Abstract
Background
Endothelial barrier dysfunction (EBD) involves microtubule disassembly and enhanced cell contractility. Histone deacetylase 6 (HDAC6) deacetylates α-tubulin, and thereby destabilizes microtubules. This study investigates a role for HDAC6 in EBD.
Methods
EBD was induced with thrombin±HDAC6 inhibitors (tubacin and MC1575), and assessed by transendothelial electrical resistance (TEER). Markers for microtubule disassembly (α-tubulin and acetylated α-tubulin) and contraction (phosporylated myosin light chain 2, P-MLC2) were measured using immunoblots and immunofluorescence.
Results and Conclusion
Thrombin induced a ~50% decrease in TEER that was abrogated by the HDAC6 inhibitors. Moreover, inhibition of HDAC6 diminished edema in the lung injured by lipopolysacchride. Lastly, inhibition of HDAC6 attenuated thrombin- induced microtubule disassembly and P-MLC2. Our results suggest that HDAC6 can be targeted to limit EBD.
Introduction
Endothelial barrier dysfunction (EBD) is a vascular reaction to inflammation, which leads to increased blood vessel permeability and contributes to the pathogenesis of a variety of life-threatening disorders, such as acute respiratory distress syndrome. Understanding the mechanisms that mediate EBD is vital for developing more effective treatments of critical conditions involving the vasculature. Microtubule disassembly is a pivotal module of the complex signaling network that orchestrates EBD [1]. Modulators of inflammation, such as TNF-α, TGF-β, and thrombin, disrupt endothelial barrier function via stimulating disassembly of microtubules and reorganization of cytoskeleton [2; 3; 4]. Nocodazole, a microtubule-destabilizing agent, is sufficient to induce EBD in pulmonary endothelial cells [5]. Moreover, microtubule disassembly mediates phosphorylation of myosin light chain 2 (MLC2), which is critical for contraction of endothelial cells [6]. Thus, microtubule disassembly emerges as an appealing therapeutic target for treatment of acute lung inflammation. Indeed, intravenous administration of a microtubule stabilizing agent, taxol, ameliorates EBD and inflammation in lipopolysacchride (LPS)-induced lung injury [7].
Histone deacetylases (HDAC) remove acetyl moieties from acetylated lysine residues, a post-translational modification that regulates fundamental cellular processes [8]. Four HDAC classes have been identified. HDAC6 belongs to class II and deacetylates mainly cytoplasmic proteins, e.g., α-tubulin [9]. A role for HDAC6 in endothelial barrier is implicated by the following findings: 1) HDAC6-mediated deacetylation of α-tubulin promotes microtubule disassembly [9]; 2) Rho, a signaling intermediate between microtubule and the actin skeleton, induces HDAC6-dependent deacetylation of α-tubulin in osteoclasts when activated [10]; 3) HDAC6 is required for TGF-β1-induced formation of stress fibers [11]. Therefore, the current study investigates whether inhibition of HDAC6 can attenuate EBD induced by thrombin.
Materials and Methods
Reagents
Tubacin, an HDAC6-specific inhibitor was kindly provided by Drs. S. L. Schreiber and Ralph Mazitschek at the Broad Institute of Harvard and MIT [12]. Thrombin and LPS (E. coli 055:B5) were purchased from Sigma (St. Louis, MO). Tautomycin, a specific inhibitor of protein phosphatases 1 & 2, was purchased from Calbiochem (La Jolla, CA). MC1575, a class II HDAC selective inhibitor, was synthesized as previously described [13].
Cell Culture and Treatments
Primary human pulmonary arterial endothelial cells (HPAEC) were purchased from Lonza (Basel, Switzerland) and cultured for experiments until passage six as we previously described [14]. Thrombin (50 nM) was used to induce EBD. In the selected exposures, HPAECs were pre-treated with the HDAC6 inhibitors tubacin (5 μM) and MC1575 (10 μM) for 6 hrs. Tautomycin (10 nM) was added to the culture 1 hr prior to treatment with thrombin+tubacin when indicated.
