-regulated in cultured human and rat cell lines following exposure to different typical and atypical antipsychotic medications (Ferno et al., 2005; Lauressergues et al., 2010; Polymeropoulos et al., 2009; Raeder et al., 2006). However, multiple dietary and hormonal factors that regulate Scd1 mRNA expression, including glucose, PUFAs, insulin, and leptin, may have mitigated antipsychotic-induced elevations in Scd1 mRNA expression in vivo (see below). Moreover, it remains possible that Scd1 mRNA expression was up-regulated in adipose tissue (Mangravite et al., 2007), peripheral blood cells (Vik-Mo et al., 2008), and/or skeletal muscle (Hulver et al., 2005). Alternatively, atypical antipsychotic medications may up-regulate Scd1 protein expression and/or enzymatic activity independent of effects on Scd1 transcription, and elevations in SCD1 protein expression in the absence of corresponding elevations in SCD1 mRNA expression have been observed previously (Garcia-Serrano et al., 2010).
We previously found that different atypical antipsychotic medications up-regulate long-chain PUFA biosynthesis in rats (McNamara et al., 2009a, 2011), and PUFAs reduce Scd1 mRNA expression at the level of transcription and mRNA stability (Landschulz et al., 1994; Ntambi, 1999; Sessler et al., 1996). In the present study, plasma DHA and AA compositions were inversely correlated with liver Scd1 mRNA expression in control rats, but not in any of the antipsychotic-treated groups. This finding suggests that elevations in PUFA biosynthesis may mitigate antipsychotic-induced elevations in liver Scd1 mRNA expression. This is supported in part by the previous finding that higher levels of dietary omega-3 fatty acid intake were associated with lower Scd1 mRNA expression in adipose fat (Muhlhausler et al., 2010). Nevertheless, among all rats plasma DHA and AA levels were inversely correlated with the plasma 18:1/18:0 ratio as well as TG and glucose levels, suggesting that PUFAs have antagonistic effects on Scd1 activity. It will be of interest to determine whether reductions in Scd1 activity mediate decreases in TG and/or glucose levels in response to omega-3 fatty acid supplementation in rodent disease models (Hassanali et al., 2010; Lu et al., 2011; Mustad et al., 2006) and in schizophrenic patients treated with atypical antipsychotic medications (Caniato et al., 2006; Peet et al., 2002). Nevertheless, these data highlight an important contribution of PUFAs to antipsychotic effects on Scd1 expression/activity, and emphasize the need to account for PUFAs in future antipsychotic studies.
Excessive weight gain and obesity are frequently observed in schizophrenic patients following chronic treatment with atypical antipsychotic medications (Henderson, 2007). Prior clinical studies have found that indices of SCD1 activity (plasma 18:1/18:0 ratio) are positively associated with excess adiposity and obesity (Flowers & Ntambi, 2009). Moreover, Scd1 mutant mice exhibit reduced adiposity independent of body weight gain and are resistant to dietary-induced obesity (Ntambi et al., 2002). In the present study, the 18:1/18:0 ratio and TG levels were not significantly correlated with body weight gain or endpoint body weight. Moreover, chronic treatment with specific doses of paliperidone and olanzapine decreased, rather than increased, body weight, and the other antipsychotics were weight neutral. Reductions in body weight gain have previously been observed in male rats following chronic olanzapine or risperidone treatment at specific doses despite greater visceral adiposity, and may be attributable to reciprocal reductions in lean muscle mass (Cooper et al., 2007; Ota et al., 2002). Because visceral adiposity and muscle mass were not determined in the present study, additional studies will be required to evaluate the relationship between Scd1 activity and greater visceral adiposity observed in male and female rats following atypical antipsychotic treatment (Cooper et al., 2007; Minet-Ringuet et al., 2006; Ota et al., 2002).
