Abstract
Studies on early development have demonstrated the profound effects of early social experience on the behavioral development and physiology of young rhesus macaques. Given these relationships, we hypothesized that rhesus macaques exposed to different nursery-rearing conditions may develop unique biobehavioral profiles. If this is true, the assessment of temperament may allow us to pinpoint successful rearing environments, thus improving the overall health of non human primates that are raised in captive environments. We conducted biobehavioral assessments in order to examine differences in the development of infants raised under four different peer-rearing conditions (continuous pairing, intermittent pairing, continuous pairing with partner rotation, and intermittent rotational pairing) and compared these animals with data from a mother-reared control group. Overall, continuous-rotationally paired animals were most similar to mother-reared controls on most behavioral and temperament measures, suggesting that more socially complex rearing environments (greater number of social partners) favor a more active behavioral style. Cortisol profiles of mother-reared controls were similar to both continuous pairing groups, and these three groups had higher cortisol concentrations than the intermittent rotational-pairing group. In addition, intermittently paired infants displayed a significantly higher frequency of self-stroke behavior during a human intruder challenge, an abnormal behavior also known as floating limb which has been shown to be a precursor of self-biting. Overall, the data are consistent with the idea that social complexity in the nursery, as operationalized in our continuous rotational pairing, leads to a biobehavioral profile that is most similar to that of infants raised by their mothers in large, socially complex, cages.
Keywords: temperament, nursery-rearing, infant development, emotionality, rhesus macaque, peer-rearing, hypothalamic-pituitary-adrenal regulation
Introduction
Rearing nonhuman primates in a nursery setting is a common husbandry practice at many primate facilities around the world for a variety of reasons. Scientifically, rearing in a nursery affords investigators maximal and safe access to animals when data must be collected frequently during early development such as, for example, when studying a disease process associated with fetal development, parturition, or early postnatal development [Abel 2009]. Nursery-rearing may also be used for derivation of animals that are free of specific pathogens (specific pathogen-free, or SPF) [Solnick et al. 1999], such as Cercopithecine herpesvirus B, which can be fatal to humans. Many of the viruses to be bred out of domestic colonies of animals in SPF programs are spread to the naive animal via social interaction (play-fighting, aggression, sexual behavior) with older, infected animals. Nursery-rearing, particularly from day of birth, ensures that animals interact only with animals that are themselves free of pathogens. (Of course, once a breeding group of SPF animals has been created, nursery-rearing of offspring for SPF derivation purposes may no longer be necessary.) Finally, nursery-rearing (or hand-rearing) is needed for situations such as maternal rejection, or maternal or infant illness that can occur in any species of captive animals, both in laboratories and zoos [Porton and Niebruegge 2006; Voelkl and Huber 2006].
Studies investigating the effects of early experience on development, however, have demonstrated that nursery-rearing of rhesus macaques (which nowadays generally involves removal around the day of birth, single-housing with some type of surrogate for a few weeks, followed by socialization with a conspecific) can have profound effects on behavioral development as well as on measures of welfare [Bellanca and Crockett 2002; Lutz et al. 2003; Novak and Petto 1991; Novak and Sackett 2006; Novak and Suomi 1988; Rommeck et al. 2009a; Rommeck et al. 2009b]. For example, nursery-reared animals have been shown to exhibit higher frequencies of abnormal behaviors such as floating limb and self-biting, compared to those reared by their mothers [Rommeck et al. 2009a], and despite advances in how nursery rearing is implemented [Sackett et al. 2006], it is considered a risk factor for developing abnormal behaviors [Bellanca and Crockett 2002; Bentson et al. 2005; Chamove 1973; Chamove et al. 1973; Champoux et al. 1991; Harlow 1958; Harlow et al. 1966; Harlow and Zimmermann 1958; Lutz et al. 2003; Lutz et al. 2007; Novak 2003; Novak and Sackett 2006; Novak and Sackett 1997; Rommeck et al. 2009a; Rommeck et al. 2009b; Roy 1981; Ruppenthal et al. 1976; Ruppenthal et al. 1991; Suomi 1991].
In addition to its effects on behavior, previous work has also demonstrated a link between nursery rearing and physiological outcomes. Shannon et al. [1998], for example, showed that surrogate-peer reared rhesus monkey infants had lower basal and stress cortisol concentrations compared to animals reared with their mothers. Similarly, Capitanio et al. [2006; 2005b] found that nursery-reared subjects displayed significantly lower cortisol levels in response to stressful challenges, as well as in response to pharmacologic challenge with dexamethasone and ACTH. These data suggested that the procedure of nursery-rearing altered one aspect of the hypothalamic-pituitary-adrenal (HPA) system, specifically the set-point around which the HPA system is regulated, with nursery-reared animals’ HPA systems having a lower overall set-point. Finally, there is evidence that nursery rearing can have an effect on immune functioning, potentially resulting in heightened cellular immune function [Capitanio in press]
The research that has documented behavioral and physiological effects of nursery-rearing is somewhat problematic, in that different laboratories utilize slightly different procedures in their nurseries (e.g., intermittent versus continuous socialization), making direct comparison of studies difficult. Nevertheless, there is considerable consistency in results, suggesting that this procedure can fundamentally alter the animal’s approach to its environment, and might best be described as having changed the animal’s temperament. In fact, in response to a challenging situation, such as a social separation, nursery-reared animals tend to show extreme patterns of behavioral responsiveness [Suomi 1991] including increases in self-directed behaviors and vocalizations, suggesting that these animals might best be characterized as having a reactive temperament. Because temperament is an enduring characteristic of an individual, and can be associated with psychiatric and somatic outcomes [Capitanio 2008; Capitanio et al. 2008; Friedman 2008; Lahey 2009; Mehta and Gosling 2008; Neeleman et al. 2002; Sloan et al. 2008; Windle 1987], it is a useful construct for describing the effects of various early experiences on patterns of responsiveness.
