Abstract
Previous work shows a relationship between measures of morning or evening preference (e.g., Morningness-Eveningness Questionnaire (MEQ) scores) and melatonin and sleep timing, body mass index (BMI) and mood. This study explores the relationship of these factors to atypical depression (ATD) symptoms, particularly increased appetite and hypersomnia, in depressed and non-depressed peri- and post-menopausal women. Participants were 19 normal control subjects and 10 depressed patients, 46 – 72 years of age. In a university hospital setting, we administered the MEQ and Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders (SIGH-SAD version), which includes a measure of ATD, 3–5 weeks before obtaining nighttime polysomnography and overnight plasma melatonin in dim light (< 30 lux). Scores on SIGH-SAD appetite-related items were significantly correlated with MEQ, dim light melatonin onset (DLMO) time and midsleep time (MST); BMI was related to MST, sleep end time, phase-angle differences between sleep and melatonin timing, and appetite measures. Results suggest that relative to women with earlier DLMOs and MSTs, depressed peri- and post-menopausal women whose DLMOs and MSTs are phase-delayed may experience increases in appetite, hypersomnia, and BMI. These symptoms might be relieved by sleep or light manipulations that advance melatonin and sleep timing parameters.
Keywords: Atypical depression, menopause, morningness-eveningness, chronotype, dim light melatonin onset, midsleep time, BMI
1. Introduction
The Morningness-Eveningness Questionnaire (MEQ) (Horne and Ostberg, 1976) measures self-rated preference for the morning versus the evening hours. Goulet et al. (2007) found an earlier sleep onset and earlier dim light melatonin onset (DLMO) in morning vs. evening-types. In studies of clinically depressed men and women, depressed patients had lower MEQ scores (greater eveningness) than non-depressed controls (Drennan et al., 1991; Hirata et al., 2007; Gaspar-Barba et al., 2009). Parry et al. (2008) reported that severity of depressed mood, as measured by the 21-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1967), was related to duration of melatonin secretion, sleep end time, body mass index (BMI) and years-past-menopause (YPM) in a group of peri- and post-menopausal women.
The present study confirms our earlier findings of greater eveningness in depressed vs. non-depressed women at menopause (Parry et al., 2008), and extends our study to an investigation of the relationship of the MEQ score, melatonin and sleep timing to two major defining symptoms of “atypical” depression (ATD) – increased appetite and hypersomnia – that contrast with the decreased appetite and insomnia characteristic of melancholic depression. Depression with atypical features may affect 15–40% of depressed individuals (Quitkin, 2002), and women are more susceptible to ATD than men (Grigoriadis and Robinson, 2007). We measured symptoms of depression using the Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders (SIGH-SAD version)(Williams et al., 1994), which includes an 8-item measure of ATD symptoms. The primary aim of the study was to determine the extent to which symptoms of ATD in women at menopause were associated with altered melatonin timing, sleep timing, and BMI. We expected lower MEQ scores (greater eveningness) to be associated with later melatonin onset time and sleep end time, higher BMI and increased severity of ATD symptoms. A second aim was to evaluate the relationship of MEQ score, DLMO, MST and BMI to scores on individual appetite-related versus non-appetite-related SIGH-SAD atypical items. We expected the SIGH-SAD appetite and hypersomnia measures to be inversely correlated with MEQ score, positively correlated with DLMO, MST and BMI, and correlated with each other to a significantly greater degree in depressed patients (DP) than in normal control (NC) women. Finally, based on our results, we include suggestions for clinical interventions that might reduce the severity of atypical symptoms.