In conclusion, this in vivo study demonstrates that atypical antipsychotic medications increase the plasma 18:1/18:0 ratio, an index of Scd1 enzyme activity, at doses found to also increase postprandial TG and glucose levels. These results are consistent with prior preclinical (Attie et al., 2002; Rahman et al., 2003; Uto et al., 2010) and clinical (Mar-Heyming et al., 2008; Paillard et al., 2008; Warensjö et al., 2007) studies finding that Scd1 activity is positively associated with TG synthesis and insulin resistance. Additionally, these data suggest that PUFA biosynthesis is an important determinant of atypical antipsychotic effects on Scd1 activity and TG and glucose levels. Future studies are warranted to investigate whether inhibition of the Scd1 enzyme, either pharmacologically of with long-chain omega-3 fatty acids, can attenuate or prevent elevated TG and glucose levels in response to chronic atypical antipsychotic treatment.
Supplementary Material
Acknowledgments
This work was supported in part by an investigator-initiated research grant from Ortho-McNeil Janssen Pharmaceuticals (R076477SCH0004) and NIH grants MH073704 (to R.K.M.), DK59630 (to P.T.), and DA017399 (to J.W.L.). The authors thank Ortho-McNeil Janssen Pharmaceuticals for providing the risperidone and paliperidone, AstraZeneca Pharmaceuticals for providing the quetiapine, and Eli-Lilly and Company for providing the olanzapine.
Abbreviations
- Scd1
Stearoyl-CoA desaturase-1
- 18:0
stearic acid
- 18:1
oleic acid
- PUFA
polyunsaturated fatty acids
- EPA
eicosapentaenoic acid, DHA, docosahexaenoic acid
- AA
arachidonic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson C, Hamer RM, Lawler CP, Mailman RB, Lieberman JA. Striatal volume changes in the rat following long-term administration of typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2002;27:143–151. doi: 10.1016/S0893-133X(02)00287-7. [DOI] [PubMed] [Google Scholar]
- Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- Best L, Yates AP, Reynolds GP. Actions of antipsychotic drugs on pancreatic beta-cell function: contrasting effects of clozapine and haloperidol. J Psychopharmacol. 2005;19:597–601. doi: 10.1177/0269881105056641. [DOI] [PubMed] [Google Scholar]
- Betz A, Ishiwari K, Wisniecki A, Huyn N, Salamone JD. Quetiapine (Seroquel) shows a pattern of behavioral effects similar to the atypical antipsychotics clozapine and olanzapine: studies with tremulous jaw movements in rats. Psychopharmacology (Berl) 2005;179:383–392. doi: 10.1007/s00213-004-2046-9. [DOI] [PubMed] [Google Scholar]
- Caniato RN, Alvarenga ME, Garcia-Alcaraz MA. Effect of omega-3 fatty acids on the lipid profile of patients taking clozapine. Aust N Z J Psychiatry. 2006;40:691–697. doi: 10.1080/j.1440-1614.2006.01869.x. [DOI] [PubMed] [Google Scholar]
- Cooper GD, Pickavance LC, Wilding JP, Harrold JA, Halford JC, Goudie AJ. Effects of olanzapine in male rats: enhanced adiposity in the absence of hyperphagia, weight gain or metabolic abnormalities. J Psychopharmacol. 2007;21:405–413. doi: 10.1177/0269881106069637. [DOI] [PubMed] [Google Scholar]
- Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. The polyunsaturated fatty acid composition of hepatic and plasma lipids differ by both sex and dietary fat intake in rats. J Nutr. 2010;140:245–250. doi: 10.3945/jn.109.115691. [DOI] [PubMed] [Google Scholar]
- Eberly LE, Stamler J, Neaton JD Multiple Risk Factor Intervention Trial Research Group. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med. 2003;163:1077–1083. doi: 10.1001/archinte.163.9.1077. [DOI] [PubMed] [Google Scholar]
- Fernø J, Raeder MB, Vik-Mo AO, Skrede S, Glambek M, Tronstad KJ, Breilid H, Løvlie R, Berge RK, Stansberg C, Steen VM. Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: a novel mechanism of action? Pharmacogenomics J. 2005;5:298–304. doi: 10.1038/sj.tpj.6500323. [DOI] [PubMed] [Google Scholar]
- Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim Biophys Acta. 2009;1791:85–91. doi: 10.1016/j.bbalip.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Serrano S, Moreno-Santos I, Garrido-Sánchez L, Gutierrez-Repiso C, García-Almeida JM, García-Arnés J, Rivas-Marín J, Gallego-Perales JL, García-Escobar E, Rojo-Martinez G, Tinahones F, Soriguer F, Macias-Gonzalez M, García-Fuentes E. Stearoyl-CoA desaturase-1 is associated with insulin resistance in morbidly obese subjects. Mol Med. 2010 doi: 10.2119/molmed.2010.00078. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman PM, Ried LD, Bengtson MA, Hsu C, McConkey JR. Effect on lipid profiles of switching from olanzapine to another second-generation antipsychotic agent in veterans with schizophrenia. J Am Pharm Assoc. 2007;47:373–378. doi: 10.1331/JAPhA.2007.06090. [DOI] [PubMed] [Google Scholar]
- Hassanali Z, Ametaj BN, Field CJ, Proctor SD, Vine DF. Dietary supplementation of n-3 PUFA reduces weight gain and improves postprandial lipaemia and the associated inflammatory response in the obese JCR:LA-cp rat. Diabetes Obes Metab. 2010;12:139–147. doi: 10.1111/j.1463-1326.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- Henderson DC. Weight gain with atypical antipsychotics: evidence and insights. J Clin Psychiatry. 2007;68:18–26. [PubMed] [Google Scholar]
- Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issandou M, Bouillot A, Brusq JM, Forest MC, Grillot D, Guillard R, Martin S, Michiels C, Sulpice T, Daugan A. Pharmacological inhibition of stearoyl-CoA desaturase 1 improves insulin sensitivity in insulin-resistant rat models. Eur J Pharmacol. 2009;618:28–36. doi: 10.1016/j.ejphar.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Landschulz KT, Jump DB, MacDougald OA, Lane MD. Transcriptional control of the stearoyl-CoA desaturase-1 gene by polyunsaturated fatty acids. Biochem Biophys Res Commun. 1994;200:763–768. doi: 10.1006/bbrc.1994.1516. [DOI] [PubMed] [Google Scholar]
- Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047–2056. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- Lauressergues E, Staels B, Valeille K, Majd Z, Hum DW, Duriez P, Cussac D. Antipsychotic drug action on SREBPs-related lipogenesis and cholesterogenesis in primary rat hepatocytes. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:427–439. doi: 10.1007/s00210-010-0499-4. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J, Borthwick F, Hassanali Z, Wang Y, Mangat R, Ruth M, Shi D, Jaeschke A, Russell JC, Field CJ, Proctor SD, Vine DF. Chronic dietary n-3 PUFA intervention improves dyslipidaemia and subsequent cardiovascular complications in the JCR:LA- cp rat model of the metabolic syndrome. Br J Nutr. 2011;31:1–11. doi: 10.1017/S0007114510005453. [DOI] [PubMed] [Google Scholar]
- Mangravite LM, Dawson K, Davis RR, Gregg JP, Krauss RM. Fatty acid desaturase regulation in adipose tissue by dietary composition is independent of weight loss and is correlated with the plasma triacylglycerol response. Am J Clin Nutr. 2007;86:759–767. doi: 10.1093/ajcn/86.3.759. [DOI] [PubMed] [Google Scholar]
- Mar-Heyming R, Miyazaki M, Weissglas-Volkov D, Kolaitis NA, Sadaat N, Plaisier C, Pajukanta P, Cantor RM, de Bruin TW, Ntambi JM, Lusis AJ. Association of stearoyl-CoA desaturase 1 activity with familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol. 2008;28:1193–1199. doi: 10.1161/ATVBAHA.107.160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Sullivan J, Richtand NM. Omega-3 fatty acid deficiency augments the development of behavioral sensitization in adult mice: Prevention by chronic lithium treatment. J Psychiatric Res. 2008;42:458–468. doi: 10.1016/j.jpsychires.2007.05.009. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Jandacek R, Rider T, Tso P. Chronic risperidone treatment preferentially increases rat erythrocyte and prefrontal cortex omega-3 fatty acid composition: Evidence for augmented biosynthesis. Schizophr Res. 2009a;107:150–157. doi: 10.1016/j.schres.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P. Gender differences in rat erythrocyte and brain docosahexaenoic acid composition: Role of ovarian hormones and dietary omega-3 fatty acid composition. Psychoneuroendocrinology. 2009b;34:532–539. doi: 10.1016/j.psyneuen.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Rider T, Jandacek R, Tso P, Cole-Strauss A, Lipton JW. Differential effects of antipsychotic medications on polyunsaturated fatty acid biosynthesis in rats: Relationship with liver delta6-desaturase expression. Schizophr Res. 2011 doi: 10.1016/j.schres.2011.03.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Davis VG, McEvoy JP, Goff DC, Nasrallah HA, Davis SM, Daumit GL, Hsiao J, Swartz MS, Stroup TS, Lieberman JA. Impact of antipsychotic treatment on nonfasting triglycerides in the CATIE Schizophrenia Trial phase 1. Schizophr Res. 2008;103:104–109. doi: 10.1016/j.schres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70:1–17. doi: 10.1016/j.schres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Meyer JM. Effects of atypical antipsychotics on weight and serum lipid levels. J Clin Psychiatry. 2001;62:27–34. [PubMed] [Google Scholar]
- Migler BM, Warawa EJ, Malick JB. Seroquel: behavioral effects in conventional and novel tests for atypical antipsychotic drug. Psychopharmacology (Berl) 1993;112:299–307. doi: 10.1007/BF02244925. [DOI] [PubMed] [Google Scholar]
- Minet-Ringuet J, Even PC, Goubern M, Tomé D, de Beaurepaire R. Long term treatment with olanzapine mixed with the food in male rats induces body fat deposition with no increase in body weight and no thermogenic alteration. Appetite. 2006;46:254–262. doi: 10.1016/j.appet.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Kim YC, Ntambi JM. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res. 2001;42:1018–1024. [PubMed] [Google Scholar]
- Muhlhausler BS, Cook-Johnson R, James M, Miljkovic D, Duthoit E, Gibson R. Opposing effects of omega-3 and omega-6 long chain polyunsaturated Fatty acids on the expression of lipogenic genes in omental and retroperitoneal adipose depots in the rat. J Nutr Metab. 2010 doi: 10.1155/2010/927836. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustad VA, Demichele S, Huang YS, Mika A, Lubbers N, Berthiaume N, Polakowski J, Zinker B. Differential effects of n-3 polyunsaturated fatty acids on metabolic control and vascular reactivity in the type 2 diabetic ob/ob mouse. Metabolism. 2006;55:1365–1374. doi: 10.1016/j.metabol.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68:8–1. [PubMed] [Google Scholar]
- Newcomer JW, Haupt DW, Fucetola R, Melson AK, Schweiger JA, Cooper BP, Selke G. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry. 2002;59:337–345. doi: 10.1001/archpsyc.59.4.337. [DOI] [PubMed] [Google Scholar]
- Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40:1549–1558. [PubMed] [Google Scholar]
- Ota M, Mori K, Nakashima A, Kaneko YS, Fujiwara K, Itoh M, Nagasaka A, Ota A. Peripheral injection of risperidone, an atypical antipsychotic, alters the bodyweight gain of rats. Clin Exp Pharmacol Physiol. 2002;29:980–989. doi: 10.1046/j.1440-1681.2002.t01-1-03755.x. [DOI] [PubMed] [Google Scholar]
- Paillard F, Catheline D, Duff FL, Bouriel M, Deugnier Y, Pouchard M, Daubert JC, Legrand P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis. 2008;18:436–440. doi: 10.1016/j.numecd.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297:28–37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet M, Horrobin DF E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002;36:7–18. doi: 10.1016/s0022-3956(01)00048-6. [DOI] [PubMed] [Google Scholar]