The goal of the present study was to evaluate the consequences of four different nursery rearing strategies on the animals’ responsiveness in challenging situations, to determine whether any of our rearing strategies produced animals with patterns of responses similar to those seen among more normally reared animals.. Unlike in our previous study on these animals [Rommeck et al. 2009b], we compared the responses of these animals with a similar number of animals raised in our large, outdoor field cages, which provide a rich social environment. For outcome measures, we utilized an ongoing BioBehavioral Assessment (BBA) program at the California National Primate Research Center (CNPRC) that is aimed at quantifying variation in biobehavioral organization or temperament – patterns of behavioral and physiological responsiveness and emotionality – by assessing responses to a 25-hr separation from companions and relocation to a novel room [Capitanio et al. 2005b; 2006]. Data from this program have already revealed behavioral and physiological differences between animals reared in a nursery setting and animals reared in field cages [Capitanio etal. 2005a; 2006]. In the present report, we ask whether different patterns of responsiveness might result from different nursery-rearing strategies.
Methods
This study was conducted at the CNPRC from May 2006 to May 2007. All research conducted and presented complied with protocols approved by the Institutional Animal Care and Use Committee at the University of California at Davis and adhered to the legal requirements of the USDA Animal Welfare Act and Regulations. This research also adhered to the American Society of Primatologists principles for the ethical treatment of nonhuman primates.
Subjects and Rearing Procedures
Subjects were selected from the CNPRC’s SPF derivation program, in which infants born to non-SPF mothers are removed at birth and raised in specific pathogen-free nurseries. Thirty-two animals were subjects in this study. The nursery-rearing protocol used to raise these infants was described in Rommeck et al. [2009b]. Briefly, infants were assigned to pairs and treatment groups as they arrived in the nursery, and socialization began after approximately 30 days of individual housing in an incubator. Young infants in the nursery are housed in individual incubators to assist them in thermoregulation and adaptation to self-feeding. Infants were gradually adapted to their quad cages beginning at the age of 21 days at which time they were also visually introduced to their future partner.
Four pairs (two were male/male, and two were mixed sex) were assigned to the continuous pairing (CP) group, which mirrored the standard nursery protocol at the CNPRC --infants were continuously paired with the same similar-aged pair mate throughout their stay in the nursery. Four pairs (three were female/female, one was mixed-sex) were assigned to the intermittent pairing (IP) group. In this condition, subjects were paired for 8 hours a day, from 7am to 3 pm, and were then separated by an opaque divider for the rest of the time. Eight infants (six males, two females) were part of the continuous rotational pairing (CRP) group. CRP subjects were continuously paired with another infant, but partners were rotated once per week within a group of four infants such that infants were exposed to three different social partners throughout their stay in the nursery. Finally, eight infants (five males, three females) were assigned to the intermittent rotational pairing (IRP) group, in which infants were paired for 8 hours a day and then separated for the rest of the time (as in the IP group). In addition, partners were rotated within a group of four infants once a week as in the CRP group.
Subjects were assigned to treatment groups as pairs as they arrived in the nursery in the order of CP, IP, CRP, and IRP. This order was repeated until all treatment groups contained 4 pairs each. Experimental rearing conditions continued until animals were 7 months of age, after which they were paired permanently and transferred to weanling rooms with adult size caging. Because subject assignment was done without regard to infant sex, a potential exists for a treatment group x sex confound. We performed a preliminary analysis on all nursery-reared animals assessed in the BBA program excepting those in the present study (n=229, 76 males) to test for the presence of sex differences on all measures used in the present report; no sex differences were found for any measure for which we report significant results below. We also note that because personality and temperament have a strong genetic component, shared parentage might influence outcomes via a parentage by rearing condition confound. All animals had different dams, and review of our colony parentage database, we found that nine of our 32 nursery-reared infants shared sires. Three sets of two infants shared one sire each and in only one of those sets were the two infants assigned to the same treatment group (CRP). Another set of three infants shared one sire, two of which were assigned to the same treatment group (CP) and one to another treatment group (IRP). Based on these analyses, we believe that effects of sex and shared parentage are not influential in our study.
Data for 8 mother-reared control animals (CON; 4 of each sex) from the outdoor field cages were used as a control. This sample of CON animals was randomly chosen from the pool of 220 field-cage-reared animals that were assessed in the BBA program during the same year. CON animals had been born and raised in outdoor, 0.2 hectare field cages. It is important to note that the CON infants were relocated from outdoor to indoor housing during testing, an additional stressor not experienced by nursery-reared animals.
Biobehavioral Assessment Procedures
Animals were between the ages of 90–115 days at the time of testing. Infants were separated from their peer (nursery) or mother (CON) and were relocated to their temporary Holding Cages at 0900 on Day 1 and were returned to the nursery (or to their mothers, in the case of CON infants) after 25 hours. Infants were transported to the test area in individual transport boxes and were housed singly in standard laboratory cages measuring 60 cm × 65 cm × 79 cm (Lab Products, Inc., Maywood, NJ). Each cage contained a towel and a stuffed surrogate identical to those used in the nursery. Food and water were available ad libitum. Three animals (one mixed sex pair from the CP group, and one male from the IRP group) could not be tested owing to scheduling conflicts (the CP animals) and health issues (the IRP animal).
