Abstract
Objectives/Hypothesis
The purpose of this study was to compare the clinical effectiveness of type I thyroplasty, injection laryngoplasty and graft implantation for the treatment of vocal fold scar and pathologic sulcus vocalis.
Study Design
Prospective, multi-arm, quasi-experimental research design.
Methods
Twenty-eight patients with newly diagnosed vocal fold scar and/or pathologic sulcus vocalis were assigned to one of three treatment modalities: Type I thyroplasty (n = 9), injection laryngoplasty (n = 9) and graft implantation (n = 10). Psychosocial, auditory-perceptual, acoustic, aerodynamic and videostroboscopic data were collected pre-treatment and at 1, 6, 12 and 18 months post-treatment.
Results
Type I thyroplasty and graft implantation both resulted in reduced voice handicap with no concomitant improvement in auditory-perceptual, acoustic, aerodynamic or vocal fold physiologic performance. Injection laryngoplasty resulted in no improvement on any vocal function index. Patients who underwent graft implantation exhibited the slowest improvement trajectory across the 18 month follow-up period.
Conclusions
A persistent challenge in this area is that no single treatment modality is successful for the majority of patients, and there is no evidence-based decision algorithm for matching a given treatment to a given patient. Progress therefore requires the identification and categorization of predictive clinical features that can drive evidence-based treatment assignment.
Keywords: larynx, phonosurgery, sulcus vocalis, treatment efficacy, vocal fold scar
Introduction
Vocal fold scar and pathologic sulcus vocalis are related but challenging clinical entities characterized by fibrotic changes to the vocal fold mucosa,1 an often irrevocable loss of tissue viscoelasticity and vibratory potential,2-4 profound dysphonia5,6 and significant voice handicap.7,8 Both conditions involve physical disruption of the vocal fold edge contour (due to scar contraction and/or the anatomic sulcus), disordered lamina propria extracellular matrix,1,9,10 and impaired glottal closure.5 The presentation and severity of these features can vary significantly between patients, and a certain subset of patients present with concomitant scar and sulcus deformities.7 Treatment of these disorders is considered difficult and complete voice restoration is highly unusual.11,12
Current surgical approaches to the treatment of chronic vocal fold scar and pathologic sulcus focus on enhancing glottal closure/efficiency via medialization, and/or improving vocal fold vibratory potential by direct manipulation of the lamina propria.13 The classic medialization approaches of type I thyroplasty and injection laryngoplasty are familiar to most surgeons and generally considered robust.14-18 Approaches to treatment of the lamina propria are more challenging and include scar/sulcus excision,19-21 undermining and release,22,23 mucosal slicing,24 superficial implantation of autologous fat, fascia or other scaffold materials,25-34 superficial injection of steroids or regenerative biomaterials,35-37 and pulsed dye laser irradiation.38
Each of these approaches, whether used individually or in combination, have demonstrated some degree of therapeutic benefit in retrospective case review and prospective, single-arm studies; however, there are currently no prospective, multi-arm comparisons of relative treatment efficacy for vocal fold scar or pathologic sulcus vocalis in the clinical literature. Such comparative studies are necessary to demonstrate relative treatment efficacy and provide data-driven guidance for clinical/surgical decision making. The purpose of this study, therefore, was to compare the clinical effectiveness of three treatment modalities (type I thyroplasty, injection laryngoplasty, graft implantation) using a prospective, non-randomized, quasi-experimental research design. We elected to compare these specific treatments as they represent the three most cited approaches to vocal fold scar and pathologic sulcus vocalis management in the clinical literature, and because they attempt to address the two primary aspects of voice impairment associated with these conditions: Glottal incompetence (type 1 thyroplasty, injection laryngoplasty) and lamina propria extracellular matrix disruption (graft implantation).13,23 We focused on direct comparison of these treatment modalities irrespective of the implant or injectate material delivered to the larynx, and followed patients for 18 months post-treatment.
Materials and Methods
Patients
Twenty-eight patients with a clinical diagnosis of vocal fold scar and/or pathologic (types II or III)23 sulcus vocalis were enrolled in this study (Table 1). All patients were enrolled during an initial diagnostic visit and had not undergone any form of intervention (surgical or behavioral) at the time of enrollment. The diagnosis and classification of sulcus vocalis were made by a laryngologist following videostroboscopic examination of the larynx performed by a speech-language pathologist, and confirmed or revised by the laryngologist during subsequent direct microlaryngoscopy. Vocal fold scar was diagnosed using the same procedures. For the purposes of this study, vocal fold scar concomitant with sulcus vocalis was defined as any degree of reduced tissue pliability and apparent fibrosis in a region distinct from the sulcus.
Table 1.
Patient demographic, diagnostic and treatment information.
| Treatment Group | Thyroplasty n = 9 |
Injection n = 9 |
Graft n = 10 |
Total n = 28 |
|---|---|---|---|---|
| Gender | ||||
| Male | 3 | 7 | 1 | 11 |
| Female | 6 | 2 | 9 | 17 |
| Mean age (SD) | 48.2 (6.6) | 58.7 (12.8) | 56.5 (12.2) | 54.5 (10.6) |
| Diagnosis | ||||
| Vocal fold scar | 2 | 2 | 1 | 6 |
| Pathologic sulcus vocalis | 2 | 3 | 3 | 7 |
| Both | 5 | 4 | 6 | 15 |
| Treatment material | ||||
| ePTFE | 9 | - | - | 9 |
| Calcium hydroxyapatite | - | 2 | - | 2 |
| BDDE crosslinked HA | - | 2 | - | 2 |
| Micronized acellular dermal matrix | - | 5 | - | 5 |
| Acellular dermal matrix | - | - | 7 | 7 |
| Autologous fascia | - | - | 3 | 3 |
BDDE, butanediol diglycidyl ether; ePTFE, expanded polytetrafluoroethylene; HA, hyaluronic acid.
Interventions
Patients were assigned to one of three treatment modalities: Type I thyroplasty (n = 9), injection laryngoplasty (n = 9) and graft implantation (n = 10). Treatment assignment was not randomized but rather determined by the laryngologist based on clinical judgment of the pathology and major factors causing the dysphonia. Generally, injection laryngoplasty was used for relatively small volumetric deficiencies with limited scar contracture. Type I thyroplasty was used for larger deficiencies where preoperative manual compression of the thyroid alae yielded a perceptible improvement in voice quality, and graft implantation was reserved for large deficiencies associated with extensive scarring and/or deep sulci. Neither the laryngologist nor the patient were blinded to the treatment condition. The implant or injectate material administered to each patient was determined by the laryngologist and, with the exception of expanded polytetrafluoroethylene (ePTFE; GoreTex®, Gore Medical, Flagstaff, AZ) thyroplasty, varied across patients within each treatment group (Table 1). Patients with unilateral pathology received unilateral treatment; patients with bilateral pathology received bilateral treatments. All surgical interventions were administered by one of two laryngologists. Post-operative voice rest was 24-48 h in all cases. All patients received routine peri-operative behavioral voice therapy (1-3 sessions) consisting of vocal hygiene education, resonant voice and accent method instruction, and (in cases of presumed compensatory hyperfunction) extrinsic laryngeal muscle reposturing. In each case all voice therapy was administered by a single speech-language pathologist.
