Abstract
Goldenseal (Hydrastis canadensis L.) is used to combat inflammation and infection. Its antibacterial activity in vitro has been attributed to its alkaloids, the most abundant of which is berberine. The goal of these studies was to compare the composition, antibacterial activity, and efflux pump inhibitory activity of ethanolic extracts prepared from roots and aerial portions of H. canadensis. Ethanolic extracts were prepared separately from roots and aerial portions of six H. canadensis plants. Extracts were analyzed for alkaloid concentration using LC-MS and tested for antimicrobial activity against Staphylococcus aureus (NCTC 8325-4) and for inhibition of ethidium bromide efflux. Synergistic antibacterial activity was observed between the aerial extract (FIC 0.375) and to a lesser extent the root extract (FIC 0.750) and berberine. The aerial extract inhibited ethidium bromide efflux from wild-type S. aureus, but had no effect on the expulsion of this compound from an isogenic derivative deleted for norA. Our studies indicate that the roots of H. canadensis contain higher levels of alkaloids than the aerial portions, but the aerial portions synergize with berberine more significantly than the roots. Furthermore, extracts from the aerial portions of H. canadensis contain efflux pump inhibitors, while efflux pump inhibitory activity was not observed for the root extract. The three most abundant H. canadensis alkaloids, berberine, hydrastine and canadine, are not responsible for the efflux pump inhibitory activity of the extracts from H. canadensis aerial portions.
Keywords: Hydrastis canadensis, Ranunculaceae, synergy, alkaloid, berberine, Staphylococcus aureus
Introduction
The rise in infections from multi-drug resistant bacteria is recognized worldwide as a major health crisis. According to the European Antimicrobial Resistance Surveillance System, as many as 52 million of the world population may carry multi-drug resistant bacteria . One such resistant strain, methicillin-resistant Staphylococcus aureus (MRSA),is estimated to be responsible for over 18,000 annual deaths [1] in the US alone. Better methods to treat infections from resistant bacteria are needed. There is a long history of the use of botanical medicines to treat inflammation and infection. It is often argued that the inherent complexity of such preparations, which may lead to synergistic interactions, may make them more effective than their pharmaceutical counterparts [2, 3]. Furthermore, if such botanical medicines act through multiple different pathways, it may make the development of resistance more difficult. For these reasons, further research into the use of botanical medicines to combat bacterial infections is warranted.
The botanical medicine goldenseal (Hydrastis canadensis L.) is the subject of this research. Goldenseal preparations are popular in the international market [4, 5], and are among the top 20 best selling botanical dietary supplements in the US [6]. Crude extracts and isolated compounds from goldenseal have demonstrated antibacterial activity in vitro and in clinical trials [7-11]. The antibacterial activity of goldenseal has typically been attributed to alkaloids, especially berberine [11, 12], which has shown activity against various Gram-positive bacteria, including MRSA [13]. However, there has been some suggestion that other compounds present in complex goldenseal preparations might enhance the antimicrobial activity of berberine [7, 14]. We have observed pronounced antimicrobial activity of extracts from the aerial portions of goldenseal, which could not be attributed to berberine alone. We hypothesize that these extracts contain efflux pump inhibitors that synergistically enhance the antimicrobial activity of berberine. Bacterial efflux pumps are membrane bound proteins that pump toxins out of bacterial cells [15]. Overexpression of efflux pumps contributes to the development of resistance in bacteria, including S. aureus [16]. Inhibition of efflux pumps may enhance the effectiveness of antimicrobial agents that are substrates for these pumps, and decrease the minimum inhibitory concentration (MIC) for the antimicrobials [17].
The goals of these studies were (1) to compare alkaloid content in extracts prepared from below ground (roots and rhizomes) and above ground (leaves and stems) portions of H. canadensis, (2) to evaluate the antibacterial activity of H. canadensis extracts in combination with the antibacterial agent berberine and (3) to determine whether H. canadensis extracts act as inhibitors of norA, the major chromosomal S. aureus efflux pump. Ultimately, our objective was to show whether synergists other than the major known alkaloids are present in H. canadensis, and to determine which part of the plant would have higher levels of such synergists. These questions are important in the manufacture and quality control of dietary supplements from H. canadensis. More broadly, these studies are relevant because we demonstrate an analytical approach by which synergistic activity can be investigated prospectively in crude extracts of botanical medicines.