Details of all tests in the BBA program have been described in detail in Golub et al., [2009], and are described briefly below. Infants were assessed in a predetermined random order that remained constant for all tests. The same behavioral catalog was used to assess infants in all tests (see Table 1), with minor exceptions that were related to the specifics of a given test (e.g., proximity to the intruder in the Human Intruder test). Interobserver reliability was computed at better than 85% agreement for behavior categories, and we note that the person recording the behaviors for the present study was blind to both the rearing conditions of the subjects, as well as the previous results from these animals [Rommeck et al. 2009b].
Table 1.
Ethogram for Biobehavioral Assessment
| States: | |
| sit: | Hindquarters are on the perch or floor; includes shifting weight slightly one step. |
| lie: | Relaxed posture with body resting on a horizontal surface. |
| stand: | Torso in a stationary position and weight is supported by 3 or 4 legs; can include steps taken that only involve one or two feet. |
| active: | Whole body movement; step, jump. |
| locomote: | Directed movement from one location to another |
| run: | Rapid movement in which at times no feet are in contact with surface |
| pace: | Repetitive rapid movement over the same path |
| crouch: | Ventral surface close to floor; head at or below the level of the shoulders. |
| sleep: | Eyes closed. |
| rock/sway: | Unbroken rhythmic movements of the upper body while the animal is sitting. |
| hang: | Holding onto ceiling or front mesh; all 4 limbs off of floor. |
| motor stereotypy: | Movement back and forth, repeatedly covering the same route. |
| Events: | |
| scratch: | Common usage. |
| self-clasp: | Hand or feet closed on fur or some body part. |
| self-bite: | Discrete biting action, usually directed to limbs and often accompanied by a threat face. |
| self-stroke: | Very gently bringing the hand or foot across the side of head or face. |
| self manip.: | Masturbation, pulling or tugging or pushing at self. |
| self groom: | Using hands or lips to pick through or part its own fur. |
| suck: | Insertion into mouth of fingers, toes, and other body parts. |
| back-flip: | Tossing the body up and backwards in a circular motion in the air. |
| convulsive jerk: | Sudden and somewhat violent contractions of the limbs and trunk. |
| cage shake/bounce | Holding onto cage and shaking it, generating a lot of noise. |
| coo: | Medium-pitched, moderately intense, clear call. |
| screech: | Intense, very high-pitched. |
| gecker: | Staccato cackling sounds. |
| bark: | Gruff, abrupt, low-pitched vocalization. |
| other | Other vocalizations not previously described. |
| lipsmack: | Rapid lip movement usually with pursed lips, accompanied by a smacking sound. |
| threat: | Scored with at least two or more of the following: open mouth stare, head bob, ear flaps, bark vocalizations. |
| fear grimace: | Exaggerated grin with teeth showing. |
| yawn: | Wide open mouth displaying teeth. |
| tooth grind: | Loud gnashing of teeth. |
| environmental explore: | Discrete manipulation by hand or mouth with the physical environment or objects in the cage. |
Holding Cage Observations
In order to assess behavioral responses to relocation and separation from pair mates, each subject was observed twice for 5 minutes, fifteen minutes after placement in the Holding Cage (0915) on Day 1, and toward the end of the assessment period, at 0700 on Day 2. Data were collected using Observer software [Noldus 1991]. The observer, who was unfamiliar to the subjects prior to testing, sat in front of the Holding Cage at a distance of approximately 2.4 m from the front of the cage without making eye contact with the animal. Behaviors were scored according to the ethogram in Table 1.
Hypothalamic Pituitary-Adrenal Regulation
Blood samples were drawn via femoral venipuncture at four time points during the testing period in order to assess the infants’ response to relocation and separation from peers and to evaluate HPA-axis regulation. Infants were awake and not sedated during venipuncture. Sample 1 (1.0 ml) was drawn after completion of the Holding Cage observations at 1100 h. Sample 2 (0.5 ml) was drawn after completion of the Human Intruder test (see below) at 1600 h. Immediately after the Sample 2 venipuncture each monkey was given an injection of 500 μg/kg dexamethasone intramuscularly. Sample 3 (0.5 ml) was taken following completion of Holding Cage observations on Day 2 at 0830 h, immediately after which subjects were administered 2.5 IU adrenocorticotropic hormone (ACTH). The final sample (0.5 ml) was drawn 30 minutes after ACTH was given [Capitanio et al. 2006]. Blood was drawn into unheparinized syringes and was immediately transferred to EDTA tubes, which were subsequently centrifuged at 1000g for 10 min at 4°C. Plasma was decanted into microtubes for storage at −80°C until assayed for cortisol concentration by RIA (Diagnostic Products Corp., Los Angeles, CA).
Human Intruder
At 1400 h on Day 1, animals were transferred from their Holding Cage to a test cage constructed of stainless steel mesh and measuring (38.7cm W × 52.0cm H × 47.0cm L). Each infant experienced four consecutive 1-minute trials. During trial 1 an unfamiliar human (the “intruder”) sat 1 m in front of the cage presenting her profile to the animal for one minute (“Profile Far”). Trial 2 consisted of the human moving to within 0.3 m of the cage front (“Profile Near”). During trial 3, the human returned to the far position and attempted to maintain eye contact with the infant (“Stare Far”). This was followed by the human returning to within 0.3 m of the cage while maintaining eye contact (“Stare Near”) in trial 4. The same technician functioned as the human intruder for all animals. Infant behavioral responses were recorded using a Panasonic video camera. The video was later coded using VideoPro software as part of the Observer program and the ethogram in Table 1 with addition of the categories Left and Right indicating the position of the infant’s head in either the left (away from the intruder) or right (near the intruder) part of the cage.