2. Methods
2.1. Participants
Details of the comprehensive recruitment, evaluation, selection and data collection procedures, described elsewhere (Parry et al., 2008), are summarized here. The University of California, San Diego Institutional Review Board approved the protocol, and all subjects gave written informed consent after the procedures had been explained fully. Prospective participants were peri- or post-menopausal, non-smokers, without significant medical illness, off antidepressants or medication that would interfere with the melatonin measures, off hormone replacement therapy for at least three months, and without alcohol abuse within the last year. Patients with bipolar illness were excluded. We recruited subjects through referral sources of the UCSD Department of Psychiatry Outpatient Services and Women’s Specialty Clinic, the Reproductive and Family Medicine Clinics, and the Internal Medicine Group. Through the UCSD public affairs office, we placed advertisements in local newspapers, mailed flyers to local San Diego zip codes, made on-line announcements for UCSD employees/students, and placed flyers or brochures at clinics, libraries and on campus bulletin boards in the San Diego area. Prospective participants who reported sleep problems or were shift-workers or current time-zone travelers were excluded. Participants followed their voluntary/usual sleep times and were asked to restrict napping on days when they did overnight testing. Of those who qualified for and completed the study, three of 19 NC women were peri-menopausal, with irregular menses for at least one year; the remaining NC women were postmenopausal, being without menses for at least one year, verified by FSH > 40 mIU/ml (Burger, 1994).
One of 10 DP was peri-menopausal; the remaining 9 DP women were postmenopausal, without menses for at least one year, who met DSM-IV criteria for a major depressive episode (APA, 2000).
Of the 31 women who completed the protocol, one DP who had claimed to be two years past menopause (24 months without a period) was dropped from the analyses because her serum FSH level was only 10.3 mIU/ml, and her serum estradiol level of 91.5 pg/ml was 2.65 SD above the group mean, suggesting that she was either not menopausal or was undergoing estrogen replacement, unreported to us. In addition, one NC subject, whose MEQ score (34) was 2.6 SD below the group mean, and who reported having severe fibromyalgia which disturbed her sleep and predisposed her toward eveningness, was also dropped. Characteristics of the remaining subjects are presented in Table 1. In all, 29 women aged 46–72 years completed the protocol satisfactorily -- 10 DP who met DSM-IV criteria (APA, 2000) for a major depressive episode, plus 19 NC subjects. Depression severity was evaluated with the Structured Interview Guide for the Hamilton Depression Rating Scale (SIGH-SAD) (Williams et al., 1994) which included the 21-item HDRS (Hamilton, 1967), an 8-item addendum of atypical items, and a 12-item Hypomania Rating Scale. Three NC women were peri-menopausal, with irregular menses during the previous year; the remaining 16 NC women were postmenopausal, being without menses for at least one year, verified by FSH > 40 mIU/ml. One DP woman was peri-menopausal; the remaining 9 DP women were postmenopausal, without menses for at least one year.
Table 1.
Mean (SD) SIGH-SAD Atypical Score, Morningness-Eveningness Questionnaire (MEQ) Score, Demographic Characteristics, Sleep and Melatonin Timing Parameters and Phase Angle Differences (PADs) in Normal Control Subjects and Depressed Patients.
| Normal Control Mean (SD) |
Depressed Patients Mean (SD) |
P (NC vs. DP) | |
|---|---|---|---|
| SIGH-SAD Atypical Score | 1.6 (1.6) | 9.7 (4.6) | 0.001 |
| MEQ Score | 61.6 (8.1) | 53.0 (9.1) | 0.015 |
| Personal History of Depression | 3/19 (15.8%) | 10/10 (100%) | 0.001 |
| Age | 56.3 (7.3) | 53.8 (3.5) | 0.328 |
| Years Past Menopause | 8.1 (7.9) | 4.3 (3.7) | 0.167 |
| BMI | 26.9 (4.8) | 28.2 (5.4) | 0.530 |
| Sleep Onset Time | 23:27 (0:55) | 23:35 (0:46) | 0.673 |
| Midsleep Time (MST) | 02:50 (0:46) | 03:10 (0:31) | 0.250 |
| Sleep End Time | 30:13 (1:00) | 30:43 (0:32) | 0.161 |
| Total Sleep Time | 315.8 (93.2) | 296.2 (95.2) | 0.597 |
| DLMO | 19:48 (1:27) | 20:25 (1:24) | 0.446 |
| PAD(DLMO – MST) | 7.0 (1.60) | 6.9 (1.30) | 0.597 |
| DLMOff | 8:42 (1:10) | 9:15 (1:20) | 0.446 |
| PAD(MST - DLMOff) | 5.9 (1.40) | 6.1 (1.30) | 0.686 |
2.2. Mood assessments
All subjects underwent a Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995) to confirm diagnoses. For study inclusion, DP had mean scores on the 21-item Hamilton Depression Rating Scale (HDRS) ≥ 14; Beck Depression Inventory (BDI) ≥ 10 (Beck et al., 1961), for two weeks. NC subjects had no clinically significant mood changes during the month, and mean HDRS ratings ≤ 8, and BDI ratings ≤ 5. Subjects completed the MEQ ratings 3–5 weeks before admission.