Type I thyroplasty
Type I thyroplasty was performed as described by Isshiki et al.39 with additional modifications according to McCulloch et al.40 After confirmation of bilateral vocal fold mobility (prior to local or intravenous anesthesia), the soft tissues of the neck were infused at the level of the inferior border of the thyroid cartilage, where a 4-5 cm incision was made. Subplatysmal flaps were raised and the strap muscles were split in the midline to expose the larynx. The sternohyoid and thyrohyoid muscles were separated, and an inferiorly-based perichondrial flap was elevated. Next, a standard thyroid cartilage window was created, with its superior limit no higher than the midpoint between the thyroid notch and the inferior border of the thyroid cartilage at the midline. The anterior limit was no closer than 6 mm from the midline, the inferior limit was no closer than 3 mm from the inferior border, and the posterior limit was generally no further than 10 mm from the anterior margin of the window. The cartilage window was incised with an oscillating saw, removed and retained for later replacement. The strut of thyroid cartilage immediately inferior to the window was liberated from its surrounding soft tissues to allow wrapping and/or suturing of the implant to that region to prevent migration. The ePTFE implant was then inserted through the thyroid cartilage window with a bias towards placement at and below the level of the true vocal fold. Implant placement was fine-tuned via auditory-perceptual judgment of voice quality produced by the awake patient and visual-perceptual judgment of medialization obtained using simultaneous transnasal flexible endoscopy of the larynx. In cases of bilateral thyroplasty, implant placement was performed following the creation of both thyroid cartilage windows. Once satisfactory medialization was achieved, the cartilage window and perichondrial flaps were replaced and the wound closed in layers over a passive drain.
Injection laryngoplasty
All injection laryngoplasty procedures were performed in the clinic setting using a transoral approach and topical and/or local anesthesia. With the patient seated upright and leaning forward, the posterior oral cavity and oropharynx were lightly anesthetized using atomized 4% topical lidocaine. Next, three doses of 0.5-1.0 mL 4% topical lidocaine were delivered to the supraglottis and glottis using an Abraham cannula while the patient produced a sustained [i] vowel, with simultaneous visualization using a 70° rigid endoscope attached to a light source and video monitoring system (RLS9100B; Kay Elemetrics, Lincoln Park, NJ). Vocal fold injections were performed using a curved orolaryngeal injector needle (Medtronic/Xomed, Minneapolis, MN) for the delivery of butanediol diglycidyl ether crosslinked hyaluronic acid (BDDE crosslinked HA; Restylane®, Medicis Aesthetics, Scottsdale, AZ; n = 2) and micronized acellular dermal matrix (Cymetra®, LifeCell, Branchburg, NJ; n = 5), and the manufacturer's proprietary needle (bent to accommodate the transoral approach) for the delivery of calcium hydroxyapatite (Radiesse® Voice, BioForm, San Mateo, CA; n= 2). Each injection targeted the paraglottic space musculature at the junction of the posterior and middle thirds of the vocal fold, to avoid the possibility of either arytenoid rotation or anterior vocal fold overcorrection. The injectate material (0.2-0.8 mL per vocal fold) was delivered to both the infraglottic and glottic regions, and the procedure was terminated when mild overcorrection was achieved at the level of the glottis.
Graft implantation
Under adequate anesthesia, a Zeitels glottiscope (Endocraft, Boston, MA) was introduced to lateralize the false vocal folds in order to expose the true vocal fold superior surface. All subsequent vocal fold dissection and implantation procedures were performed using an operating microscope. A linear incision was placed laterally with a sharp sickle knife and extended parallel to the vocal fold edge, extending just beyond the length of the scar/sulcus deformity. This incision afforded the development of a lateral flap, identification of the vocal ligament, and placement of a securing suture away from the vocal fold medial edge. A sharp dissection technique was used to maintain flap integrity. The flap was developed by extending the dissection 2-3 mm inferior to the plane of the scar/sulcus. In each case, the intention was to create a sufficiently large pocket to allow straightforward placement of the graft followed by closure of the mucosal incision with minimal tension. Temporalis fascia, harvested from the retroauricular area (n = 3), and acellular dermal matrix (AlloDerm®, LifeCell, Branchburg, NJ; n = 7) graft materials were used. The implantion procedure was identical for both materials. The graft material was trimmed to fit inside the pocket without tension and a single 6-0 catgut suture was employed to coapt the free edges of the flap.
Vocal Function Measures
Psychosocial, auditory-perceptual, acoustic, aerodynamic and videostroboscopic data were collected less than 1 month prior to treatment, and 1, 6, 12 and 18 months post-treatment. A single speech-language pathologist with doctoral-level training and 10 years of experience evaluating patients with voice disorders conducted all data collection sessions.
Psychosocial analysis
The Voice Handicap Index (VHI) was presented in the format described by Jacobson et al.41 The VHI total score was calculated and then recalculated to eliminate tallying errors. The VHI emotional, physical and functional subscales were not employed.
Auditory-perceptual analysis
Patients were instructed to produce a 3-4 s sustained [a] vowel token and the standard phrase “the blue spot is on the key again” at comfortable pitch and loudness levels. Voice samples were recorded using a unidirectional cardioid microphone (SM58; Shure, Niles IL) placed 10 cm from the lips at a 45° angle, connected to a preamplifier (Bluetube DP; PreSonus, Baton Rouge, LA) and digital audio tape (DAT) recorder (Fostex D-5; Foster Electric, Schaumberg, IL). Digitization was performed using a 44.1 kHz sampling rate and 16-bit quantization.
A digital copy of each sample was transferred from DAT to desktop computer, sample order was randomized, and sample identity was masked prior to analysis. Auditory-perceptual ratings were performed in a quiet room and samples (sustained vowels and connected speech) were presented at ∼70 dB SPL using headphones (HD 238; Sennheiser, Old Lyme, CT). Ratings were performed using the using the Grade component of the GRBAS scale42 by a doctoral-level speech-language pathologist with 7 years of experience evaluating patients with voice disorders. A second speech-language pathologist with 10 years of comparable experience performed reliability ratings on 10% of samples. Inter-rater agreement (Spearman's ρ) was 0.81.
Acoustic analysis
Pitch range was elicited using the recording set-up reported above. Patients were instructed to produce both ascending and descending pitch glides beginning at a comfortable pitch. Each task was repeated until the speech-language pathologist was satisfied maximum performance was achieved. Maximum and minimum F0 were measured using CSpeech 4.0 (Paul Milenkovic, Madison, WI). Maximum F0 was defined as including falsetto register; minimum F0 was defined as excluding pulse register. F0 range was calculated and then converted to pitch range in semitones (ST).