Temperament Ratings
Just prior to the end of the 25 hour testing period, the technician who performed all testing rated the temperament of each animal based on her total experience observing, handling, and interacting with the animals, in order to gain an overall impression of the infants’ behavioral characteristics. The observer rated each animal on 16 traits, using a 7 point Likert-type scale ranging from “total absence” to “extremely large amount.” Factor analysis (described in detail in Golub at al. [2009]) identified four factors, named for the trait that loaded highest on each factor: Vigilant (vigilant, not depressed, not tense, not timid), Gentle (gentle, calm, flexible, curious), Confident (confident, bold, active, curious, playful), and Nervous (nervous, fearful, timid, not calm, not confident). Factor scores were calculated by summing the z-scores for all adjective items loading on a given factor, and then computing a z-score for each facator. Cronbach’s alpha values for the factors ranged from 0.6 to 0.9.
Statistical Analyses
Owing to slight variations in the length of the observation periods, duration measures were converted to a proportion of the total observation time, and frequencies of states and events were converted to a rate per 60 s. Data were analyzed (using SPSS, version 14) with multivariate analysis of variance for each data set (Holding Cage, Cortisol, Human Intruder, Temperament), followed by univariate ANOVAs in the event of a significant multivariate effect. For all analyses, rearing condition was a between-subjects factor, and within-subjects factors were included for the Holding Cage data (Day 1 vs. Day 2), Cortisol data (four samples), and Human Intruder data (Profile Far, Profile Near, Stare Far, and Stare Near positions). We analyzed the following behaviors for Holding Cage: rates of convulsive jerk, self-clasp, environmental explore, fear grimace, self-bite, scratch, self-stroke, and distress vocalizations, and proportions of time spent in crouch, hang, locomotion, and pace. For the Human Intruder test, we examined rates of self-clasp, self-stroke, convulsive jerk, lipsmack, threat, and fear grimace, as well as coo, screech, gecker, bark, and other vocalizations, and proportions of time spent: activity, crouch, motor stereotypy, pace. For all analyses, pairwise comparisons were made to identify specific rearing condition differences when there was a significant main effect of rearing condition. Confidence intervals and alpha levels for multiple pairwise comparisons were adjusted using Sidak correction.
Results
Holding Cage Observation
Rearing groups did not differ on any measure for this assessment, as indicated by non- significant multivariate effects for rearing condition, or for the interaction of rearing condition by day. A significant day effect was found (F11,22=2.413, p<0.05), and univariate tests showed that distress vocalizations (F1,32=7.843, p<0.01) were higher on Day 1 compared to Day 2, and scratch (F1,32=10.961, p<0.01) showed higher frequencies on Day 2.
Hypothalamic Pituitary-Adrenal Regulation
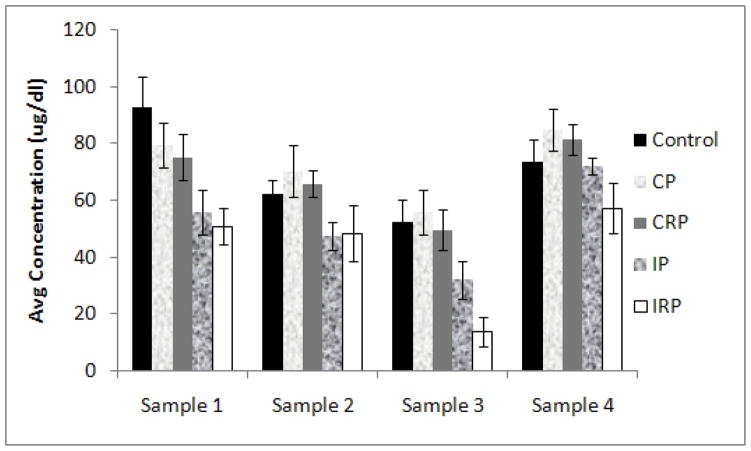
Both continuously paired groups (CP, CRP) were most like the mother reared controls (CON) in cortisol patterns, while the intermittent pairing groups (IP, IRP) showed patterns that were most different from those groups. Results of the multivariate ANOVA indicated that there was a main effect of sample (F3,96=42.482, p<0.001) and rearing condition (F4,32=5.667, p=0.001), with CON, CP, and CRP animals showing higher cortisol levels compared to animals in the IRP condition. There was also a significant sample by rearing condition interaction (F12, 96 = 1.986, p<0.05; see Figure 1). Examination of the interaction effect revealed no significant difference between CON, CP and CRP animals for any sample. In response to the initial separation and relocation (Sample 1), CON monkeys had significantly higher cortisol concentrations compared to IP (p<0.05) but not IRP monkeys. For Sample 2, there were no rearing condition differences. In response to dexamethasone (Sample 3), CON, CP, and CRP monkeys had higher cortisol concentrations than did IRP (p=0.001, p=0.001, and p<0.01, respectively), with the cortisol concentrations of IP animals not different from any of the other rearing conditions. Finally, for the ACTH-stimulated sample (Sample 4), CP and CRP animals had significantly higher concentrations than did IRP animals (p<0.05, and p<0.05, respectively), but there were no differences among any of the other rearing conditions.
Figure 1.
Effects of rearing condition on plasma cortisol concentrations at the 4 different sampling times. Significant main effect of rearing condition (CON, CP, CRP > IRP, p<0.05). Significant rearing x sample interaction (Sample 1: CON > IP, p<0.05; Sample 3: CON, CP, CRP > IRP, all p<0.01; Sample 4: CP, CRP > IRP, both p<0.05).
