Abstract
Gestational alcohol exposure leads to a spectrum of neurological symptoms which range from severe mental retardation caused by high dose exposure, to subtle cognitive and neuropsychiatric symptoms caused by low-to-moderate doses. We and other investigators have demonstrated that exposure to moderate levels of alcohol throughout gestation leads to impaired neurogenesis in the adult hippocampus, although the mechanisms by which this occurs are not known. To begin to distinguish cell-intrinsic from microenvironmental contributions to impaired adult neurogenesis, we isolated neural stem progenitor cells (NSPCs) from the adult SVZ of mice exposed to moderate levels of alcohol throughout gestation. We found that NSPCs isolated from fetal alcohol exposed (FAE) mice displayed reduced neurosphere formation in culture, as assessed by a serial passage neurosphere assay, and reduced neuronal differentiation upon growth factor withdrawal. These studies suggest that gestational alcohol exposure leads to long-lasting NSPC-intrinsic dysregulation, which may underlie in vivo neurogenic deficits.
Alcohol is a potent teratogen that targets the developing CNS, resulting in long-lasting disabilities that can persist into adulthood. Heavy or binge patterns of drinking during pregnancy lead to fetal alcohol syndrome (FAS), which is associated with severe structural defects in brain development and mental retardation (Clarren and Smith, 1978, Lemoine, et al., 1968). In contrast, low or moderate levels of alcohol consumption during pregnancy lead to more subtle mental disabilities without overt structural changes in the CNS. These include deficits in learning and memory (Willford, et al., 2006, Willford, et al., 2004), maladaptive behavior (Streissguth, et al., 1994), and mental illness such as depression and antisocial disorder (Famy, et al., 1998). Fetal alcohol spectrum disorder (FASD) encompasses the range of clinical disabilities that result from gestational alcohol exposure (Sokol, et al., 2003), with an estimated prevalence in children of 2–5% in the United States (May, et al., 2009).
Adult neurogenesis represents a novel form of CNS plasticity associated with both cognitive and neuropsychiatric function (DeCarolis and Eisch, 2010, Leuner and Gould, 2010). It is well-established that embryonic neurogenesis is a target of alcohol toxicity during fetal development (Goodlett, et al., 2005, Miller, 1986). Acute alcohol exposure during adolescence or adulthood has also been shown to impair neurogenesis (Nixon, et al., 2010). Furthermore, several studies have now demonstrated that gestational or early postnatal alcohol exposure can result in long-lasting deficits in neurogenesis that persist within the adult brain long after cessation of gestational exposure (Choi, et al., 2005, Gil-Mohapel, et al., 2010, Ieraci and Herrera, 2007, Klintsova, et al., 2007, Redila, et al., 2006, Sliwowska, et al., 2010, Uban, et al., 2010). There is growing interest in this area of research, since impaired adult neurogenesis may partially underlie cognitive and neuropsychiatric aspects of FASD. If so, therapeutic stimulation of adult neurogenesis could potentially reverse these deficits.
Adult neurogenesis is regulated by both the intrinsic neurogenic potential of NSPCs and by microenvironmental cues within neurogenic niches of the adult brain. NSPC-intrinsic regulators include cell surface receptors and intracellular signaling cascades (Johnson, et al., 2009). Microenvironmental extrinsic regulators include cytokines, growth factors, hormones, extracellular matrix and cell-cell interactions within the neurogenic niche (Ables, et al., 2010, Alvarez-Buylla and Lim, 2004, Gao, et al., 2009, Kokovay, et al., 2010). To begin to distinguish NSPC vs. microenvironmental factors that might underlie the negative impact of prenatal alcohol exposure on adult neurogenesis, we studied the neurogenic potential of NSPCs in culture following isolation from adult brain of FAE mice. Our results suggest that previously reported in vivo deficits in adult neurogenesis following gestational alcohol exposure may be due, in part, to cell-intrinsic dysregulation of NSPC function that persists into adulthood.