Acoustic perturbation values (percent jitter, percent shimmer, signal-to-noise ratio [SNR]) were extracted from a 1 s steady-state portion of the same sustained [a] vowel used for auditory-perceptual analysis. Analyses were performed using CSpeech 4.0. The correlation dimension (D2) acoustic parameter was also measured, based on recent work demonstrating the value of nonlinear dynamic analysis in the characterization of severely aperiodic voice signals.43-46 D2 was measured using a previously reported algorithm.47,48
Aerodynamic analysis
Phonation threshold pressure (Pth) data were collected using a custom built aerodynamic measurement system. A 3 mm-bore pressure tube was placed inside the mouth perpendicular to the airstream (slightly past the front incisors), passed through a pneumotachograph mask, and attached to a narrow-band pressure transducer. A second (wide-band) pressure transducer was used to measure flow output from the mask. A microphone was positioned adjacent to the mask to record the acoustic voice signal.
Patients were trained to produce a series of repeated [pi] syllables on a single breath, starting at a whisper and gradually increasing subglottal pressure (Ps) until phonation was initiated. Once the speech-language pathologist was satisfied with patient training, the pneumotachograph mask was set in place and the [pi] syllable train task was performed five times. All productions occurred at a comfortable pitch level and were paced at approximately 1.5 syllables per s.
Pressure and flow calibration were performed before each data collection session. Signals were digitized at 8.33 Hz and 12-bit quantization using a desktop computer and CSpeech 4.0. Pressure peaks were investigated for suitable waveform morphology, and extremely sharp, shallow or irregular shaped peaks were eliminated from the analysis. The pressure peak in each train that corresponded with the initiation of phonation (as observed on the acoustic channel) was used for the analysis. Mean Pth was calculated by averaging the maximum pressure at this peak across the five trains.
Videostroboscopic analysis
Laryngeal imaging was performed using a Kay Elemetrics RLS 9100B system attached to a 70° rigid endoscope. Laryngeal maneuvers were observed using both halogen and stroboscopic light. Phonation was elicited at low, comfortable, and high pitches, and with gradual increases in loudness. The larynx was also observed at rest, during deep inspiration, and during inhalation phonation.
Videostroboscopic ratings were performed independently by the same two speech-language pathologists who performed the auditory-perceptual ratings. Patient identity and pre- or post-treatment time point were masked in all samples. Presentation order was randomized. To validate comparison across measurement points, all ratings were confined to phonation segments produced at comfortable pitch and loudness levels.
Ratings focused on vocal fold vibratory amplitude and mucosal wave excursion and were performed using the SERF instrument.49 These indices were selected as they are direct physiological correlates of mucosal pliability and can be expressed quantitatively as a percentage of vocal fold width in the medial-lateral plane. Percentage excursion for each parameter was rated using a scale resolution of 10% of total vocal fold medial-lateral width. Values were then averaged across left and right vocal folds. Inter-rater agreement (Spearman's ρ) was 0.85.
Statistical Analysis
Pre-treatment baseline value, mean post-treatment value across an 18 month period, best post-treatment value within this 18 month period, number of vocal function indices showing post-treatment improvement/deterioration/no change, and duration to best post-treatment value were calculated for all indices to facilitate visual and statistical comparisons. Individual patient data were analyzed using graphing and visual inspection and group data were analyzed using one-way ANOVAs with treatment modality as a fixed effect. An α-level of .05 was employed for all statistical testing; all p-values were two-sided.
Results
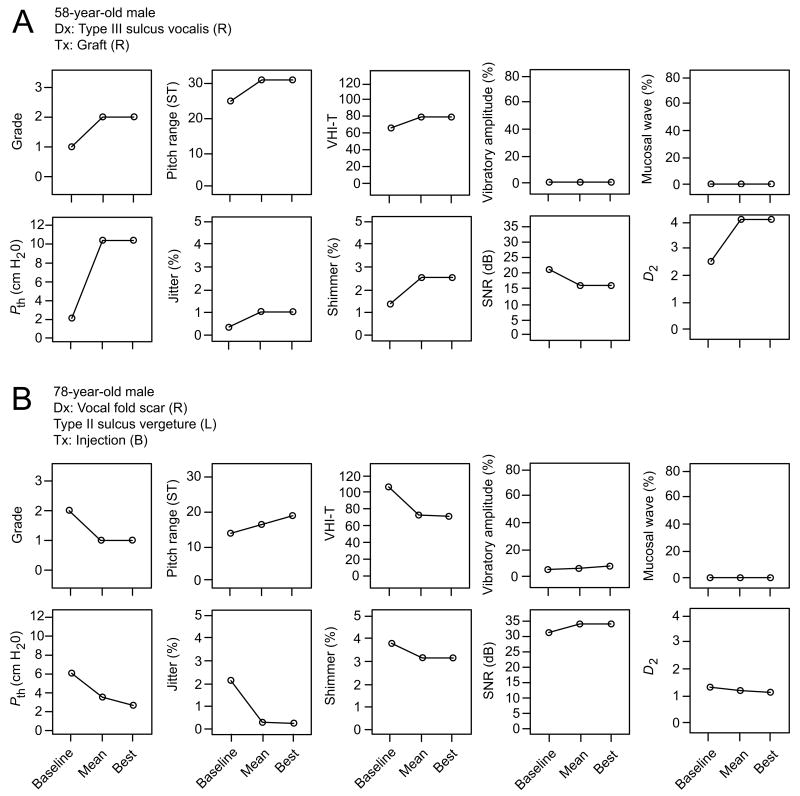
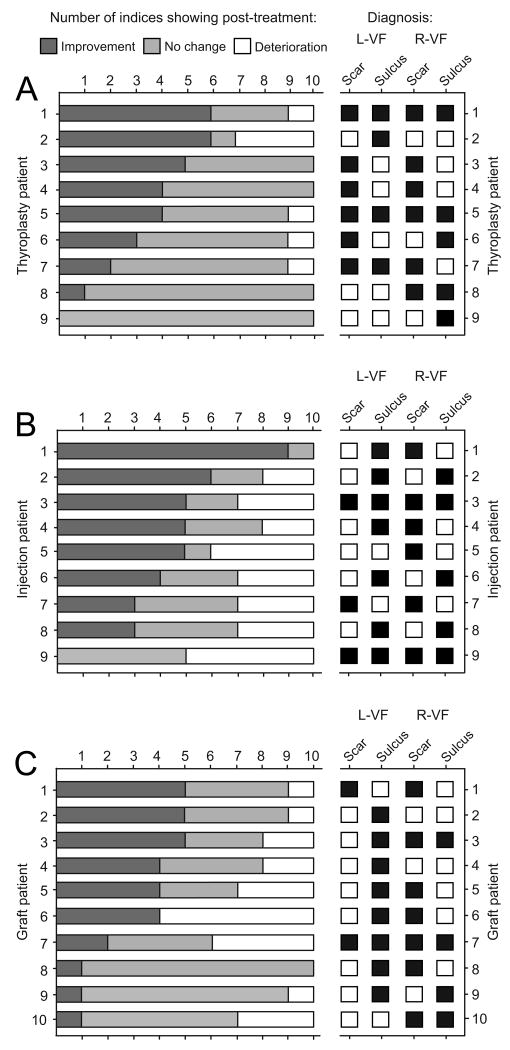
We observed a wide variation in treatment response across individual patients and treatment groups. Certain patients exhibited general deterioration reflected by the majority of vocal function indices (for a representative case, see Figure 1A), whereas others experienced robust improvement across indices (for a representative case, see Figure 1B). Eleven patients (three thyroplasty, five injection, three graft) demonstrated improvement in at least five of ten indices; two patients (one injection, one graft) demonstrated deterioration in at least five of ten indices; twenty one patients (seven thyroplasty, eleven injection, three graft) demonstrated no change in at least five of ten indices (Figure 2). Overall positive or negative outcome did not appear to correspond to pathologic sulcus subtype, the presence of sulcus with or without concomitant scar, or the presence of unilateral versus bilateral pathology (Figure 2).