Human Intruder
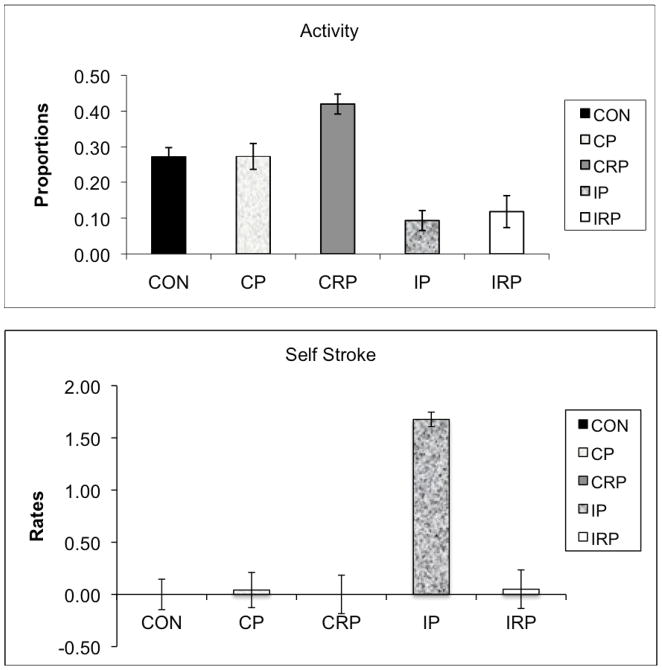
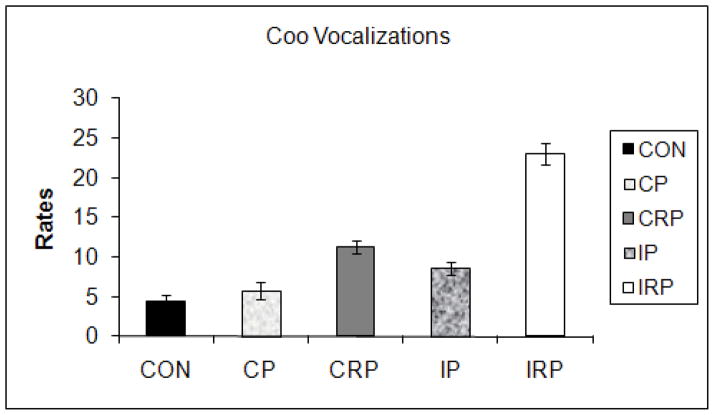
The two continuously-reared groups were again most similar to the controls, while the intermittently-reared animals were different. These results were indicated by a significant multivariate main effect of rearing condition (F56,88=1.619, p<0.05); the rearing condition by intruder position interaction was not significant. Univariate analyses revealed significant effects for activity (F4,32=3.235, p<0.05), with CRP more active than IP (p<0.05) and IRP (p=0.054, see Figure 2A), self stroke (F4,32=3.345, p<0.05), with IP monkeys showing higher rates of self- stroke than CON (p=0.055) and CRP (p=0.055) animals, with rates for CP and IRP not significantly different from any group (see Figure 2B), and coo vocalization (F4,32=4.496, p<0.01), with higher rates of vocalizations in IRP monkeys than in CON (p<0.01) and CP (p<0.05) animals (see Figure 2C).
Figure 2.
Effects of rearing condition on the proportion of time active (A), rate of self-stroke (B), and rate of cooing (C) during the Human Intruder test. Significant main effect of rearing condition (Activity: CRP > IP (p<0.05), IRP (p=0.054); Self-stroke: IP>CON (p=0.055), CRP (p=0.055); Coo: IRP > CON (p<0.01), CP (p<0.05)).
A significant multivariate effect of intruder position was found (F42,255=1.619, p<0.05). Rates of coo and screech vocalizations were significantly greater in the Stare Near position compared to Profile Far (p<0.05 and p<0.05, respectively), Profile Near (p<0.01 and p<0.05, respectively), and Stare Far (p<0.05 for coo vocalizations only) positions. In addition, rates of fear grimacing were greater in the Stare Near position compared to Profile Far (p <0.01), Profile Near (p<0.05), and Stare Far (p=0.057) positions.
Temperament Ratings
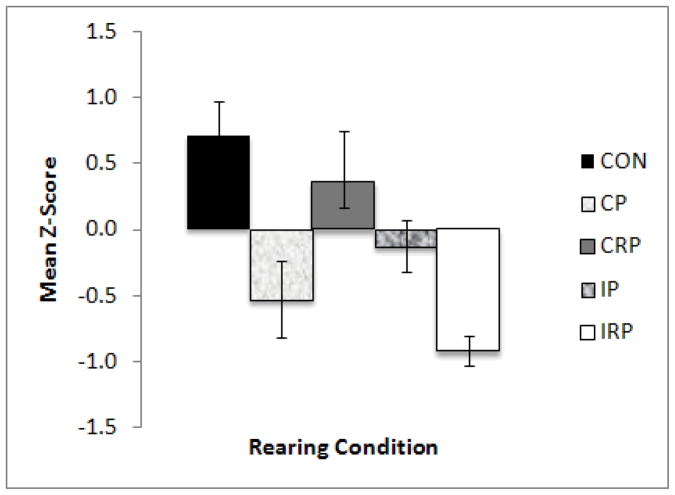
Differences in Confidence were found among rearing conditions, with CON and CRP monkeys showing the most similar values, and IRP monkeys showing the lowest Confidence. These results were indicated by a significant multivariate effect of rearing condition (F16,128=1.981, p<0.05) for the four temperament factors. Univariate follow-up analyses showed a significant difference only for the Confident factor (F4,32=6.892, p<0.001; Figure 3). Post-hoc analysis showed that CON and CRP animals were more Confident than IRP (p<0.001) monkeys, and CON animals were also rated as more Confident compared to CP (p<0.05) animals.
Figure 3.
Effect of rearing condition on the Confident temperament factor. Significant main effect of rearing condition (CON > IRP (p<0.001), CP (p<0.05); CRP > IRP, p<0.001).