Methods
Gestational Alcohol exposure
For these studies, FAE and saccharine control mice were generated as previously described (Allan, et al., 2003). Briefly, female C57Bl6 female mice were individually housed and offered 22 hr free access to either 0.066% saccharine or water for two weeks. Ethanol was then added to the saccharine tube for the experimental group, whereas the control group continued to drink saccharine alone. The concentration of ethanol was gradually increased over four days to reach the final concentration of 10% (w/v). After two weeks of 10% ethanol, a male was introduced into the cage and remained until the female was determined to be pregnant. Mice consumed an average of 8.0 ± 2.5 g alcohol/kg/day during pregnancy, which generated a mean blood alcohol concentration of 84 ± 4.5 mg/dl (n=6.) in a separate cohort of similarly drinking mice, as assessed using methods described in Allan et al., (2003). Following birth, ethanol concentration was gradually lowered from 10% to 0% over a period of four days as previously described (Allan et al., 2003). This drinking paradigm has been demonstrated not to affect litter size or maternal care (Allan et al., 2003). At weaning, offspring were gender separated and standard housed in groups of 2–3 until sacrificed at 1–2 months for neural stem cell isolation as described below. All mice were maintained in 12h: 12h reverse light: dark with food and water available ad libitum. Animal procedures were approved by the University of New Mexico Animal Care and Use Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
NSPC isolation
NSPCs were isolated from microdissected subventricular zone of one month old FAE or saccharine (control) offspring, as previously described (Harms, et al., 2010). Both male and female offspring were used for these studies. Briefly, mice were overdosed with fluorothane, and brains were quickly removed and sliced in 600 μM coronal sections. The SVZ regions were microdissected with the aid of a microscope and pooled across 4–5 mice per litter, enzymatically dissociated using MACS Neural tissue dissociation kit (Miltenyi Biotec), as adopted from (Smrt, et al., 2007) and expanded for 7–10 days in defined serum-free DMEM/F12 medium containing 15 mM HEPES, 2.5 mM L-glutamine (Gibco), 3 mM sodium bicarbonate, 25 μg/ml insulin, 16 μg/ml putrescine, 30 nM sodium selenite, 100 μg/ml apo-transferrin and 20 μM progesterone plus 10 ng/ml EGF and 10 ng/ml bFGF (Invitrogen) (Rietze and Reynolds, 2006). NSPCs were expanded as non-adherent culture in uncoated tissue culture dishes for neurosphere assay, or as adherent cell culture on poly-L-lysine-coated plates for assessing neuronal differentiation.
Neurosphere assay
Neurosphere assays were performed as described in (Groszer, et al., 2006). Isolated SVZ-NSPCs were resuspended in serum-free DMEM/F12 medium containing 10 ng/ml EGF and 10 ng/ml bFGF, plated on uncoated 6-well cell culture dishes, and fresh growth factors added every three days. Primary neurospheres (passage 0; P0) that formed within 1 week of cell culture were collected, enzymatically dissociated and re-plated onto uncoated 6-well dishes at a density of 8–10 cells/μl. The neurospheres were passaged every seven days, using this method. Beginning at passage 2, neurospheres from 3 wells of the 6-well dish were collected and used for quantification, by placing them in a poly-L-lysine-coated 12 well culture dish that contained a counting grid on the bottom surface. The total number of neurospheres per well was determined within 20 min using a phase contrast microscope. Neurosphere number was averaged across 10 wells. Only neurospheres <50 μm diameter were counted.
NSPC differentiation
To induce NSPC differentiation, the cells were plated onto poly-L-lysine coated coverslips and grown in DMEM/F12 medium with supplements as described above, but lacking EGF or FGF. After seven days in growth factor free media, the NSPCs were prepared for immunocytochemical analysis to assess phenotypic fate as previously described, (Roitbak, et al., 2008). The following primary antibodies were used to identify astrocytes, neuroblasts, and immature neurons, respectively: mouse monoclonal anti-GFAP (1:1000, Accurate Chem. & Sci. Corp.), goat polyclonal anti-doublecortin (1:300, Santa Cruz Biotech), and mouse monoclonal anti Tuj-1 (1:300, Promega, Madison, WI). Secondary antibodies were used at a common dilution of 1:200, and included FITC-conjugated anti-mouse and Cy3-conjugated anti-goat (Jackson ImmunoResearch Laboratories). DAPI staining was used for nuclei. Samples were imaged on a Zeiss LSM510-META confocal imaging system. To quantify the percent neuronal and glial differentiation, 10 images per coverslips (6–10 coverslips/group/experiment) were visualized using only the DAPI channel (UV Diod filter) and randomly chosen for laser confocal analysis. Images were acquired at 40X magnification. The percentage of DAPI+ cells that expressed Dcx or GFAP was determined by a separate observer blinded to treatment group using LSM Image Browser software.