Materials and Methods
Cell lines, chemicals and biochemicals
Staphyloccus aureus NCTC 8325-4 [18] and its isogenic norA deletion mutant K1758 [19] were used. Müeller Hinton broth, carbonyl cyanide m-chloro-phenylhydrazone (CCCP, purity >98% by TLC), berberine (purity >98% by HPLC), (1R,9S)-(−)-β-hydrastine (purity >98% by HPLC) and DMSO were purchased from Sigma Aldrich (Saint Louis, MO, USA) and canadine (tetrahydroberberine, purity >98% by HPLC, stereochemistry unconfirmed) was obtained from Sequoia Research (Pangbourne, UK). Acetic acid was purchased from Fisher Chemical (Pittsburgh, PA, USA). Ethanol (95%), HPLC grade acetonitrile, and HPLC grade methanol were obtained from Pharmaco-AAPER (Shelbyville, KY, USA). Nanopure water was prepared with a nanodiamond water purification system from Barnstead (Dubuque, IA, USA).
Plant material
Hydrastis canadensis L. (Ranunculaceae) was cultivated in its native habitat (a hardwood forest in Hendersonville, NC, N 35° 24.277′, W 082° 20.993′, 702.4 m elevation) and harvested in September of 2008. A voucher specimen was deposited at the Herbarium of the University of North Carolina at Chapel Hill (NCU583414) and identified by Dr. Alan S. Weakly. Individual H. canadensis plants were harvested and numbered (Table 1).
Table 1.
Quantity of alkaloids in extracts from the root and rhizome or leaf and stem (aerial) portions of 6 individual H. canadensis plants and 2 “pooled samples.” Concentrations were calculated from calibration curves for standards of each alkaloid analyzed with LC-MS in the positive ion mode. The “pooled root” and “pooled leaf” extracts were prepared from larger batches of plant material (including multiple individual plants) and used for antimicrobial testing.
| Extract | Berberine (mM) | Hydrastine (mM) | Canadine (mM) |
|---|---|---|---|
| Root 1 | 29.9 ± 0.4 | 2.30 ± 0.04 | 0.069 ± 0.011 |
| Root 2 | 25.3 ± 0.5 | 2.08 ± 0.04 | 0.061 ± 0.010 |
| Root 3 | 39.6 ± 0.5 | 3.34 ± 0.06 | 0.078 ± 0.013 |
| Root 4 | 70.0 ± 0.9 | 5.23 ± 0.10 | 0.056 ± 0.009 |
| Root 5 | 25.0 ± 0.3 | 3.50 ± 0.07 | 0.074 ± 0.011 |
| Root 6 | 28.9 ± 0.4 | 2.42 ± 0.05 | 0.074 ± 0.012 |
| Average Root | 36.5 ± 0.5 | 3.15 ± 0.06 | 0.069 ± 0.011 |
| Aerial 1 | 8.5 ± 0.1 | 1.23 ± 0.02 | 0.042 ± 0.007 |
| Aerial 2 | 8.3 ± 0.1 | 1.58 ± 0.03 | 0.046 ± 0.007 |
| Aerial 3 | 8.8 ± 0.1 | 1.13 ± 0.02 | 0.029 ± 0.004 |
| Aerial 4 | 9.2 ± 0.1 | 0.77 ± 0.01 | 0.050 ± 0.008 |
| Aerial 5 | 6.2 ± 0.8 | 1.20 ± 0.02 | 0.059 ± 0.009 |
| Aerial 6 | 5.7 ± 0.1 | 0.83 ± 0.02 | 0.044 ± 0.007 |
| Average Aerial | 7.8 ± 0.1 | 1.12 ± 0.02 | 0.045 ± 0.007 |
| Pooled Sample Root | 16.6 ± 0.3 | 3.79 ± 0.07 | 0.069 ± 0.011 |
| Pooled Sample Aerial | 8.0 ± 0.1 | 2.61 ± 0.05 | 0.054 ± 0.008 |
Extraction and LC-MS
The extracts (50% ethanol: 50% nanopure water) were prepared using 1 g of powdered leaves and stems or roots and rhizomes per 5 mL of solvent (1:5 w:v) according to the standard procedures in the US dietary supplements industry [20]. Henceforth, the root and rhizome extract will be designated “root” and the leaf and stem extract will be designated “aerial”. Plant material was blended with solvent, macerated for 24 hr, and filtered under vacuum. Extracts were stored in amber bottles at room temperature. Biological activity and HPLC profile was retained in these storage conditions for >1 year. An entire below ground portion (roots and rhizome) and its corresponding aerial portions (leaves and stems) of six individual plants were extracted separately. The extracts were numbered so that in each case, the number for the root and aerial extract of a single plant match. Two larger “pooled extracts” were also prepared, one using 100 g of root/rhizome material and one using 100 g of aerial portions. All bioassay data presented here were collected using these “pooled extracts”.