Discussion
Our data suggest that monkeys that were nursery-reared with the continuous rotational pairing (CRP) procedure showed a biobehavioral profile that was most similar to that seen for the infants reared in a rich social environment in large, outdoor field cages (CON). In contrast, nursery-reared animals that were given intermittent socialization showed the greatest differences from both CON and CRP animals on a variety of measures. Below we discuss the results from the various data sets (since no rearing group effects were found for the Holding Cage observations, these data are not discussed).
Hypothalamic pituitary adrenal axis responses
The cortisol data suggest that animals that are reared with continuously-available companions (CON, CRP, CP) show concentrations that are not different from each other, but are significantly different from concentrations shown by animals that are given only intermittent access to companions on a rotational basis (IRP); although not statistically significant, inspection of Figure 1 shows that cortisol concentrations for IP animals are generally more similar to those of the IRP animals than to the other animals, particularly for the first three samples. In addition, while all groups showed the expected decrease in cortisol following dexamethasone suppression and increase following ACTH administration, IRP monkeys appeared to have the greatest decline in response to dexamethasone (compare Sample 2 to Sample 3) and the largest adrenal response to ACTH. Together, these results are consistent with an earlier suggestion by Capitanio et al. [2005b] that the amount of early social experience is associated with the “set-point” of the hypothalamic-pituitary-adrenal system. In that earlier study, nursery-reared animals that were given intermittent access to peers (similar to the IP animals in the present study) had lower cortisol concentrations compared to nursery-reared animals given continuous access to peers (equivalent to the CP animals in the present study). The present results suggest that, however the intermittent rearing strategy exerts its effects on the HPA system, this effect is even more pronounced when intermittent access to companions is accompanied by a strategy that involves rotating partners on a regular basis. Whether this effect persists into later age periods, or has implications for other physiological systems (e.g., the immune system) is unknown, but we believe these results deserve considerable follow-up.
Human Intruder
Data from the human intruder assessment are consistent with the picture of greater similarity in responses between CON, CRP, and CP animals compared to the intermittently paired animals. One interesting rearing effect found during this test was the higher rate of self-stroke in IP individuals. We note that the behavioral measure of self-stroke (see Table 1) is similar to our definition of floating limb in previous reports [Rommeck et al. 2009b]. Floating limb behavior consists of a peculiar behavioral pattern defined in our most recent paper as: “A limb moving seemingly of its own accord, in that the animal is not attending to or even aware of limb movement; often incorporates a slow stroking of the animal’s own body” [Rommeck et al. 2009b]. While this behavior does not cause physical harm to the animal, it has been associated with [Bentson et al. 2010; Bentson et al. 2005] and even shown to be predictive of [Rommeck et al. 2009b] self-biting in rhesus macaques. The present result is consistent with our previous finding of a significant overall increase in floating limb behavior with an intermittent pairing strategy. It is unclear whether floating limb is simply a larger part of an IP infant’s behavioral repertoire overall or whether it was performed specifically as a coping behavior in response to the human intruder challenge.
Temperament Ratings
In contrast to the results for cortisol, the temperament rating data suggest that the three groups that experienced continuous socialization were not equivalent. Specifically, while CON and CRP animals had scores for Confident temperament that were not significantly different from each other, both groups had scores that were higher than those of the CP animals (significantly so for the CON animals). Among intermittently-reared animals, the biggest differences were evident for the IRP animals – they were significantly less Confident than CON and CRP animals.
In the BioBehavioral Assessment program, temperament ratings provide an overall assessment of adaptation during the technicians’ experiences with the animals. These experiences include not only performing the behavioral observations during the formal assessments, but also handling the animals as they are relocated to the testing cages and for blood sampling and handling during the processes of feeding and changing the towels in their cages, etc. It may be the case that the similarity between CRP and CON animals in their temperament ratings, and their difference from CP animals, is a reflection of the adjustments that such individuals make regularly to a variety of animals (as opposed to only a single, other animal) that they interact with – perhaps they learn greater flexibility, and that they can better control outcomes. Although IRP animals also interact with the same number of different individuals as CRP animals, the shorter-time that IRP animals are together, combined with the inevitable separation that occurs every day, might contribute to a more intense and anxious style of social experience, that could be expressed as less confidence during the BBA situation.
General Discussion
Altogether, these findings suggest that among nursery-reared animals, continuous access to peers, and in particular continuous-rotational pairing, produces the most species-typical pattern of biobehavioral responsiveness to stressful situations. In our recent paper examining the effects of these rearing strategies on the frequency of abnormal behaviors, we found that the CRP method also produced the most behaviorally normal animals [Rommeck et al. 2009b]. Intermittent pairing (without respect to partner rotation), in contrast, was associated in the present study with more self-stroking and coo vocalizations in the human intruder test, and lower confidence ratings, and in our earlier paper, IP and IRP animals showed the highest frequencies of abnormal behaviors such as floating limb and self-biting [Rommeck et al., 2009b]. Further research is needed to assess any associations between our rearing-conditions and later correlates to physical health. Given previous research [Lahey 2009; Mehta and Gosling 2008; Neeleman et al. 2002; Sloan et al. 2008] and our present findings we would predict that intermittently paired animals might be most susceptible, and CRP animals least susceptible, to poor health outcomes.
We are aware that our findings are inconsistent with previous studies by Novak and Sackett [1997] and Sackett et al. [2002]. It is, however, difficult to compare our results to this earlier work because of a number of methodological differences including different rearing approaches (e.g., socialization occurring with multiple peers for 30-min per day in a playroom setting) and a difference in subject species (Macaca nemestrina versus Macaca mulatta). Another major difference in the early rearing experience of the M. nemestrina subjects was the frequent and early human handling and cognitive testing experienced by these subjects. Handling and cognitive testing introduces elements of physical and mental stimulation that may be enriching enough to counteract the development of some abnormal behaviors in captive primates and therefore warrants further study.