All quantitative data represent means ± S.E.M across 3–5 separate experiments (n=3–5, as indicated in figure legend), utilizing a separate litter for each experiment. . Data were subjected to statistical analysis using ANOVA and post-hoc analyses as indicated, , with p <0.05 considered significant.
Results
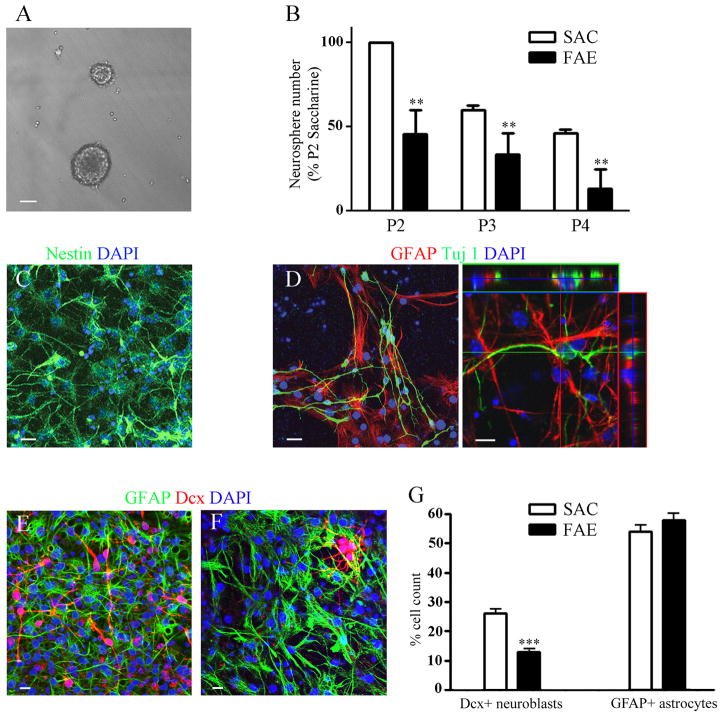
As demonstrated in Figure 1B, gestational exposure to alcohol reduced neurosphere-forming capacity of postnatal NSPCs, as indicated by fewer neurospheres over serial passage at clonal density. NSPCs isolated from FAE mice generated approximately 50% fewer neurospheres at every passage, compared to NSPCs isolated from saccharine controls. Statistical analysis revealed a significant passage effect [(F(1,8) = 21.3, p=0.002], a significant effect of prenatal exposure [F(2,8) = 10.2, p=0.006], and no treatment interaction.
Figure 1. NSPCs derived from SVZ of postnatal FAE mice display less neurosphere-forming capacity and impaired neurogenesis in vitro.
A) Phase contrast image of neurospheres cultured from NSPCs of saccharine control mice in serum-free medium with EGF/FGF. Bar = 50 μm. B) Serial passage neurosphere formation from NSPCs isolated from SVZ of postnatal day 30 FAE or Sac control mice. Data represent the average number of neurospheres per well ± S.E.M., n=3 separate experiments (litters) with 10–12 replicates per passage for each experiment. Two way ANOVA revealed significant passage and FAE effects, as described in text, **p<0.01. C) NSPCs from saccharine control mice expanded as adherent monolayer cultures in the presence of EGF/FGF and immunostained for nestin (green) and counterstained with DAPI nuclear dye (blue). D) NSPCs expanded as monolayer cultures and differentiated by seven days of growth factor withdrawal gave rise to both Tuj1(green) and GFAP (red)immunopositive cells. Cultures are counterstained with DAPI nuclear dye (blue). Orthogonal confocal image is shown on right panel. E and F) Representative NSPC cultures isolated from saccharine (E) or FAE (F) mice following seven days of differentiation and immunostained for GFAP (green) and Dcx (red). Cultures were counterstained with DAPI nuclear dye (blue). Bars on C–F = 10 μm. G) Percentage of saccharine NSPCs (open bars) or FAE NSPCs (filled bars) that express Dcx or GFAP after seven days of in vitro differentiation. Data are expressed as mean percentage of total nuclei expressing each marker ± S.E.M., *** p<0.001, n=3–5 experiments per group, 10–12 coverslips per treatment for each experiment. For each experiment 4 mice were pooled from the same litter.