Analysis of alkaloid content was accomplished with liquid chromatography – mass spectrometry (LC-MS) using an ion trap mass spectrometer with electrospray source (LCQ Advantage, Thermo, San Jose, CA, USA) coupled to reversed phase HPLC (HP1100, Agilent, Santa Clara, CA, USA). A C-18 column (50 mm × 2.1 mm, 3 μm particle size, 110 Å pore size, Prevail packing, Grace, Deerfield, IL, USA and 0.5 μm precolumn filter, MacMod Analytical, Chadds Ford, PA, USA) was employed for the analysis of all extracts, using a 0.2 mL/min flow rate and a 10 μL injection volume. The samples were analyzed using the following gradient, where A = 1% acetic acid in nanopure water and B = HPLC grade acetonitrile: 100%-68% A from 0 to 5 min; 68% -0% A from 5 to 20 min; 0% A from 20 to 25 min., 100%B from 25-40 min. Mass spectrometric detection was conducted in the positive ion mode with a scan range of 50-2000 m/z. Capillary temperature was 275°C, sheath gas pressure was 20 (arbitrary units), and spray, capillary, and tube lens voltages were 4.5 kV, 3 V, and 60 V, respectively.
Standards of the alkaloids berberine, hydrastine, and canadine were prepared at stock concentrations of 1 mg/mL in methanol. Calibration curves over a concentration range of 0.05 to 100 μM were plotted as peak area of the relevant selected ion trace versus concentration. Extracts were diluted as necessary in 50:50 ethanol:water to provide alkaloid concentrations within the linear range of the calibration curve. Alkaloids were identified in the extracts by matching retention time and fragmentation patterns with the relevant standards.
Checkerboard Assay with Staphyloccus aureus NCTC 8325-4
Extracts were dried under nitrogen and redissolved in Müeller Hinton broth containing 10% DMSO and filtered through a 0.45 μm PVDF filter. Broth microdilution MIC assays were performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines [21]. Extracts were standardized to berberine and tested in combination with berberine with a checkerboard assay [22] over a concentration range of 5 to 300.00 μg/mL (where these represent concentration of berberine either in the standard or the extract). A purified standard of the berberine, which has well documented antimicrobial activity [23-25], served as the positive control. The assay was performed in triplicate in 96-well plates with 1 × 105 CFU/mL S. aureus, 2% final DMSO content, and a final well volume of 250 μL. The negative (vehicle) control consisted of 2% DMSO in broth. MIC values were determined at the point at which there was no significant difference between OD600 of the vehicle control and the treatment. OD600 for wells containing only extracts or berberine (without bacteria) was subtracted from the relevant assay wells to minimize interference. FIC indices were calculated according to equation 1, where A and B are the compounds (or extracts) tested in combination, MICA is defined as the minimum inhibitory concentration of A alone, MICB is defined as the minimum inhibitory concentration of B alone, and [B] is defined as the MIC of B in the presence of a A, and [A] is defined as the MIC of A in the presence of B.