Figure 1.
Representative clinical cases illustrating negative and positive outcomes following treatment for vocal fold scarring and pathologic sulcus vocalis. Panel A contains vocal function data collected from a 58-year-old male patient with right-sided Type III sulcus vocalis treated with an acellular dermal matrix graft. This patient demonstrated improved pitch range and no change/deterioration on all other indices. Videostroboscopic closure pattern was spindle-shaped on both pre-treatment and all post-treatment examinations. Panel B contains vocal function data collected from a 78-year-old male patient with right-sided vocal fold scar and left-sided Type II sulcus vergeture treated with bilateral calcium hydroxyapatite injections. This patient demonstrated improvement on all indices except mucosal wave excursion. Videostroboscopic closure pattern was spindle-shaped on both pre-treatment and all post-treatment examinations. Vocal function data are graphed as pre-treatment baseline value, mean post-treatment value across an 18 month period, and best post-treatment value at any time point within this 18 month period. B, bilateral; D2, correlation dimension; Dx, diagnosis; L, left; Pth, phonation threshold pressure; R, right; SNR, signal-to-noise ratio; ST, semitone; Tx, treatment; VHI-T, voice handicap index total score.
Figure 2.
Distribution of the number of vocal function indices showing mean post-treatment improvement, no change or deterioration following type I thyroplasty (A; n = 9), injection laryngoplasty (B; n = 9) and graft implantation (C; n = 10) treatments for vocal fold scarring and pathologic sulcus vocalis. Data are presented by individual patient on the left; key diagnostic information for each patient is presented on the right. Mean post-treatment change reflects an 18 month follow-up period. L-VF, left vocal fold; R-VF, right vocal fold.
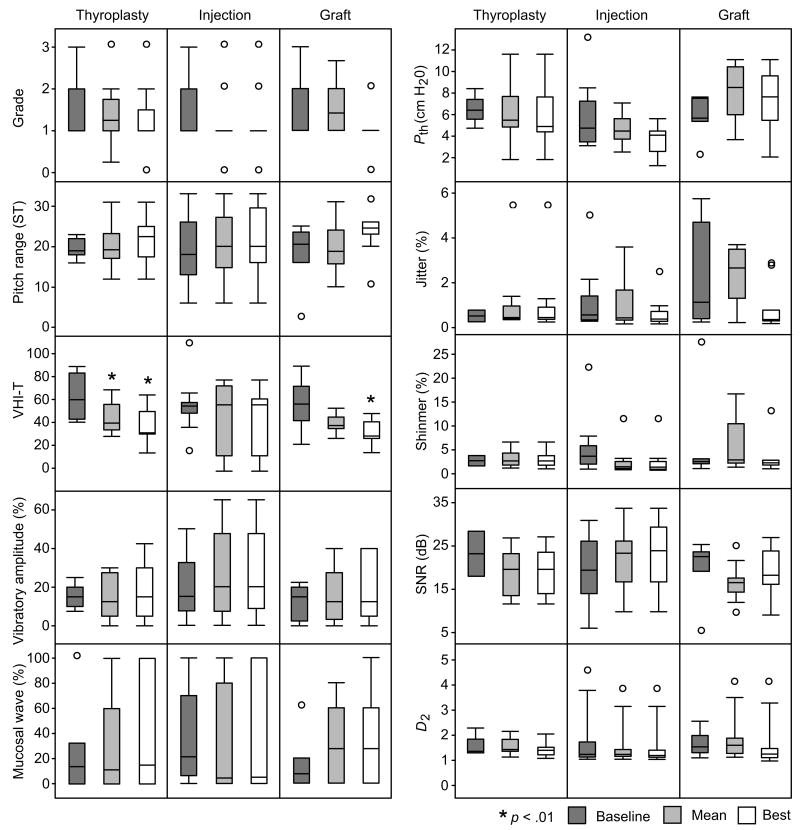
Analysis of group data revealed statistically significant improvement in mean and best post-treatment VHI total scores for the thyroplasty group, and best post-treatment VHI total score for the graft group (Figure 3). All other vocal fold function indices demonstrated non-significant post-treatment changes. Mucosal wave excursion and vibratory amplitude exhibited the greatest variance across patients.
Figure 3.
Group analysis of outcomes following type I thyroplasty (n = 9), injection laryngoplasty (n = 9) and graft implantation (n = 10) treatments for vocal fold scarring and pathologic sulcus vocalis. Vocal function data are graphed as pre-treatment baseline value, mean post-treatment value across an 18 month period, and best post-treatment value at any time point within this 18 month period. D2, correlation dimension; Pth, phonation threshold pressure; SNR, signal-to-noise ratio; ST, semitone; VHI-T, voice handicap index total score.