In conclusion, the BBA program revealed significant changes in physiology as well as temperament and behavioral responses to challenges between treatment groups. Our results suggest that intermittent pairing of infants in the nursery produces animals that cope less effectively with stress and are less confident compared to nursery-reared animals that are continuously paired. Specifically, infants raised under the continuous rotational condition appeared to be most similar to mother-reared controls and showed a temperamental profile that has been associated with favorable health outcomes in previous studies [Cederblad et al. 1995]. This suggests that time spent alone during the first months of life is a significant contributor to developing both behavioral as well as physiological abnormalities while exposure to a greater diversity of social partners seems to produce more psychologically and physiologically normal animals and therefore shows promise as an improved nursery rearing strategy.
Acknowledgments
We thank the California National Primate Research Center for supporting this project with a pilot study grant funded by the National Institute of Health grant P51 RR000169. This work was also supported by RR019970 to JPC. Special appreciation also goes to the primate center nursery staff, especially nursery supervisor Kelly Weaver, and to Laura DelRosso and Laura Calonder for collecting all BBA data. The first author also thanks Dr. Joy Mench for her comments on the Masters thesis on which this paper was based. Finally we thank Dr. Nicholas Lerche for his support throughout this project. All research conducted and presented complied with protocols approved by the Institutional Animal Care and Use Committee at the University of California at Davis and adhered to the legal requirements of the USDA Animal Welfare Act and Regulations.
References
- Abel K. The rhesus macaque pediatric SIV infection model-a valuable tool in understanding infant HIV pathogenesis and for designing pediatric HIV prevention strategies. Current HIV research. 2009;7(1):2. doi: 10.2174/157016209787048528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. American Journal of Primatology. 2002;58(2):57–69. doi: 10.1002/ajp.10052. [DOI] [PubMed] [Google Scholar]
- Bentson K, Crockett C, Wahl K, Runeson E, Bellanca R, Lee G, Thom J, Montgomery H, Yi M, McComas J. Floating limb behaviors and self biting are associated in laboratory monkeys. American journal of primatology. 2010;72(8):725–733. doi: 10.1002/ajp.20831. [DOI] [PubMed] [Google Scholar]
- Bentson K, Montgomery H, Bellanca R, Lee G, Thom J, Crockett C. Floating limb activity is associated with self-biting in four monkey species (Macaca mulatta, M. fascicularis, M. nemestrina, and Papio cynocephalus) American Journal of Primatology. 2005;66(Suppl):181. [Google Scholar]
- Capitanio J. Personality and disease. Brain Behavior and Immunity. 2008;22(5):647–650. doi: 10.1016/j.bbi.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio J, Abel K, Mendoza S, Blozis S, McChesney M, Cole S, Mason W. Personality and serotonin transporter genotype interact with social context to affect immunity and viral set-point in simian immunodeficiency virus disease. Brain Behavior and Immunity. 2008;22(5):676–689. doi: 10.1016/j.bbi.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP. Nonhuman primate personality and immunity: Mechanisms of health and disease. In: Weiss A, King JE, Murray L, editors. Personality, Temperament, and Behavioral Syndromes in Nonhuman Primates. New York: Springer; in press. [Google Scholar]
- Capitanio JP, Lowe E, DelRosso L. Biobehavioral Characterization for Management and Research Purposes: Behavioral Catalog for Rhesus Macaque Infants. 2005a. [Google Scholar]
- Capitanio JP, Mason W, Mendoza SP, DelRosso L, Roberts JA. Nursery Rearing and Biobehavioral Organization. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery Rearing in the 21st Century. Chicago, Illinois: Springer; 2006. pp. 191–214. [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005b;46(4):318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Cederblad M, Dahlin L, Hagnell O, Hansson K. Intelligence and temperament as protective factors for mental health. A cross-sectional and prospective epidemiological study. European archives of psychiatry and clinical neuroscience. 1995;245(1):11–19. doi: 10.1007/BF02191539. [DOI] [PubMed] [Google Scholar]
- Chamove AS. Rearing infant rhesus together. Behaviour. 1973;47(1):48–66. doi: 10.1163/156853973x00274. [DOI] [PubMed] [Google Scholar]
- Chamove AS, Rosenblum LA, Harlow HF. Monkeys (Macaca mulatta) raised only with peers. A pilot study. Anim Behav. 1973;21(2):316–25. doi: 10.1016/s0003-3472(73)80073-9. [DOI] [PubMed] [Google Scholar]
- Champoux M, Metz B, Suomi SJ. Behavior of nursery/peer-reared and mother-reared rhesus monkeys from birth through 2 years of age. Primates. 1991;32(4):509–514. [Google Scholar]
- Friedman H. The multiple linkages of personality and disease. Brain Behavior and Immunity. 2008;22(5):668–675. doi: 10.1016/j.bbi.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub M, Hogrefe C, Widaman K, Capitanio J. Iron deficiency anemia and affective response in rhesus monkey infants. Developmental Psychobiology. 2009;51(1):47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF. The Nature of Love. American Psychological Association; 1958. [Google Scholar]
- Harlow HF, Harlow MK, Dodsworth RO, Arling GL. Maternal Behavior of Rhesus Monkeys Deprived of Mothering and Peer Associations in Infancy. Proceedings of the American Philosophical Society. 1966;110(1):58–66. [Google Scholar]