| (equation 1) |
Efflux pump assay
Wild type S. aureus and its isogenic norA deletion mutant (NCTC 8325-4 and K1758, respectively) were grown in Müeller Hinton broth to OD600 = 0.740. Ethidium bromide and CCCP were added to final concentrations of 25 μM and 100 μM, respectively, and the solution was incubated at 20°C with agitation (300 RPM) for 20 min. This solution was diluted to OD600 = 0.400 with fresh Müeller Hinton broth containing 10 μg/mL ethidium bromide and 100 μM CCCP. The solution was spun down in 1 mL aliquots at 20°C for 5 min at 13,000 × g in a Spectrafuge 24D centrifuge (Labnet, Woodbridge NJ, USA). The supernatant was discarded and the red pellets placed on ice. At time of measurement, these pellets were thawed for 5 min and resuspended with 1 mL fresh Müeller Hinton broth containing 2% DMSO with or without inhibitor. Fluorescence measurements were made using a Spex FluoroMax-2 spectrofluorometer (Instruments S. A., Inc, Edison, NJ, USA) every second for 300 s, with λex = 530, λemiss = 600, and slit widths of 5 mm.
Statistics
Statistical significance of means versus control was evaluated using single factor ANOVA (calculated with Microsoft Excel version 2007) with p < 0.05 considered significant.
Results
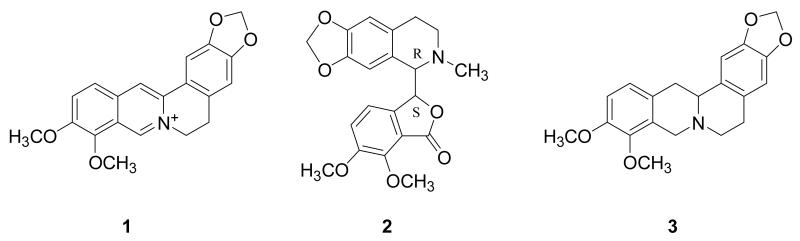
The alkaloids berberine, hydrastine, and canadine (Figure 1) were detected in all extracts, as confirmed based on matching retention times, molecular weights, and CID fragmentation patterns with standard compounds (Figure 2, Supporting Information). The configuration of hydrastine indicated in Figure 1 is that reported for the standard, but the configuration of the canadine standard was not confirmed. The isolation of a +/− mixture of canadine from H. canadensis has been previously reported [26], but it was not possible with the LC-MS method employed to distinguish stereoisomers of hydrastine or canadine.
Figure 1.
Structures of major alkaloid constituents of Hydrastis canadensis, berberine (1), (1R,9S)-(−)-β-hydrastine (2) and canadine (tetrahydroberberine) (3).
Alkaloids were found in higher concentrations in root than in leaf extracts (Table 1). This was true for each of the individual root and leaf samples, and average values among the 6 root and 6 aerial portion samples. For berberine, the root extracts contained an average of 5 times more berberine (36.45 ± 0.47 mM in roots versus 7.77 ± 0.10 mM in aerial portions, p = 0.002), 3 times more hydrastine (3.145 ± 0.058 mM in roots versus 1.123 ± 0.021 mM in aerial portions, p = 0.002), and 1.5 times more canadine (0.069± 0.011 mM in roots versus 0.045 ± 0.007 in aerial portions, p = 0.001) than did the aerial extracts. Consistent with previous reports [27], berberine was the most abundant of the alkaloids (an average of 6.12% in roots, 1.31% in aerial portions), followed by hydrastine (0.60% in roots, 0.22% in aerial portions) and, finally, canadine (0.11 % in roots, 0.008% in aerial portions).