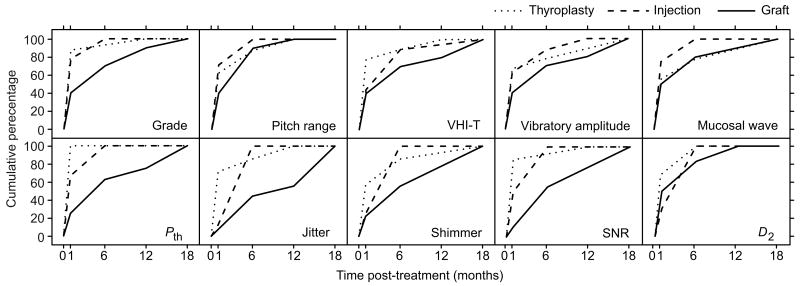
Analysis of duration to best post-treatment value revealed clear differences between treatment modalities. Patients in the graft group exhibited the slowest trajectory of improvement across all vocal function indices: 100% of these patients obtained maximum improvement in eight of ten indices at 18 months post-treatment (Figure 4); the exceptional indices were pitch range and D2, which reached the 100% plateau at 12 months post-treatment. In contrast, 100% of patients in the injection group obtained maximum improvement in the majority of indices (eight of ten) by six months post-treatment, and 100% of patients in the thyroplasty group obtained maximum improvement in the majority of indices (seven of ten) by 12 months post-treatment. Pth demonstrated the most rapid course to stability of any outcome measure in any group, exhibiting maximum improvement in 100% of thyroplasty patients at one month post-treatment.
Figure 4.
Duration to best post-treatment value following type I thyroplasty (n = 9), injection laryngoplasty (n = 9) and graft implantation (n = 10) treatments for vocal fold scarring and pathologic sulcus vocalis. Best post-treatment value reflects an 18 month follow-up period. Data are presented as cumulative percentage of total patient number within each treatment group. D2, correlation dimension; Pth, phonation threshold pressure; SNR, signal-to-noise ratio; VHI-T, voice handicap index total score.
Discussion
The purpose of this study was to compare the clinical effectiveness of type I thyroplasty, injection laryngoplasty and graft implantation for the treatment of vocal fold scar and pathologic sulcus vocalis, using a prospective, quasi-experimental research design. To our knowledge, this study represents the first multi-arm treatment comparison for these disorders in the clinical literature. No single treatment modality demonstrated clear superiority across the majority of vocal function indices used. Type I thyroplasty and graft implantation both resulted in improved VHI with no concomitant improvement in auditory-perceptual, acoustic, aerodynamic or vocal fold physiologic performance. Injection laryngoplasty resulted in no improvement on any vocal function index. Patients who underwent graft implantation exhibited the slowest trajectory of improvement across the 18 month follow-up period.
Type I thyroplasty resulted in improved mean and best post-treatment VHI total scores, whereas graft implementation resulted in improved best post-treatment score only. This discrepancy was most likely due to the relatively slow trajectory of improvement in the graft group, leading to significantly improved VHI scores at a smaller number of (later occurring) post-operative timepoints, and a relatively smaller magnitude change in mean outcome. Given that VHI outcome at 18 months post-treatment was equivalent, but recovery trajectory was slower, our data do not support graft implantation over type I thyroplasty as a primary treatment modality for patients with vocal fold scar/sulcus. A prolonged and gradual recovery period is well documented following graft implantation to the lamina propria;25,29,30,32 as such, it is possible that a number of patients in the graft group experienced meaningful improvement beyond 18 months post-treatment. If so, there may be therapeutic value with this approach that was not captured in our dataset.
Discordance between the VHI and other vocal fold function measures, as seen in our dataset, has been reported in a number of studies. Cheng & Woo50 identified significant improvement in VHI total score following surgical removal of benign vocal fold lesions in 21 patients, but no change in 12 out of 13 acoustic and aerodynamic parameters. The majority of these instrumental parameters did not correlate with the VHI. Lau et al.51 compared VHI total score and various videostroboscopic parameters in 28 patients before and after injection laryngoplasty for unilateral vocal fold paralysis. VHI total score exhibited a moderately strong correlation with closed phase duration and weak correlations with all other parameters. These findings align with the majority of descriptive (non-treatment) studies that have reported weak to moderately strong correlations between the VHI and commonly used acoustic and aerodynamic measures.52-55 It is intuitive that individuals with dysphonia have varying personalities, personal circumstances, social and occupational demands, and consequently perceive different degrees of voice-related handicap; because of this, it has been suggested there is a nonlinear relationship between voice impairment, disability and handicap.54 Overall, the VHI captures a unique aspect of voice-related psychosocial function that is independent of (and complementary to) acoustic, aerodynamic and physiologic voice function. In one sense, improved voice handicap represents one of the most meaningful aspects of treatment-induced change.
Our study design and implementation hold several limitations. First, we did not randomize patients to the three treatment groups. This allowed the laryngologist to assign patients to the presumed most-appropriate treatment based on presentation, therefore reflecting standard clinical practice; nevertheless, our findings do not carry the weight of a randomized clinical trial. Second, we did not control for implant or injectate material, but rather focused exclusively on treatment modality. This may represent a source of intra-treatment variability in the dataset. Third, we administered routine perioperative voice therapy to all patients, which again reflects standard of care but also may have influenced treatment outcome. Fourth, our recruitment success and associated sample size were limited by the decision to exclude patients who had undergone prior surgical or behavioral intervention. Due to our sample size, we were unable to delineate and categorize patient subgroups based on potentially important variables such as scar/sulcus location, unilateral versus bilateral pathology, pathologic sulcus subtype, and the presence of aggravating factors and other comorbidities. This degree of patient hetereogeneity, inherent to almost any clinical scar/sulcus study, represents another source of variability. Future research efforts in this area may benefit from multi-institutional recruitment, in addition to patient randomization and (although controversial) consideration of a placebo control (i.e., sham surgery) condition. Additional experimental precautions, such as single- or double-blinding of treatment group assignment, are difficult to implement, as in-office injection laryngoplasty does not require general anesthesia, and patients/researchers can easily identify the incision sites used for thyroplasty window creation and autologous fat/fascia harvest.
We focused on three singular treatment modalities in this study, primarily due to their prominence and reported value in the clinical literature. Consequently, it is unknown how type I thyroplasty, injection laryngoplasty and graft implantation outcomes compare to other proposed approaches such as mucosal slicing,24 pulsed dye laser irradiation,38 steroid injection,35 and superficial injection/implantation of hyaluronic acid-based biomaterials36,37 or autologous fat.27,28,33,34 Further, we did not include combinative treatments, such as CO2 laser excision followed by collagen injection,19,20 fascia implantation followed by fat injection,32 or gelatin sponge implantation followed by fat injection;26 each of which holds the conceptual appeal of simultaneously or sequentially tackling glottal incompetence and lamina propria issues, with possibly compounded benefit. Finally, a number of emerging therapies involving growth factors,56-60 phytochemical and pharmacological agents,61,62 tunable biomaterials63-70 and various cell types71-79 await future clinical translation. Appropriate evaluation of these treatment strategies requires careful investigation in appropriately powered clinical trials.