Transendothelial Electrical Resistance Assay
Endothelial barrier was assessed using transendothelial electrical resistance assays (TEER) as described elsewhere [2; 3; 4]. TEER was measured every 30 min through 2 hrs post exposure to thrombin. The results are presented as the means and standard deviations obtained from at least 8 data points from 2 independent experiments.
Cell Fractionation and Immunoblots
Polymerized (for measurement of microtubules) and non-polymerized α-tubulins were fractionated as described elsewhere [15]. Immunoblots for total and fractionated proteins were carried out as previously described [16]. The following primary antibodies were used for immunoblots: rabbit polyclonal antibodies specific for α-tubulin and diphospho-MLC2 (CS-2144 and CS-3674, Cell Signaling Technology, Danvers MA); and a mouse monoclonal antibody specific for acetylated α-tubulin (Ac-α-tubulin) (6-11B-1, Sigma). The secondary antibodies conjugated with IRDye800 were detected using an Odyssey Infrared Imaging System (LiCor Biosciences, Lincoln NB). The blots shown in the figures were representatives from at least two independent experiments.
Immunofluorescence and Image Analysis
Ac-α-tubulin positive microtubules were assessed using immunofluorescence and compared across the treated groups as described elsewhere with minor modifications [2; 3; 4]. Briefly, the images were captured in monochrome at 400 x using an Olympus epifluorescence microscope. The raw images were processed with background subtraction, digital deconvolution, and particle analysis for Ac-α-tubulin using identical parameters across the groups using MBF Image J (a collection of plug-ins and macros of image J that are collated and organised by the McMaster Biophotonics Facility). The percentage of area covered by microtubules within a single cell was determined by comparing the values of the area covered by Ac-α-tubulin positive microtubules (gray) with uncovered areas (black). Eight random fields (~15 cells/field) from 2 independent experiments were included in the statistical analysis.
Assessment of LPS-induced Edema in the Lung
C57BL/6 mice (10-week old, Charles River, Wilmington MA) were exposed to LPS as described elsewhere with minor modifications [17]. Briefly, LPS (2 mg/kg) was administered i.p. on Day 0. Tubacin (40 mg/kg) was administered i.p. daily starting two days prior to LPS exposure until sacrifice of the mice on Day 2 post-exposure to LPS. The vehicle groups received PBS or DMSO in place of LPS or tubacin. Edema in the lung was assessed by measuring wet/dry weight ratios as described elsewhere [17]. Total protein was extracted from the exposed lungs to assess the levels of Ac-α-tubulin. The animal protocol was approved by the Tulane University Institutional Animal Care and Use Committee.
Statistical Analysis
Means and standard error were obtained from at least 3 independent experiments. A P value < 0.05 as determined via an unpaired two-tailed Student’s T-test was considered significant (GraphPad Prism, Version 5).
Results
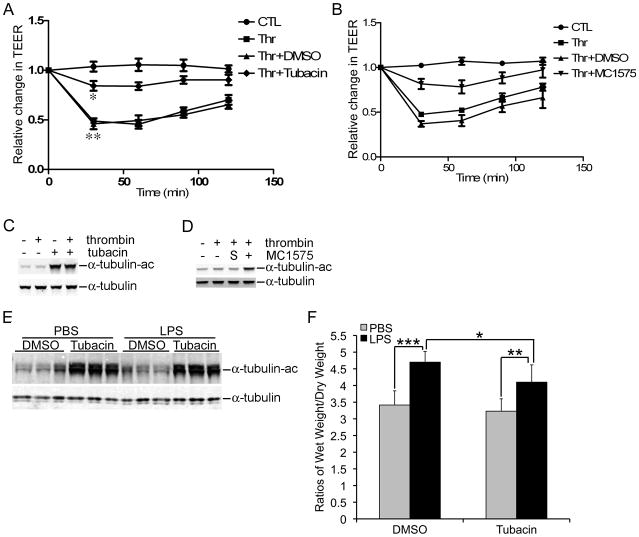
Confluent monolayer of HPAECs were treated with thrombin, one of the most potent inducers of EBD [18], in the presence or absence of tubacin, an HDAC6 specific inhibitor. The integrity of the monolayer was assessed using TEER assays. Thrombin (50 nM) caused a ~50% reduction in TEER as early as 30 min post-exposure, which was sustained through 2 hrs (Fig 1A, P < 0.01). Pretreatment of HPAECs for 6 hrs with tubacin (5 μM), restored TEER to approximately 85% of the control (Fig 1A, P < 0.05). Similar to tubacin, MC1575 (20 μM), a class II HDAC selective inhibitor, attenuated thrombin-induced EBD (Fig 1B). Concurrently, tubacin and MC1575 increased the protein levels of Ac-α-tubulin in HPAECs (Fig 1, C & D). Unlike a previous report regarding TGF-β1-induced EBD, thrombin-induced EBD was not accompanied by a substantial decrease in Ac-α-tubulin [4].