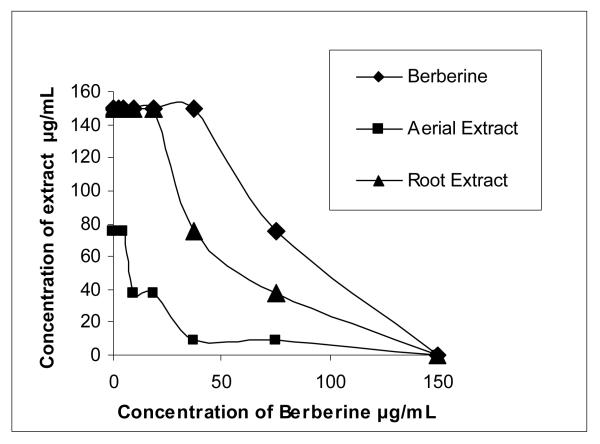
As indicated by the FIC value of 0.375 (Table 2) and by the convex shape of the isobologram (Figure 3), the extract prepared from a pooled sample of H. canadensis leaves synergistically enhanced the antimicrobial activity of berberine. A suggestion of synergistic enhancement by the root extract was observed as well (Figure 3), but the results did not comply with the stringent guidelines of an FIC < 0.5 indicating synergy [28]. For validation purposes, berberine was tested in combination with itself (Figure 3) and the effect was additive (FIC of 1.00, linear isobologram). The berberine standard also served as the positive control for the antimicrobial assays, and gave an MIC of 150 μg/mL, consistent with literature values [29-32].
Table 2.
MIC and FIC indices of Hydrastis canadensis extracts or isolated alkaloids in combination with berberine. MIC indicates the minimum inhibitory concentration of the compound alone, while FIC (calculated with equation 1), indicates the degree of interaction between the given compound or extract and berberine.
| MIC (μg/mL) | FIC Index | |
|---|---|---|
| Berberine | 150 | 1.00 |
| Hydrastine | >300 | 0 |
| Canadine | >300 | 0 |
| Root Extract | 150 | 0.750 |
| Aerial Extract | 75 | 0.375 |
Figure 3.
Isobolograms indicating inhibition of bacteria growth by H. canadensis extracts and added berberine. Berberine alone was also included in the assay for validation and to serve as a positive control. Extracts were dissolved in 2% DMSO in Müeller Hinton broth. Assays were performed in triplicate in a 96-well plate with 5 × 105 CFU/mL S. aureus.
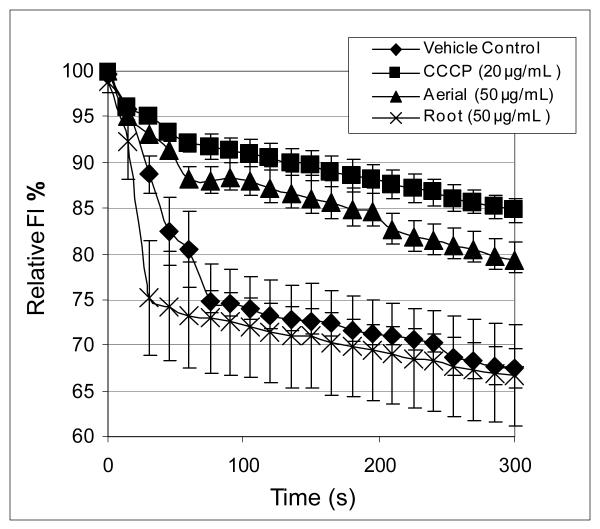
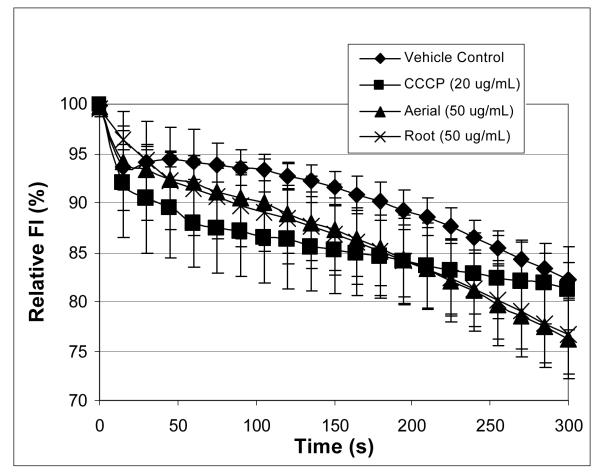
The extract prepared from pooled aerial portions of H. canadensis was also shown to inhibit efflux of ethidium bromide from wild type S. aureus (Figure 4), but not its norA-deleted derivative (Figure 5). For wild-type S. aureus, the percent of ethidum bromide fluorescence after 3 minutes compared to the initial reading for bacteria in broth alone was 66.7 % ± 5.5 % for the root extract (50 μg/mL), 79.2 % ± 1.2 % for the aerial extract (50 μg/mL), and 84.73 % ± 1.29 % for the positive control (CCCP at 20 μg/mL). The results for the CCCP and the aerial extract were significantly different from that of the negative control (ethidium bromide loaded bacteria in Müeller Hinton broth with 2% DMSO), with p values of 0.002 and 0.02, respectively. There was no significant difference in % fluorescence between the root extract and the negative control after 3 minutes. For the norA-deleted S. aureus, no significant inhibition of ethidium bromide efflux was observed for any of the treatments (H. canadensis aerial extract, H. canadensis root extract, or CCCP) after 3 min (Figure 5).