Conclusions
Our data suggest that type I thyroplasty and graft implantation lead to reduced voice handicap in patients with vocal fold scar and pathologic sulcus vocalis; however, the improvement trajectory for patients undergoing graft implementation is relatively slow. Although select patients exhibit benefit, none of the treatment approaches evaluated in this study reliably improve auditory-perceptual, acoustic, aerodynamic or vocal fold physiologic function. A persistent challenge in this area is that no single treatment modality is successful for the majority of patients, and there is no evidence-based decision algorithm for matching a given treatment to a given patient. Progress therefore requires the identification and categorization of predictive clinical features that can drive evidence-based treatment assignment. In the meantime the clinician must rely on careful assessment of the pathology so that the most critical aspects are addressed during initial treatment. Where there is extensive tissue loss and glottal incompetence, tissue replacement and medialization seem appropriate first steps. Additional gains may be seen as emerging therapies are translated to clinical practice.
Acknowledgments
This research was performed with approval of the Health Sciences Institutional Review Board of the University of Wisconsin-Madison. The work was supported by grant R01 DC004428 from the National Institute on Deafness and other Communicative Disorders.
Footnotes
Accepted for presentation at the 133rd Annual Meeting of the American Laryngological Association, April 27th-28th 2011, Chicago, IL.
The authors hold no financial or other conflicts of interest.
References
- 1.Hirano S, Minamiguchi S, Yamashita M, Ohno T, Kanemaru S, Kitamura M. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. doi: 10.1016/j.jvoice.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 3.Rousseau B, Hirano S, Scheidt TD, et al. Characterization of vocal fold scarring in a canine model. Laryngoscope. 2003;113:620–627. doi: 10.1097/00005537-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Welham NV, Montequin DW, Tateya I, Tateya T, Choi SH, Bless DM. A rat excised larynx model of vocal fold scar. J Speech Lang Hear Res. 2009;52:1008–1020. doi: 10.1044/1092-4388(2009/08-0049). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano M, Yoshida T, Tanaka S, Hibi S. Sulcus vocalis: functional aspects. Ann Otol Rhinol Laryngol. 1990;99:679–683. doi: 10.1177/000348949009900901. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto Y, Hirano S, Tateya I, Kanemaru S, Ito J. Temporal changes in vocal functions of human scarred vocal folds after cordectomy. Laryngoscope. 2010;120:1597–1601. doi: 10.1002/lary.21016. [DOI] [PubMed] [Google Scholar]
- 7.Welham NV, Dailey SH, Ford CN, Bless DM. Voice handicap evaluation of patients with pathologic sulcus vocalis. Ann Otol Rhinol Laryngol. 2007;116:411–417. doi: 10.1177/000348940711600604. [DOI] [PubMed] [Google Scholar]
- 8.Rosen CA, Lee AS, Osborne J, Zullo T, Murry T. Development and validation of the voice handicap index-10. Laryngoscope. 2004;114:1549–1556. doi: 10.1097/00005537-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Hsiung MW, Woo P, Wang HW, Su WY. A clinical classification and histopathological study of sulcus vocalis. Eur Arch Otorhinolaryngol. 2000;257:466–468. doi: 10.1007/s004050000254. [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Hirano M. Electron microscopic investigation of sulcus vocalis. Ann Otol Rhinol Laryngol. 1998;107:56–60. doi: 10.1177/000348949810700111. [DOI] [PubMed] [Google Scholar]
- 11.Benninger MS, Alessi D, Archer S, et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996;115:474–482. doi: 10.1177/019459989611500521. [DOI] [PubMed] [Google Scholar]
- 12.Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg. 2005;13:143–147. doi: 10.1097/01.moo.0000162261.49739.b7. [DOI] [PubMed] [Google Scholar]
- 13.Dailey SH, Ford CN. Surgical management of sulcus vocalis and vocal fold scarring. Otolaryngol Clin North Am. 2006;39:23–42. doi: 10.1016/j.otc.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Koufman JA, Isaacson G. Laryngoplastic phonosurgery. Otolaryngol Clin North Am. 1991;24:1151–1177. [PubMed] [Google Scholar]
- 15.Zeitels SM, Mauri M, Dailey SH. Medialization laryngoplasty with Gore-Tex for voice restoration secondary to glottal incompetence: indications and observations. Ann Otol Rhinol Laryngol. 2003;112:180–184. doi: 10.1177/000348940311200213. [DOI] [PubMed] [Google Scholar]
- 16.Su CY, Tsai SS, Chiu JF, Cheng CA. Medialization laryngoplasty with strap muscle transposition for vocal fold atrophy with or without sulcus vocalis. Laryngoscope. 2004;114:1106–1112. doi: 10.1097/00005537-200406000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Bjorck G, D'Agata L, Hertegard S. Vibratory capacity and voice outcome in patients with scarred vocal folds treated with collagen injections - case studies. Logoped Phoniatr Vocol. 2002;27:4–11. doi: 10.1080/140154302760146925. [DOI] [PubMed] [Google Scholar]
- 18.Ford CN, Bless DM, Loftus JM. Role of injectable collagen in the treatment of glottic insufficiency: a study of 119 patients. Ann Otol Rhinol Laryngol. 1992;101:237–247. doi: 10.1177/000348949210100307. [DOI] [PubMed] [Google Scholar]
- 19.Martinez Arias A, Remacle M, Lawson G. Treatment of vocal fold scar by carbon dioxide laser and collagen injection: retrospective study on 12 patients. Eur Arch Otorhinolaryngol. 2010;267:1409–1414. doi: 10.1007/s00405-010-1231-1. [DOI] [PubMed] [Google Scholar]
- 20.Remacle M, Lawson G, Degols JC, Evrard I, Jamart J. Microsurgery of sulcus vergeture with carbon dioxide laser and injectable collagen. Ann Otol Rhinol Laryngol. 2000;109:141–148. doi: 10.1177/000348940010900206. [DOI] [PubMed] [Google Scholar]
- 21.Welham NV, Rousseau B, Ford CN, Bless DM. Tracking outcomes after phonosurgery for sulcus vocalis: a case report. J Voice. 2003;17:571–578. doi: 10.1067/s0892-1997(03)00086-9. [DOI] [PubMed] [Google Scholar]
- 22.Bouchayer M, Cornut G, Witzig E, Loire R, Roch JB, Bastian RW. Epidermoid cysts, sulci, and mucosal bridges of the true vocal cord: a report of 157 cases. Laryngoscope. 1985;95:1087–1094. [PubMed] [Google Scholar]
- 23.Ford CN, Inagi K, Khidr A, Bless DM, Gilchrist KW. Sulcus vocalis: a rational analytical approach to diagnosis and management. Ann Otol Rhinol Laryngol. 1996;105:189–200. doi: 10.1177/000348949610500304. [DOI] [PubMed] [Google Scholar]