- Pérez C, Canal JR, Domínguez E, Campillo JE, Guillén M, Torres MD. Individual housing influences certain biochemical parameters in the rat. Lab Anim. 1997;31:357–361. doi: 10.1258/002367797780596158. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Licamele L, Volpi S, Mack K, Mitkus SN, Carstea ED, Getoor L, Thompson A, Lavedan C. Common effect of antipsychotics on the biosynthesis and regulation of fatty acids and cholesterol supports a key role of lipid homeostasis in schizophrenia. Schizophr Res. 2009;108:134–142. doi: 10.1016/j.schres.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Raeder MB, Fernø J, Vik-Mo AO, Steen VM. SREBP activation by antipsychotic- and antidepressant-drugs in cultured human liver cells: relevance for metabolic side-effects? Mol Cell Biochem. 2006;289:167–173. doi: 10.1007/s11010-006-9160-4. [DOI] [PubMed] [Google Scholar]
- Rahman SM, Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc Natl Acad Sci U S A. 2003;100:11110–11115. doi: 10.1073/pnas.1934571100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Iwase M, Uchizono Y, Nakamura U, Imoto H, Abe S, Iida M. The atypical antipsychotic clozapine impairs insulin secretion by inhibiting glucose metabolism and distal steps in rat pancreatic islets. Diabetologia. 2006;49:2930–2938. doi: 10.1007/s00125-006-0446-6. [DOI] [PubMed] [Google Scholar]
- Sessler AM, Kaur N, Palta JP, Ntambi JM. Regulation of stearoyl-CoA desaturase 1 mRNA stability by polyunsaturated fatty acids in 3T3-L1 adipocytes. J Biol Chem. 1996;271:29854–29858. doi: 10.1074/jbc.271.47.29854. [DOI] [PubMed] [Google Scholar]
- Smith RC, Lindenmayer JP, Davis JM, Kelly E, Viviano TF, Cornwell J, Hu Q, Khan A, Vaidhyanathaswamy S. Effects of olanzapine and risperidone on glucose metabolism and insulin sensitivity in chronic schizophrenic patients with long-term antipsychotic treatment: a randomized 5-month study. J Clin Psychiatry. 2009;70:1501–1513. doi: 10.4088/JCP.08m04446yel. [DOI] [PubMed] [Google Scholar]
- Smith RC, Lindenmayer JP, Hu Q, Kelly E, Viviano TF, Cornwell J, Vaidhyanathaswamy S, Marcovina S, Davis JM. Effects of olanzapine and risperidone on lipid metabolism in chronic schizophrenic patients with long-term antipsychotic treatment: a randomized five month study. Schizophr Res. 2010;120:204–209. doi: 10.1016/j.schres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on serotonin 1A, 2A and 2C receptors in rat forebrain regions. Psychopharmacology (Berl) 2002;161:263–270. doi: 10.1007/s00213-002-1016-3. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Mahadik SP, Warsi S, Davis LW, Waller JL. Chronic exposure to typical or atypical antipsychotics in rodents: temporal effects on central alpha7 nicotinic acetylcholine receptors. Neuroscience. 2005;136:519–529. doi: 10.1016/j.neuroscience.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Uto Y, Ogata T, Kiyotsuka Y, Ueno Y, Miyazawa Y, Kurata H, Deguchi T, Watanabe N, Konishi M, Okuyama R, Kurikawa N, Takagi T, Wakimoto S, Ohsumi J. Novel benzoylpiperidine-based stearoyl-CoA desaturase-1 inhibitors: Identification of 6-[4-(2-methylbenzoyl)piperidin-1-yl]pyridazine-3-carboxylic acid (2-hydroxy-2-pyridin-3-ylethyl) amide and its plasma triglyceride-lowering effects in Zucker fatty rats. Bioorg Med Chem Lett. 2010;20:341–345. doi: 10.1016/j.bmcl.2009.10.101. [DOI] [PubMed] [Google Scholar]
- Vik-Mo AO, Birkenaes AB, Fernø J, Jonsdottir H, Andreassen OA, Steen VM. Increased expression of lipid biosynthesis genes in peripheral blood cells of olanzapine-treated patients. Int J Neuropsychopharmacol. 2008;11:679–684. doi: 10.1017/S1461145708008468. [DOI] [PubMed] [Google Scholar]
- Warensjö E, Ingelsson E, Lundmark P, Lannfelt L, Syvänen AC, Vessby B, Risérus U. Polymorphisms in the SCD1 gene: associations with body fat distribution and insulin sensitivity. Obesity. 2007;15:1732–1740. doi: 10.1038/oby.2007.206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.