Figure 4.
Percent fluorescence over time for S. aureus (NCTC 8325-4) loaded with ethidium bromide and treated with various extracts and controls. Treatments included the known efflux pump inhibitor CCCP (positive control), and extracts from the roots and aerial portions of H. canadensis. All extracts and CCCP were dissolved in Müeller Hinton broth containing 2% DMSO. Data points represent the average of three separate experiments (using 3 different pellets of S. aureus). Error bars are +/− standard error.
Figure 5.
Percent fluorescence over time for the norA-deleted S. aureus (K1758). Aside from the use of a different strain of bacteria, conditions were identical to those employed for Figure 4.
Discussion
Goldenseal roots (rather than aerial portions) are more commonly employed medicinally because of their higher alkaloid content [27]. Our results do show higher alkaloid content in the root extracts, but we show that when the extracts are standardized to berberine content, extracts from the aerial portions of the plants synergize the antimicrobial activity of berberine (Figure 3). This finding indicates that some constituent(s) other than berberine in the extract from H. canadensis aerial portions synergistically enhance the antimicrobial activity of berberine. This synergistic enhancement is not due to hydrastine or canadine in the leaf extracts. Purified standards of these compounds exhibited no significant antimicrobial activity (MIC values of >300 μg/mL, Table 2), and did not enhance the antimicrobial activity of berberine (FIC = 0, Table 2).
On the basis of literature reporting the presence of efflux pump inhibitors in berberine containing plants [33], we hypothesized that the synergists present in the extracts of H. canadensis aerial portions might act as inhibitors of the norA efflux pump, the major chromosomal efflux pump expressed by S. aureus. Indeed, our data (Figures 4 and 5) show that extracts from H. canadensis aerial portions do contain norA efflux pump inhibitors. Ethidium bromide is a known substrate of norA, and fluoresces strongly (due to intercalation with DNA) when inside bacterial cells [33]. With the ethidium bromide efflux assay, cells are loaded with ethidium bromide and fluorescence is monitored over time. A decrease in fluorescence indicates efflux of ethidium bromide from the cells, and in the presence of an efflux pump inhibitor, fluorescence should decrease more slowly. This effect is observed with CCCP, a known efflux pump inhibitor, and with the extract prepared from aerial portions of H. canadensis (Figure 4). If inhibition of norA is responsible for the altered behavior of wild type S. aureus in the presence of CCCP and H. canadensis extracts, no effect should be observed with a norA-deleted strain. This, indeed, was the case (Figure 5). The efflux pump inhibitory activity observed was not due to the presence of berberine, hydrastine, or canadine, as evidenced by the fact that the root extract, which had higher alkaloid concentration, did not show activity in the assay (Figure 4). To verify this, the alkaloids were each tested individually, and showed no significant inhibition of ethidium bromide efflux (Figure 6, Supplemental Information).