- Harlow HF, Zimmermann RR. The Development of Affectional Responses in Infant Monkeys. Proceedings of the American Philosophical Society. 1958;102(5):501–509. [Google Scholar]
- Lahey B. Public health significance of neuroticism. American Psychologist. 2009;64(4):241–256. doi: 10.1037/a0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. American Journal of Primatology. 2003;60(1):1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Davis EB, Ruggiero AM, Suomi SJ. Early predictors of self-biting in socially- housed rhesus macaques (Macaca mulatta) Am J Primatol. 2007;69(5):584–90. doi: 10.1002/ajp.20370. [DOI] [PubMed] [Google Scholar]
- Mehta P, Gosling S. Bridging human and animal research: A comparative approach to studies of personality and health. Brain Behavior and Immunity. 2008;22(5):651–661. doi: 10.1016/j.bbi.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Neeleman J, Sytema S, Wadsworth M. Propensity to psychiatric and somatic ill-health: evidence from a birth cohort. Psychological medicine. 2002;32(05):793–803. doi: 10.1017/s0033291702005901. [DOI] [PubMed] [Google Scholar]
- Noldus L. The observer: a software system for collection and analysis of observational data. Behav Res Methods Instrum Comput. 1991;23(3):415–429. [Google Scholar]
- Novak MA. INVITED PAPER Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. American Journal of Primatology. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Novak MA, Petto AJ. Perspectives on psychological well-being in captive primates: Through the looking glass. In: Novak MA, Petto AJ, editors. Through the looking glass: Issues of psychological well-being in captive nonhuman primates. 1991. pp. 1–7. [Google Scholar]
- Novak MA, Sackett GP. The Effects of Rearing Experiences: The Early Years. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. Chicago, Illinois: Springer; 2006. pp. 5–19. [Google Scholar]
- Novak MA, Suomi SJ. Psychological well-being of primates in captivity. American Psychologist. 1988;43(10):765–773. doi: 10.1037//0003-066x.43.10.765. [DOI] [PubMed] [Google Scholar]
- Novak MF, Sackett GP. Pair-rearing infant monkeys (Macaca nemestrina) using a” rotating-peer” strategy. Am J Primatol. 1997;41(2):141–9. doi: 10.1002/(SICI)1098-2345(1997)41:2<141::AID-AJP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Porton I, Niebruegge K. The Changing Role of Hand Rearing in Zoo-Based Primate Breeding Programs. In: Sackett GP, Ruppenthal G, Elias K, editors. Nursery rearing of nonhuman primates in the 21st century. New York: Springer; 2006. pp. 21–31. [Google Scholar]
- Rommeck I, Anderson K, Heagerty A, Cameron A, McCowan B. Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. Journal of Animal Welfare Science. 2009a;12(1):61–72. doi: 10.1080/10888700802536798. [DOI] [PubMed] [Google Scholar]
- Rommeck I, Gottlieb DH, Strand SC, McCowan B. The effects of four nursery-rearing strategies on infant behavioral development in rhesus macaques (Macaca mulatta) Journal of the American Assiciation of Laboratory Animal Science. 2009b;48(4):395–401. [PMC free article] [PubMed] [Google Scholar]
- Roy MA. Abnormal Behaviors in Nursery-Reared Squirrel Monkeys (Sairniri sciureus) American Journal of Primatology. 1981;1:35–42. doi: 10.1002/ajp.1350010105. [DOI] [PubMed] [Google Scholar]
- Ruppenthal GC, Arling GL, Harlow HF, Sackett GP, Suomi SJ. A 10-year perspective of motherless-mother monkey behavior. J Abnorm Psychol. 1976;85(4):341–349. doi: 10.1037//0021-843x.85.4.341. [DOI] [PubMed] [Google Scholar]
- Ruppenthal GC, Walker CG, Sackett GP. Rearing infant monkeys (Macaca nemestrina) in pairs produces deficient social development compared with rearing in single cages. American Journal of Primatology. 1991;25:103–113. doi: 10.1002/ajp.1350250204. [DOI] [PubMed] [Google Scholar]
- Sackett G, Ruppenthal G, Elias K. Nursery rearing of nonhuman primates in the 21st century. Springer Verlag; 2006. [Google Scholar]
- Sackett GP, Ruppenthal GC, Davis AE. Survival, growth, health, and reproduction following nursery rearing compared with mother rearing in pigtailed monkeys(Macaca nemestrina) American Journal of Primatology. 2002;56(3):165–183. doi: 10.1002/ajp.1072. [DOI] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. BRIEF REPORTS Rearing Condition and Plasma Cortisol in Rhesus Monkey Infants. American Journal of Primatology. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Sloan E, Capitanio J, Tarara R, Cole S. Social temperament and lymph node innervation. Brain Behavior and Immunity. 2008;22(5):717–726. doi: 10.1016/j.bbi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnick J, Canfield D, Yang S, Parsonnet J. Rhesus monkey (Macaca mulatta) model of Helicobacter pylori: noninvasive detection and derivation of specific-pathogen-free monkeys. Laboratory animal science. 1999;49(2):197. [PubMed] [Google Scholar]
- Suomi SJ. Early stress and adult emotional reactivity in rhesus monkeys. Ciba Found Symp. 1991:171–83. doi: 10.1002/9780470514047.ch11. [DOI] [PubMed] [Google Scholar]
- Voelkl B, Huber L. Hand Rearing of Infant Common Marmosets. In: Sackett GP, Ruppenthal G, Elias K, editors. Nursery rearing of nonhuman primates in the 21st century. New York: Springer; 2006. pp. 121–129. [Google Scholar]
- Windle M. Stressful life events, general mental health, and temperament among late adolescent females. Journal of Adolescent Research. 1987;2(1):13. [Google Scholar]