- 24.Pontes P, Behlau M. Treatment of sulcus vocalis: auditory perceptual and acoustical analysis of the slicing mucosa surgical technique. J Voice. 1993;7:365–376. doi: 10.1016/s0892-1997(05)80260-7. [DOI] [PubMed] [Google Scholar]
- 25.Kishimoto Y, Hirano S, Kojima T, Kanemaru S, Ito J. Implantation of an atelocollagen sheet for the treatment of vocal fold scarring and sulcus vocalis. Ann Otol Rhinol Laryngol. 2009;118:613–620. doi: 10.1177/000348940911800902. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Sprecher AJ, Wei C, Jiang JJ. Implantation of gelatin sponge combined with injection of autologous fat for sulcus vocalis. Otolaryngol Head Neck Surg. 2010;143:198–203. doi: 10.1016/j.otohns.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Hsiung MW, Woo P, Minasian A, Schaefer Mojica J. Fat augmentation for glottic insufficiency. Laryngoscope. 2000;110:1026–1033. doi: 10.1097/00005537-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 28.Paniello RC, Sulica L, Khosla SM, Smith ME. Clinical experience with Gray's minithyrotomy procedure. Ann Otol Rhinol Laryngol. 2008;117:437–442. doi: 10.1177/000348940811700606. [DOI] [PubMed] [Google Scholar]
- 29.Tsunoda K, Baer T, Niimi S. Autologous transplantation of fascia into the vocal fold: long-term results of a new phonosurgical technique for glottal incompetence. Laryngoscope. 2001;111:453–457. doi: 10.1097/00005537-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Tsunoda K, Kondou K, Kaga K, et al. Autologous transplantation of fascia into the vocal fold: Long-term result of type-1 transplantation and the future. Laryngoscope. 2005;115:1–10. doi: 10.1097/01.mlg.0000183966.72921.31. [DOI] [PubMed] [Google Scholar]
- 31.Pinto JA, da Silva Freitas ML, Carpes AF, Zimath P, Marquis V, Godoy L. Autologous grafts for treatment of vocal sulcus and atrophy. Otolaryngol Head Neck Surg. 2007;137:785–791. doi: 10.1016/j.otohns.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 32.Hsiung MW, Kang BH, Pai L, Su WF, Lin YH. Combination of fascia transplantation and fat injection into the vocal fold for sulcus vocalis: Long-term results. Ann Otol Rhinol Laryngol. 2004;113:359–366. doi: 10.1177/000348940411300504. [DOI] [PubMed] [Google Scholar]
- 33.Sataloff RT, Spiegel JR, Hawkshaw M, Rosen DC, Heuer RJ. Autologous fat implantation for vocal fold scar: a preliminary report. J Voice. 1997;11:238–246. [PubMed] [Google Scholar]
- 34.Neuenschwander MC, Sataloff RT, Abaza MM, Hawkshaw MJ, Reiter D, Spiegel JR. Management of vocal fold scar with autologous fat implantation: perceptual results. J Voice. 2001;15:295–304. doi: 10.1016/S0892-1997(01)00031-5. [DOI] [PubMed] [Google Scholar]
- 35.Mortensen M, Woo P. Office steroid injections of the larynx. Laryngoscope. 2006;116:1735–1739. doi: 10.1097/01.mlg.0000231455.19183.8c. [DOI] [PubMed] [Google Scholar]
- 36.Molteni G, Bergamini G, Ricci-Maccarini A, et al. Auto-crosslinked hyaluronan gel injections in phonosurgery. Otolaryngol Head Neck Surg. 2010;142:547–553. doi: 10.1016/j.otohns.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 37.Hertegard S, Hallen L, Laurent C, et al. Cross-linked hyaluronan used as augmentation substance for treatment of glottal insufficiency: safety aspects and vocal fold function. Laryngoscope. 2002;112:2211–2219. doi: 10.1097/00005537-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Mortensen MM, Woo P, Ivey C, Thompson C, Carroll L, Altman K. The use of the pulse dye laser in the treatment of vocal fold scar: a preliminary study. Laryngoscope. 2008;118:1884–1888. doi: 10.1097/MLG.0b013e31817d7546. [DOI] [PubMed] [Google Scholar]
- 39.Isshiki N, Morita H, Okamura H, Hiramoto M. Thyroplasty as a new phonosurgical technique. Acta Otolaryngol. 1974;78:451–457. doi: 10.3109/00016487409126379. [DOI] [PubMed] [Google Scholar]
- 40.McCulloch TM, Hoffman HT, Andrews BT, Karnell MP. Arytenoid adduction combined with Gore-Tex medialization thyroplasty. Laryngoscope. 2000;110:1306–1311. doi: 10.1097/00005537-200008000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson BH, Johnson A, Grywalski C, et al. The voice handicap index (VHI): Development and validation. Am J Speech Lang Pathol. 1997;6:66–70. [Google Scholar]
- 42.Hirano M. Clinical examination of voice. New York: Springer-Verlag; 1981. [Google Scholar]
- 43.Jiang JJ, Zhang Y, MacCallum J, Sprecher A, Zhou L. Objective acoustic analysis of pathological voices from patients with vocal nodules and polyps. Folia Phoniatr Logop. 2009;61:342–349. doi: 10.1159/000252851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang JJ, Zhang Y, McGilligan C. Chaos in voice, from modeling to measurement. J Voice. 2006;20:2–17. doi: 10.1016/j.jvoice.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Maccallum JK, Cai L, Zhou L, Zhang Y, Jiang JJ. Acoustic analysis of aperiodic voice: perturbation and nonlinear dynamic properties in esophageal phonation. J Voice. 2009;23:283–290. doi: 10.1016/j.jvoice.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Jiang JJ. Acoustic analyses of sustained and running voices from patients with laryngeal pathologies. J Voice. 2008;22:1–9. doi: 10.1016/j.jvoice.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, McGilligan C, Zhou L, Vig M, Jiang JJ. Nonlinear dynamic analysis of voices before and after surgical excision of vocal polyps. J Acoust Soc Am. 2004;115:2270–2277. doi: 10.1121/1.1699392. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Jiang JJ, Wallace SM, Zhou L. Comparison of nonlinear dynamic methods and perturbation methods for voice analysis. J Acoust Soc Am. 2005;118:2551–2560. doi: 10.1121/1.2005907. [DOI] [PubMed] [Google Scholar]
- 49.Poburka BJ. A new stroboscopy rating form. J Voice. 1999;13:403–413. doi: 10.1016/s0892-1997(99)80045-9. [DOI] [PubMed] [Google Scholar]
- 50.Cheng J, Woo P. Correlation between the Voice Handicap Index and voice laboratory measurements after phonosurgery. Ear Nose Throat J. 2010;89:183–188. [PubMed] [Google Scholar]
- 51.Lau DP, Zhang EZ, Wong SM, Lee G, Chan YH. Correlating voice handicap index and quantitative videostroboscopy following injection laryngoplasty for unilateral vocal paralysis. Otolaryngol Head Neck Surg. 2010;143:190–197. doi: 10.1016/j.otohns.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Hsiung MW, Pai L, Wang HW. Correlation between voice handicap index and voice laboratory measurements in dysphonic patients. Eur Arch Otorhinolaryngol. 2002;259:97–99. doi: 10.1007/s004050100405. [DOI] [PubMed] [Google Scholar]