Proponents of the use of botanical dietary supplements often argue that their complexity leads to enhanced efficacy due to synergistic interactions among multiple constituents. Here we demonstrate that extracts from the aerial portions of Hydrastis canadensis contain both the known antimicrobial agent berberine and other compounds (hitherto unidentified) that synergistically increase the antimicrobial activity of berberine. We also show that this enhancement of antimicrobial activity is likely to occur through inhibition of the norA efflux pump. This finding is particularly interesting given that Hydrastis canadensis is listed as endangered by CITES in much of its native habitat. Our results show that the aerial portions of this plant, which can be harvested sustainably, are a good source of antibacterial compounds. Furthermore, we demonstrate that berberine is effective in vitro at lower dosages when used in the complex phytochemical matrix of the plant aerial portions than in the root portions. Follow up studies to evaluate the toxicity of extracts prepared from roots versus aerial portions of H. canadensis and to test their antibacterial activity in vivo are warranted.
Supplementary Material
Acknowledgments
This publication was made possible by Grant Number 1 R15 AT005005-01 from the National Center for Complementary and Alternative Medicine (NCCAM), a component of the National Institutes of Health (NIH). We thank William Burch for providing goldenseal plant material for this study, and Dr. Robert Cannon, Dr. Joseph Falkinham and Dr. Nicholas Oberlies for helpful advice.
References
- 1.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1711. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 2.Spelman K, Duke JA, Bogenschutz-Godwin MJ. The synergy principle at work with plants, pathogens, insects, herbivores and humans. In: Cseke L, Kirakosyan A, Kaufman PB, et al., editors. Natural Products from Plants. 2nd edition CRC Taylor and Francis; Boca Raton: 2006. pp. 475–501. [Google Scholar]
- 3.Wagner H, Ulrich-Merzenich G. Synergy Research: Approaching a new generation of phytopharmaceuticals. Phytomed. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Bannerman JE. Goldenseal in world trade: Pressures and potentials. Herbalgram. 1997;2:51–53. [Google Scholar]
- 5.Sinclair A, Catling PM. Cultivating the increasingly popular medicinal plant, Goldenseal: review and update. Am J Alternative Agr. 2001;16:131–140. [Google Scholar]
- 6.Blumenthal M. Herb market levels after five years of boom. Herbalgram. 1999;47:64–65. [Google Scholar]
- 7.Hwang BY, Roberts SK, Chadwick L, Wu CD, Kinghorn DA. Antimicrobial constitutents from goldenseal (the rhizomes of Hydrastis canadensis) against selected oral pathogens. Planta Med. 2003;69:623–627. doi: 10.1055/s-2003-41115. [DOI] [PubMed] [Google Scholar]
- 8.Khosla PK, Neeraj VI, Gupta SK, Satpathy G. Berberine, a potential drug for trachoma. Rev Int Trach Pathol Ocul Trop Subtrop Sante Publique. 1992;69:147–165. [PubMed] [Google Scholar]
- 9.Knight SE. Goldenseal (Hydrastis canadensis) versus penicillin: a comparison of effects on Staphylococcus aureus, Streptococcus pyogenes, and Pseudomonas aeruginosa. Bios. 1999;70:3–10. [Google Scholar]
- 10.Mahady GB, Pendland SL, Stoia A, Chadwick L. In vitro suceptibility of Helicobacter pylori to isoquinoline alkaloids from Sanguinaria canadensis and Hydrastis canadensis. Phytother Res. 2003;17:217–221. doi: 10.1002/ptr.1108. [DOI] [PubMed] [Google Scholar]
- 11.Scazzocchino F, Cometa MF, Tomassini L, Palmery M. Antibacterial activity of Hydrastis canadensis extract and its major isolated alkaloids. Planta Med. 2001;67:561–564. doi: 10.1055/s-2001-16493. [DOI] [PubMed] [Google Scholar]
- 12.Chadwick LR, Wu CD, Kinghorn AD. Isolation of alkaloids from goldenseal (Hydrastis canadensis rhizomes) using pH-zone refining countercurrent chromatography. J Liq Chrom Rel Technol. 2001;24:2245–2453. [Google Scholar]