Figure 1.
Attenuation of Thrombin-induced Decreases in TEER by Inhibition of HDAC6. A) HPAECs were exposed to thrombin in the presence or absence of tubacin. TEER was measured at the indicated time points post-exposure. **, a comparison between the control and the thrombin treated groups; *, a comparison between the thrombin+DMSO and the thrombin+tubacin groups. B) An experiment that is similar to part A except that MC1575 replaced tubacin. C) The culture conditions were identical to A. Protein levels of Ac-α-tubulin and α-tubulin were determined using immunoblots. D) An experiment that is similar to part A except that MC1575 replaced tubacin. “S” refers to DMSO, the solvent for MC1575. E) Total protein was extracted from mouse lungs exposed to LPS with or without tubacin. The protein levels of Ac-α-tubulin and α-tubulin were determined. F) LPS-exposed mouse lungs were dissected and wet/dry weight ratios were measured as described in the Methods Section. Means and standard errors were obtained from 7 mice per group. *, **, and *** denote a P value < 0.05, 0.01, and 0.001, respectively.
In a well-characterized murine model of acute lung injury, tubacin ameliorated LPS- induced edema in the lung. The LPS-induced increase in the lung wet/dry weight ratio (4.7 in LPS vs 3.4 in PBS) was significantly reduced by tubacin (4.1 in LPS+tubacin) (Fig. 1F). As expected, tubacin caused a robust increase in Ac-α-tubulin in the lung without any appreciable alteration in the quantity of α-tubulin (Fig. 1E).
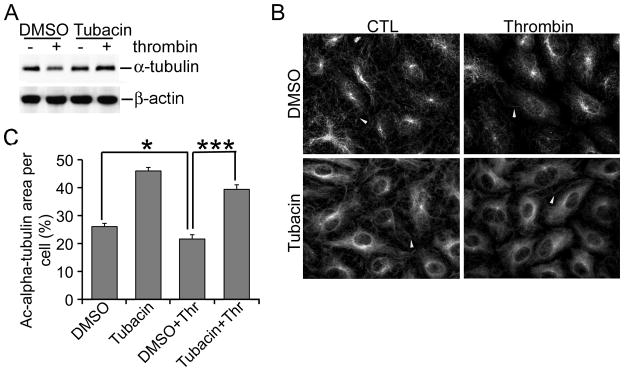
Disassembly of microtubules has been reported to mediate EBD [2; 4]. Therefore, we investigated whether inhibition of HDAC6 stabilized microtubules. To this end, we examined the amount of polymerized α-tubulin in thrombin-induced EBD with or without inhibition of HDAC6. We employed a previously published fractionation method that recovers the polymerized α-tubulin in the insoluble fraction after cell lysis [15]. The non-polymerized α-tubulin can only be recovered efficiently from the soluble fraction if cells are pretreated with a microtubule stabilizing agent, which is not suitable for our experiments since microtubule stabilizing agents alone are sufficient to inhibit EBD [7]. In contrast to a minimal change in α-tubulin and Ac-α-tubulin in the total cell lysates (Fig. 1, C & D), HPAECs exhibited a noticeable decrease in α-tubulin at 30 min post-exposure to thrombin in the polymerized fraction (Fig. 2A). The thrombin-induced decrease in α-tubulin was completely reversed by tubacin. In accordance, thrombin caused a modest, yet statistically significant decrease in the percentage of cell area covered by Ac-α-tubulin, from 26% in the control group to 21% in the thrombin treated group (Fig. 2, B & C, P < 0.05). Tubacin abrogated the thrombin-induced decrease in area coverage as 39% of a cells’ area was covered by Ac-α-tubulin in the presence of thrombin (Fig. 2, B & C, P < 0.001). Tubacin alone also increased Ac-α-tubulin coverage to 46%. Consistently, total intensity of Ac-α-tubulin per cell was significantly reduced by thrombin (18,460±1186 in DMSO vs 14,660±459.5 in thrombin, data not shown) and this reduction was completely blocked by tubacin (25,010±1236 in thrombin+tubacin, data not shown). These results suggest that HDAC6 inhibition antagonizes thrombin-induced microtubule disassembly.