- 53.Schindler A, Mozzanica F, Vedrody M, Maruzzi P, Ottaviani F. Correlation between the Voice Handicap Index and voice measurements in four groups of patients with dysphonia. Otolaryngol Head Neck Surg. 2009;141:762–769. doi: 10.1016/j.otohns.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 54.Wheeler KM, Collins SP, Sapienza CM. The relationship between VHI scores and specific acoustic measures of mildly disordered voice production. J Voice. 2006;20:308–317. doi: 10.1016/j.jvoice.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Woisard V, Bodin S, Yardeni E, Puech M. The Voice Handicap Index: Correlation between subjective patient response and quantitative assessment of voice. J Voice. 2007;21:623–631. doi: 10.1016/j.jvoice.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Hirano S, Bless DM, Rousseau B, et al. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope. 2004;114:548–556. doi: 10.1097/00005537-200403000-00030. [DOI] [PubMed] [Google Scholar]
- 57.Hirano S, Bless DM, Nagai H, et al. Growth factor therapy for vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2004;113:777–785. doi: 10.1177/000348940411301002. [DOI] [PubMed] [Google Scholar]
- 58.Ohno T, Hirano S, Kanemaru S, et al. Drug delivery system of hepatocyte growth factor for the treatment of vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2007;116:762–769. doi: 10.1177/000348940711601008. [DOI] [PubMed] [Google Scholar]
- 59.Kishimoto Y, Hirano S, Kitani Y, et al. Chronic vocal fold scar restoration with hepatocyte growth factor hydrogel. Laryngoscope. 2009;120:108–113. doi: 10.1002/lary.20642. [DOI] [PubMed] [Google Scholar]
- 60.Luo Y, Kobler JB, Zeitels SM, Langer R. Effects of growth factors on extracellular matrix production by vocal fold fibroblasts in 3-dimensional culture. Tissue Eng. 2006;12:3365–3374. doi: 10.1089/ten.2006.12.3365. [DOI] [PubMed] [Google Scholar]
- 61.Rousseau B, Tateya I, Lim X, Munoz-Del-Rio A, Bless DM. Investigation of antihyaluronidase treatment on vocal fold wound healing. J Voice. 2006;20:443–451. doi: 10.1016/j.jvoice.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Akdogan O, Selcuk A, Ozcan I, et al. Activation of vocal fold healing with topical vitamin A in rabbits. Acta Otolaryngol. 2009;129:220–224. doi: 10.1080/00016480802087219. [DOI] [PubMed] [Google Scholar]
- 63.Hansen JK, Thibeault SL, Walsh JF, Shu XZ, Prestwich GD. In vivo engineering of the vocal fold extracellular matrix with injectable hyaluronic acid hydrogels: early effects on tissue repair and biomechanics in a rabbit model. Ann Otol Rhinol Laryngol. 2005;114:662–670. doi: 10.1177/000348940511400902. [DOI] [PubMed] [Google Scholar]
- 64.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 65.Thibeault SL, Klemuk SA, Smith ME, Leugers C, Prestwich G. In vivo comparison of biomimetic approaches for tissue regeneration of the scarred vocal fold. Tissue Eng Part A. 2009;15:1481–1487. doi: 10.1089/ten.tea.2008.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jia X, Yeo Y, Clifton RJ, et al. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 67.Hahn MS, Teply BA, Stevens MM, Zeitels SM, Langer R. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials. 2006;27:1104–1109. doi: 10.1016/j.biomaterials.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 68.Munoz-Pinto DJ, Jimenez-Vergara AC, Gelves LM, McMahon RE, Guiza-Arguello V, Hahn MS. Probing vocal fold fibroblast response to hyaluronan in 3D contexts. Biotechnol Bioeng. 2009;104:821–831. doi: 10.1002/bit.22436. [DOI] [PubMed] [Google Scholar]
- 69.Kutty JK, Webb K. Mechanomimetic hydrogels for vocal fold lamina propria regeneration. J Biomater Sci Polym Ed. 2009;20:737–756. doi: 10.1163/156856209X426763. [DOI] [PubMed] [Google Scholar]
- 70.Kutty JK, Webb K. Vibration stimulates vocal mucosa-like matrix expression by hydrogel-encapsulated fibroblasts. J Tissue Eng Regen Med. 2009;4:62–72. doi: 10.1002/term.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krishna P, Rosen CA, Branski RC, Wells A, Hebda PA. Primed fibroblasts and exogenous decorin: potential treatments for subacute vocal fold scar. Otolaryngol Head Neck Surg. 2006;135:937–945. doi: 10.1016/j.otohns.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 72.Chhetri DK, Head C, Revazova E, Hart S, Bhuta S, Berke GS. Lamina propria replacement therapy with cultured autologous fibroblasts for vocal fold scars. Otolaryngol Head Neck Surg. 2004;131:864–870. doi: 10.1016/j.otohns.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 73.Long JL, Zuk P, Berke GS, Chhetri DK. Epithelial differentiation of adiposederived stem cells for laryngeal tissue engineering. Laryngoscope. 2009;120:125–131. doi: 10.1002/lary.20719. [DOI] [PubMed] [Google Scholar]
- 74.Long JL, Neubauer J, Zhang Z, Zuk P, Berke GS, Chhetri DK. Functional testing of a tissue-engineered vocal fold cover replacement. Otolaryngol Head Neck Surg. 2010;142:438–440. doi: 10.1016/j.otohns.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Johnson BQ, Fox R, Chen X, Thibeault S. Tissue regeneration of the vocal fold using bone marrow mesenchymal stem cells and synthetic extracellular matrix injections in rats. Laryngoscope. 2010;120:537–545. doi: 10.1002/lary.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hertegard S, Cedervall J, Svensson B, et al. Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection. Laryngoscope. 2006;116:1248–1254. doi: 10.1097/01.mlg.0000224548.68499.35. [DOI] [PubMed] [Google Scholar]
- 77.Cedervall J, Ahrlund-Richter L, Svensson B, et al. Injection of embryonic stem cells Into scarred rabbit vocal folds enhances healing and improves viscoelasticity: Short-term results. Laryngoscope. 2007;117:2075–2081. doi: 10.1097/MLG.0b013e3181379c7c. [DOI] [PubMed] [Google Scholar]
- 78.Svensson B, Nagubothu RS, Cedervall J, et al. Injection of human mesenchymal stem cells improves healing of scarred vocal folds: analysis using a xenograft model. Laryngoscope. 2010;120:1370–1375. doi: 10.1002/lary.20926. [DOI] [PubMed] [Google Scholar]
- 79.Kanemaru S, Nakamura T, Omori K, et al. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol. 2003;112:915–920. doi: 10.1177/000348940311201101. [DOI] [PubMed] [Google Scholar]