2.3. Admissions
Participants were admitted to the General Clinical Research Center (GCRC) of the University of California, San Diego Medical Center. The first night was an adaptation to the sleep laboratory in which oximetry and periodic leg movements were recorded to rule out sleep disorders. On the second night, polysomnographic (PSG) recordings were obtained. On the third night, serial blood samples for melatonin were collected every 30 minutes from 18:00-11:00 h. Serum samples for estradiol, progesterone, and FSH were drawn at 18:00 and 06:00 h. Sleep and testing took place under dim light (< 30 lux). Nurses and sleep technicians entered the room only when necessary (recorded by infrared camera or telecom), using a pen-size dim red flashlight. During sleep times on nights of frequent blood sampling, GCRC nurses threaded the intravenous catheter through a porthole in the wall and drew samples from an adjoining room to minimize sleep disturbances. The routine is best described as “semi-constant.” Since data reported here were collected only during a single overnight (from 16:00 – 12:00 h the following day), participants did not leave the hospital during testing; participants followed voluntary/usual sleep times and were asked to restrict napping on days when they did overnight testing.
Assays are described in previously published methods (Anderson et al., 1976; Yoon et al., 2004; Kripke et al., 2005). Plasma melatonin concentrations were measured by radioimmunoassay with kits manufactured by IBL Immuno-Biological Laboratories, Hamburg, Germany. The assay has an intra-assay coefficient of variation of approximately 8% with an inter-assay coefficient of variation of approximately 15%. The standard range is from 12.9 – 1290 pmol/L, with an assay sensitivity of 10.8 pmol/L. The dim light melatonin onset (DLMO) and offset (DLMOff) were determined by visual inspection of individual melatonin profiles, as described previously (Parry et al., 2008).
2.4. Sleep measures
All-night PSG recordings (EEG, EOG, sub-mental EMG) were digitized, stored on optical discs and scored visually in 30 second epochs without knowledge of conditions for sleep stages according to Rechtschaffen and Kales (1968) criteria by sleep technicians (inter-rater reliability κ coefficient 0.85) trained in the J. Christian Gillin Laboratory of Sleep and Chronobiology. We measured PSG-derived total sleep time (TST), sleep latency, sleep efficiency, wake after sleep onset, and focus on sleep onset time (SOT), midsleep time (MST) and sleep end time (SET) in this report.
2.5. Statistical Analyses
We evaluated differences between NC and DP using analysis of variance (ANOVA) and analysis of covariance (ANCOVA) with age or DLMO as the covariate in those cases where age or DLMO were significantly correlated (p < 0.05) with the dependent variable of interest. Two-tailed (non-directional) hypotheses were assumed for all comparisons. We calculated phase-angle difference (PAD) scores to describe temporal relationships of melatonin timing parameters to SOT, MST and SET, including: DLMO-SOT, DLMO-MST, DLMO-SET; SOT-DLMOff, MST-DLMOff, and SET-DLMOff. For simplicity, each value was assigned a positive sign. To confirm correlational results, we performed median splits on the MEQ score, DLMO, MST, BMI and PAD scores prior to performing ANOVA to test effects of these variables on SIGH-SAD scores and other variables. Pearson correlations were calculated for SIGH-SAD atypical appetite-related items, PSG variables, and other variables of interest. Partial correlation coefficients derived from “backward” linear regression analyses were calculated in cases where variables were significantly inter-correlated with each other.