To determine whether prenatal alcohol exposure impairs neuronal differentiation, NSPCs were grown as monolayer cultures in the presence or absence of EGF and FGF for seven days. As shown in Figure 1C, undifferentiated NSPCs grown in the presence of growth factors were uniformly immunopositive for the neural stem cell marker, nestin. Growth factor withdrawal resulted in spontaneous differentiation into both neuronal and glial lineages within seven days, with the appearance of doublecortin (Dcx+) neuroblasts (Figure 1E), βIII-tubulin (Tuj1+) immature neurons (Figure 1D) and GFAP+ astrocytes (Figure 1D, E). Importantly, the percent neuronal differentiation was decreased by ~50% in NSPC cultures derived from FAE vs. saccharine control mice (Figure 1E–F). Although the percent astrocyte differentiation was slightly increased in FAE vs. saccharine control NSPC cultures, this did not reach statistical significance.
Discussion
Our finding that FAE-NSPCs display reduced neurosphere-forming capacity over serial passage could be explained by fewer total NSPCs at initial plating and/or more limited ability of FAE-NSPCs to undergo self-renewal over time. Although our previous study suggested that FAE mice have normal numbers of proliferating NSPCs in hippocampus (Choi, et al., 2005), recent studies have shown that prenatal alcohol exposure leads to lower numbers of slowly proliferating primitive neural stem cells, as assessed by quantitative analysis of prominin+ cells in neural stem cell isolates from adult brain (Singh, et al., 2009). Neurosphere assays have previously been criticized for unfaithful readout of absolute neural stem cell number, since neurospheres contain cells at various stages of differentiation, including stem cells, proliferating neural progenitor cells and postmitotic glia (Jensen and Parmar, 2006; Reynolds and Rietze, 2005). Nevertheless, serial passage analysis is useful for determining the effects of treatments or genotypes on self-renewal capability of neural stem cells, albeit less useful for enumerating absolute stem cell number (Groszer, et al., 2006, Louis, et al., 2008).
Reduced neurosphere forming capacity by FAE-NSPCs was accompanied by an approximate 50% reduction in neuronal differentiation upon growth factor withdrawal. A recent study by Singh et al., (2009), suggests that prenatal alcohol may shift differentiation potential toward gliogenesis. On the other hand, Urban et al., (2010), recently reported that both adult hippocampal neurogenesis and gliogenesis are reduced in mice exposed to alcohol throughout gestation.
Alcohol’s impact on embryonic neurogenesis has been well documented; however, the long-term impact of prenatal or early postnatal alcohol exposure on adult neurogenesis has only recently begun to be explored. Despite broad variations in alcohol dose, timing, mode of alcohol administration, species, in vivo labeling methods and environmental parameters, most studies consistently demonstrate a suppression of adult hippocampal neurogenesis following embryonic or early postnatal (third trimester equivalent) exposures in rodents (Choi, et al., 2008, Ieraci and Herrera, 2007, Klintsova, et al., 2007, Redila, et al., 2006, Sliwowska, et al., 2010, Uban, et al., 2010). For example, binge-like exposures in rat pups resulted in an 18% decrease in survival of newly generated dentate granule cells at postnatal day 50 (Klintsova et al., 2007), and a single high dose of alcohol at postnatal day 7 resulted in decreased proliferation of adult neural stem cells and impaired survival of newly born neurons in adult rat (Ieraci and Herrera, 2007). Using our mouse model of moderate gestational alcohol, we found no impairment of adult hippocampal neurogenesis under standard housing conditions, but complete impairment of the neurogenic response to enriched environment (Choi et al., 2005). Interestingly, Sliwowska et al; 2010, recently reported that gestational alcohol exposure attenuates stress-induced suppression of neurogenesis.
The underlying mechanisms by which gestational exposure to moderate levels of alcohol can lead to long-lasting effects on neurogenesis in adult brain are currently unknown, but may involve epigenetic programming events. Exposure to ethanol in utero stimulates global DNA methylation changes in embryos (Garro et al., 1991) and alters expression of the epigenetically-sensitive Agouti allele in adults (Kaminen-Ahola et al., 2010). Acute exposure of NSPCs to ethanol in culture induces hypermethylation of growth factor and cell cycle regulatory genes in cultured NSPCs (Hicks et al., 2010) and changes in microRNA expression that are associated with reduced neuronal differentiation (Miranda et al., 2010). Such changes in the epigenotype of the neural stem lineage during development could conceivably persist into adulthood and affect intrinsic properties of self-renewal and phenotypic fate in response to environmental cues. It is also important to note that prenatal alcohol exposure can also result in dysregulation of the hypothalamic-pituitary-adrenal axis (HPA) throughout life. HPA dysregulation can involve increased HPA tone and over-production of corticosterone (Hellemans et al., 2010), an adrenal steroid that has suppressive effects on adult hippocampal neurogenesis (Schoenfeld and Gould, 2011).