Figure 2.
Tubacin Interferes with Thrombin-induced Microtubule Disassembly. A) Culture conditions were identical to Fig. 1. Protein levels of polymerized α-tubulin and βactin were measured using immunoblots. B) Similar to part A except that microtubules were assessed using immunofluorescence for Ac-α-tubulin. White arrowheads indicate microtubules in the periphery of a cell. C) The percentage of Ac-α-tubulin covered area per cell was determined as described in Methods. * and *** denote a P value < 0.05 and 0.001, respectively.
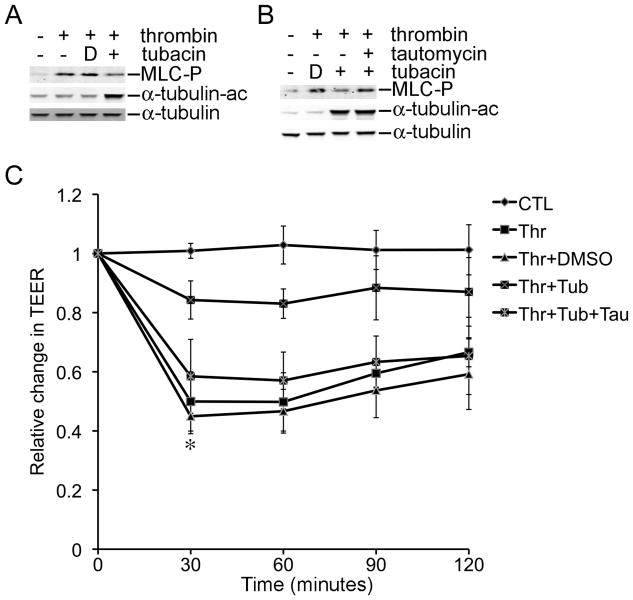
Phosphorylation of MLC2 is another important event that is promoted by microtubule disassembly during EBD [6]. Thus, we investigated whether inhibition of HDAC6 reduced phosphorylation of MLC2 in EBD. As expected, thrombin increased phosphorylation of MLC2 in HPAECs and this increase was nearly eliminated by tubacin and by MC1575 (Fig. 3A and data not shown). Moreover, co-treatment of the cells with tautomycin (10 nM), an inhibitor of protein phosphatases 1 & 2, restored thrombin-induced phosphorylation of MLC2 in the presence of tubacin (Fig 3B). Lastly, tautomycin restored thrombin-induced EBD in the presence of tubacin (Fig. 3C, P < 0.01, thrombin+tubacin vs thrombin+tubacin+tautomycin). TEER in HPAECs exposed to thrombin+tubacin+tautomycin was indistinguishable from that of HPAECs exposed to thrombin alone. In addition, tautomycin treatment alone did not alter EBD (data not shown). These results suggest that HDAC6 inhibition interferes with the activation of the lung endothelial cell contraction machinery, a pivotal component of EBD.
Figure 3.
Rescue of Thrombin-induced EBD by Tautomycin in the Presence of Tubacin. A) The protein levels of phosphorylated MLC2 were measured in HPAECs at 30 min post-exposure to thrombin±Tubacin. B) Similar to part A except that HPAECs were pretreated with tautomycin for 1 hr as indicated. C) TEER was measured in HPAECs exposed to various combinations of thrombin, Tubacin, and tautomycin. Thr, thrombin; Tub, Tubacin; Tau, tautomycin; MLC-P, phosphorylated MLC2; α-tubulin-ac, acetylated α-tubulin. ** denotes a P value < 0.01 when the thrombin+Tubacin treated group is compared with the thrombin+tubacin+tautomycin treated group. “D” in parts A and B refers to DMSO, the solvent that was used for the inhibitors.