- 13.Villinski JR, Dumas ER, Chai HB, Pezzuto JM, Angerhofer CK, Gafner S. Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and Hydrastis canadensis. Pharm Biol. 2003;41:551–557. [Google Scholar]
- 14.Gentry EJ, Jampani HB, Keshavarz-Shokri A, Morton MD, Velde DV, Telikepalli H, Mitscher LA, Shawar R, Humble D, Baker W. Antibubercular natural products: Berberine from the roots of commercial Hydrastis canadensis powder. Isolation of inactive 8-oxotetrahydrothalifendine, canadine, β-hydrastine, and two new quinic acid esters, hycandinic acid esters-1 and 2. J Nat Prod. 1998;61:1187–1193. doi: 10.1021/np9701889. [DOI] [PubMed] [Google Scholar]
- 15.Nikaido H. Multiple antibiotic resistance and efflux. Curr Opin Microbiol. 1998;1:516–523. doi: 10.1016/s1369-5274(98)80083-0. [DOI] [PubMed] [Google Scholar]
- 16.Webber MA, Piddock LJV. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother. 2003;51:9–11. doi: 10.1093/jac/dkg050. [DOI] [PubMed] [Google Scholar]
- 17.Lewis K, Ausubel FM. Prospects for plant-derived antibacterials. Nature Biotechnol. 2006;24:1504–1507. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
- 18.Novick R. Properties of cryptic high-frequency transducing phage of Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 19.Price CTD, Kaatz GW, Gustafson JE. The multidrug efflux pump NorA is not required for salicylate induced reduction in drug accumulation by Staphylococcus aureus. Int J Antimicrob Agents. 2002;20:206–213. doi: 10.1016/s0924-8579(02)00162-0. [DOI] [PubMed] [Google Scholar]
- 20.Cech RA. Making Plant Medicine. Horizon Herbs; Williams: 2000. p. 276. [Google Scholar]
- 21.Approved Standard. Seventh Ed. Vol. 26. Clinical and Laboratory Standards Institute; 2006. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; pp. M7–A7. [Google Scholar]
- 22.Eliopolous GM, Moellering RC. Antimicrobial Combinations. In: Lorian V, editor. Antibiotics in Laboratory Medicine. Williams and Wilkins; Baltimore, MD: 1996. pp. 330–396. [Google Scholar]
- 23.Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate: antimicrobial activity, bioassay, and mode of action. Can J Microbiol. 1969;15:1067–1076. doi: 10.1139/m69-190. [DOI] [PubMed] [Google Scholar]
- 24.Hyeon-Hee Y, Kang-Ju K, Jeong-Dan C, Hae-Kyong K, Young-Eun L, Na-Young C, Yong-Ouk Y. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8:454–461. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 25.Scazzocchio F, Cometa MF, Tomassini L, Palmery M. Antibacterial activity of Hydrastis canadensis extract and its major isolated alkaloids. Planta Med. 2001;67:561–56. doi: 10.1055/s-2001-16493. [DOI] [PubMed] [Google Scholar]
- 26.Monkovic I, Spenser ID. Biosynthesis of Berberine and Berverastine. Can J Chem. 1965;43:2017–2026. [Google Scholar]
- 27.Upton R. Goldenseal root Hydrastis canadensis: Standards of analysis, quality control, and therapeutics. American Herbal Pharmacopoeia; Santa Cruz: 2001. [Google Scholar]
- 28.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1–1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 29.Stermitz FR, Tawara-Matsuda J, Lorenz P, Mueller P, Zenewicz LA, Lewis K. 5′Methoxyhydnocarpin-D and phenophorbide A: Berberis species components that potentiate berberine growth inhibition of resistant Staphylococcus aureus. J Nat Prod. 2000;63:1146–1149. doi: 10.1021/np990639k. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Yu L, Xiang H, Fan J, He L, Guo N, Feng H, Deng X. Global transcriptional profiles of Staphylococcus aureus treated with berberine chloride. FEMS Microbiol Lett. 2008;279:217–225. doi: 10.1111/j.1574-6968.2007.01031.x. [DOI] [PubMed] [Google Scholar]
- 31.Yu H-H, Kim K-J, Cha J-D, Kim H-K, Lee Y-E, Choi N-Y, You Y-O. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8:454–461. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 32.Tegos G, Stermitz FR, Lomovskaya O, Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob Agents Chemother. 2002;46:3133–3141. doi: 10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Nat Acad Sci. 2000;97:1433–1437. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.