Overall, our findings suggest that previously reported in vivo deficits in adult neurogenesis following gestational alcohol exposure may be due, in part, to intrinsic dysregulation of NSPC function that persists into early adulthood. Future studies aimed at characterizing the mechanism of this effect may lead to therapeutic targeting of adult neurogenesis to ameliorate cognitive and emotional aspects of FASD symptoms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan AM, Chynoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcohol Clin Exp Res. 2003;27:2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 4.Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcohol Clin Exp Res. 2005;29:2053–2062. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- 5.Choi SH, Veeraraghavalu K, Lazarov O, Marler S, Ransohoff RM, Ramirez JM, Sisodia SS. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59:568–580. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarren SK, Smith DW. The fetal alcohol syndrome. Lamp. 1978;35:4–7. [PubMed] [Google Scholar]
- 7.DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry. 1998;155:552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- 9.Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev. 2010;64:283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- 12.Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci U S A. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harms KM, Li L, Cunningham LA. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling. PLoS One. 2010;5:e9767. doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ieraci A, Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiol Dis. 2007;26:597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MA, Ables JL, Eisch AJ. Cell-intrinsic signals that regulate adult neurogenesis in vivo: insights from inducible approaches. BMB Rep. 2009;42:245–259. doi: 10.5483/bmbrep.2009.42.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res. 2007;31:2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 18.Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemoine P, Haronsseau H, Borteryu PP, Menuet JC. Les enfants de parents alcooliques: anomalies observees a propos de 127 cas. Quest Med. 1968;25:476–482. [Google Scholar]
- 20.Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;61:111–140. C111–113. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis SA, Rietze RL, Deleyrolle L, Wagey RE, Thomas TE, Eaves AC, Reynolds BA. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 2008;26:988–996. doi: 10.1634/stemcells.2007-0867. [DOI] [PubMed] [Google Scholar]
- 22.May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- 23.Miller MW. Effects of alcohol on the generation and migration of cerebral cortical neurons. Science. 1986;233:1308–1311. doi: 10.1126/science.3749878. [DOI] [PubMed] [Google Scholar]
- 24.Nixon K, Morris SA, Liput DJ, Kelso ML. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol. 2010;44:39–56. doi: 10.1016/j.alcohol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16:305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- 26.Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods Enzymol. 2006;419:3–23. doi: 10.1016/S0076-6879(06)19001-1. [DOI] [PubMed] [Google Scholar]
- 27.Roitbak T, Li L, Cunningham LA. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling. J Cereb Blood Flow Metab. 2008;28:1530–1542. doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoenfeld T, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol Epub ahead of print. 2011 Jan 31; doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh AK, Gupta S, Jiang Y, Younus M, Ramzan M. In vitro neurogenesis from neural progenitor cells isolated from the hippocampus region of the brain of adult rats exposed to ethanol during early development through their alcohol-drinking mothers. Alcohol Alcohol. 2009;44:185–198. doi: 10.1093/alcalc/agn109. [DOI] [PubMed] [Google Scholar]
- 30.Sliwowska JH, Barker JM, Barha CK, Lan N, Weinberg J, Galea LA. Stress-induced suppression of hippocampal neurogenesis in adult male rats is altered by prenatal ethanol exposure. Stress. 2010;13:301–313. doi: 10.3109/10253890903531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smrt RD, Eaves-Egenes J, Barkho BZ, Santistevan NJ, Zhao C, Aimone JB, Gage FH, Zhao X. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. Jama. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- 33.Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, Feldman J, Mirsky AF. Maternal drinking during pregnancy: attention and short-term memory in 14-year-old offspring--a longitudinal prospective study. Alcohol Clin Exp Res. 1994;18:202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 34.Uban KA, Sliwowska JH, Lieblich S, Ellis LA, Yu WK, Weinberg J, Galea LA. Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res. 2006;30:1051–1059. doi: 10.1111/j.1530-0277.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 36.Willford JA, Richardson GA, Leech SL, Day NL. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28:497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]