Discussion
The current study demonstrates that inhibition of HDAC6 can attenuate thrombin-induced EBD and indicates that the HDAC6 inhibitors target microtubule disassembly and phosphorylation of MLC2 induced by thrombin. These results suggest a therapeutic potential of HDAC6-specific inhibitors in EBD in vitro, and also in a well-characterized murine model of acute lung injury.
Our findings provide novel mechanistic insight to the two previous publications that report protection of LPS-induced acute lung injury by either broad HDAC inhibitors butyrate and trichostatin A or by a microtubule stabilizing agent taxol [7; 19; 20]. An apparent link between two disparate modalities is HDAC6 that is potentially targeted by both reagents. Our results indicate that similar to microtubule stabilizing agents, pharmacological inhibition of HDAC6 attenuates EBD and phosphorylation of MLC2 (Fig. 1 & 3) [6]. Although the previous reports identified activation of NF-κB as a target of HDAC inhibitors in LPS-induced acute lung injury, it is conceivable that HDAC6 is one of the direct targets of HDAC inhibition because the HDAC6-specific inhibitor tubacin can attenuate LPS-induced edema in the lung (Fig. 1F) [7; 19; 20]. It is noteworthy that the tubacin-mediated reduction in edema is only partial because LPS-induced elevation in wet/dry weight ratios in the presence of tubacin is still significantly higher than that in the DMSO or the tubacin alone groups (Fig. 1F, P < 0.05). This partial protection might be attributed to incomplete inhibition of HDAC6 in endothelial cells in vivo, and may be addressed by future optimization in dosage or route of administration.
Consistent with the previous report that microtubule disassembly promotes phosphorylation of MLC2, our findings correlate tubacin-mediated microtubule stabilization with diminished phosphorylation of MLC2 (Fig. 2 & 3) [6]. However, additional mechanisms might be involved in HDAC6-mediated regulation of phosphorylation of MLC2. Our results indicate that tubacin inhibits phosphorylation of MLC2 and EBD in a protein phosphatase dependent fashion (Fig. 3). Interestingly, HDAC6 has been demonstrated to inhibit protein phosphatase activity via direct binding and thereby promote phosphorylation of Akt [21; 22]. It is plausible that HDAC6-mediated regulation of MLC2 phosphorylation involves direct binding of HDAC6 to the protein phosphatases. Which specific protein phosphatase that is targeted by HDAC6 awaits further exploration. Lastly, it remains to be determined whether HDAC6-mediated regulation of EBD involves direct deacetylation of acetylated lysines in proteins other than α-tubulin. A recent proteomic survey has identified thousands of lysine-acetylated proteins that reside in not only the chromatin and nucleus, but also a variety of subcellular organells and functional cytoskeletal complexes [23]. HDAC6 is expressed predominantly in the cytoplasm, so it is highly likely that HDAC6 regulates EBD via deacetylation of cytoskeletal protein components and/or their modulators.
In conclusion, our findings indicate that inhibition of HDAC6 abrogates thrombin-induced EBD via enhancing acetylation of α-tubulin, stabilizing microtubules, and inhibiting phosphorylation of MLC2. As supported by our study, HDAC6 is a potential therapeutic target for pathological conditions that involve lung EBD. Our results warrant further investigation of the role for HDAC6 in diverse preclinical models of EBD and the molecular mechanisms that are involved in HDAC6-mediated EBD.
Acknowledgments
This work is supported in part by NIH HL083480 awarded to JAL, Louisiana Board of Regents LEQSF(2008-10)-RD-A-26 awarded to BS, and NIH CA132603 awarded to BS and DS. We are grateful to Dr. Ralph Mazitschek and Dr. Stuart L. Schreiber (Broad Institute of Harvard University and MIT Chemical Biology Program), Initiative for Chemical Genetics-NCI, for providing tubacin. We are also grateful to Dr. Thomas Voss (Department of Microbiology, Tulane University Health Sciences Center) for his assistance with measurement of TEER and to Ms. Carol Gadwaw for her excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bogatcheva NV, Verin AD. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res. 2008;76:202–7. doi: 10.1016/j.mvr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol. 2003;28:574–81. doi: 10.1165/rcmb.2002-0075OC. [DOI] [PubMed] [Google Scholar]
- 3.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol. 2005;204:934–47. doi: 10.1002/jcp.20359. [DOI] [PubMed] [Google Scholar]
- 5.Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol. 2004;201:55–70. doi: 10.1002/jcp.20055. [DOI] [PubMed] [Google Scholar]
- 6.Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AD. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006;290:L540–8. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- 7.Mirzapoiazova T, Kolosova IA, Moreno L, Sammani S, Garcia JG, Verin AD. Suppression of endotoxin-induced inflammation by taxol. Eur Respir J. 2007;30:429–35. doi: 10.1183/09031936.00154206. [DOI] [PubMed] [Google Scholar]
- 8.Norris KL, Lee JY, Yao TP. Acetylation goes global: the emergence of acetylation biology. Sci Signal. 2009;2:pe76. doi: 10.1126/scisignal.297pe76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–8. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 10.Destaing O, Saltel F, Gilquin B, Chabadel A, Khochbin S, Ory S, Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci. 2005;118:2901–11. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- 11.Shan B, Yao TP, Nguyen HT, Zhuo Y, Levy DR, Klingsberg RC, Tao H, Palmer ML, Holder KN, Lasky JA. Requirement of HDAC6 for transforming growth factor-beta1-induced epithelial-mesenchymal transition. J Biol Chem. 2008;283:21065–73. doi: 10.1074/jbc.M802786200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A. 2003;100:4389–94. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mai A, Massa S, Pezzi R, Rotili D, Loidl P, Brosch G. Discovery of (aryloxopropenyl)pyrrolyl hydroxyamides as selective inhibitors of class IIa histone deacetylase homologue HD1-A. J Med Chem. 2003;46:4826–9. doi: 10.1021/jm034167p. [DOI] [PubMed] [Google Scholar]
- 14.Shan B, Morris CA, Zhuo Y, Shelby BD, Levy DR, Lasky JA. Activation of proMMP-2 and Src by HHV8 vGPCR in human pulmonary arterial endothelial cells. J Mol Cell Cardiol. 2007;42:517–25. doi: 10.1016/j.yjmcc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, Horinouchi S, Yoshida M. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–31. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhuang Y, Nguyen HT, Lasky JA, Cao S, Li C, Hu J, Guo X, Burow ME, Shan B. Requirement of a novel splicing variant of human histone deacetylase 6 for TGF-beta1-mediated gene activation. Biochem Biophys Res Commun. 392:608–13. doi: 10.1016/j.bbrc.2010.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, Nakajima H. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med. 2001;163:762–9. doi: 10.1164/ajrccm.163.3.2003065. [DOI] [PubMed] [Google Scholar]
- 18.Bogatcheva NV, Garcia JG, Verin AD. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochemistry (Mosc) 2002;67:75–84. doi: 10.1023/a:1013904231324. [DOI] [PubMed] [Google Scholar]
- 19.Ni YF, Wang J, Yan XL, Tian F, Zhao JB, Wang YJ, Jiang T. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respir Res. 11:33. doi: 10.1186/1465-9921-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Jin S, Wang C, Jiang R, Wan J. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J Surg. 34:1676–83. doi: 10.1007/s00268-010-0493-5. [DOI] [PubMed] [Google Scholar]
- 21.Brush MH, Guardiola A, Connor JH, Yao TP, Shenolikar S. Deactylase inhibitors disrupt cellular complexes containing protein phosphatases and deacetylases. J Biol Chem. 2004;279:7685–91. doi: 10.1074/jbc.M310997200. [DOI] [PubMed] [Google Scholar]
- 22.Chen CS, Weng SC, Tseng PH, Lin HP. Histone acetylation-independent effect of histone deacetylase inhibitors on Akt through the reshuffling of protein phosphatase 1 complexes. J Biol Chem. 2005;280:38879–87. doi: 10.1074/jbc.M505733200. [DOI] [PubMed] [Google Scholar]
- 23.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–40. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]