For analyses on the individual SIGH-SAD atypical items, we used the mean of four administrations obtained during clinical evaluations for each of the 8 SIGH-SAD atypical items. We calculated Pearson correlations for MEQ score, DLMO, MST, PAD values and BMI with each appetite-related item (Increased Appetite, Increased Eating, Carbohydrate Craving, Weight Gain) and their mean, plus the non-appetite-related items (Fatigue, Social Withdrawal, Hypersomnia, Anger), and their mean, separately, in NC vs. DP. We tested the significance of the differences between the NC and DP correlations with the Fisher r-to-z transformation (two-tailed tests). To evaluate the effects of ambient light exposure, we added daylight hours, sunrise time, and sunset time as covariates in our analyses; we found no effect of these covariates.
3. Results
As expected, the mean SIGH-SAD atypical score was significantly higher in DP vs. NC, F(1,27) = 50.4, p = 0.0001, and mean MEQ score was significantly lower (greater eveningness) in DP vs. NC, F(1,27) = 6.76, p = 0.015 (see Table 1). Of the DP, all 10 had had multiple prior episodes of depression (mean = 3.0 episodes; range = 2–5); of the 19 NC subjects, two had had a single prior episode and one had had 4 prior episodes. Groups were not significantly different in any of the other demographic, melatonin timing or PSG measures (all p > 0.05). A Chi Square analysis of the distribution of MEQ scores in the NC vs. DP groups showed DP subjects were more often “neither” or “evening” types than NC subjects were (Χ2 = 6.56, p = 0.038), with morning-types occurring more than three times as often among NC as among DP (63% vs. 20%).
3.1. Correlations in NC + DP combined: SIGH-SAD atypical score with MEQ score, melatonin timing, sleep timing, BMI and years past menopause
In NC + DP combined, multiple linear regression analyses showed MEQ score was correlated significantly and negatively with DLMO (partial r = −0.636, p = 0.001), SET (partial r = −0.547, p = 0.004), and BMI (partial r = −0.633, p = 0.001), but positively with YPM (partial r = 0.397, p = 0.045), indicating that lower MEQ score (greater eveningness) was associated with later melatonin onset, later sleep end time, greater BMI, and fewer years-past-menopause. (When we substituted age for YPM, age did not contribute significantly to the model (partial r = 0.198, p = 0.332)). The MEQ score also was correlated negatively to a numerically smaller degree with MST (partial r = −0.337, p = 0.085) than with SET, but the difference between these correlations was not statistically significant (Fisher r-to-z transformation, p > 0.05). Sleep onset time and dim light melatonin offset time (DLMOff) were not significantly related to MEQ score (all p > 0.05). Notably, the sleep quality measures (TST, sleep latency, sleep efficiency and wake after sleep onset) also were not related significantly to SIGH-SAD atypical scores, melatonin timing, or BMI in NC alone, DP alone, or NC+DP combined (all p > 0.05; data not shown).
3.2. Correlations in NC vs. DP, separately: Relationships of SIGH-SAD atypical depression scores and BMI to melatonin timing, sleep timing and phase-angle difference scores
When the groups were analyzed separately, the DLMO was correlated significantly and negatively with MEQ score in both NC (r = −0.555, p = 0.017) and DP (r = −0.716, p = 0.022). Thus, whether they were DP or NC subjects, the DLMO was phase-delayed in women who endorsed greater eveningness relative to those endorsing greater morningness. Furthermore, while MST was not correlated significantly with MEQ score in NC or DP (both p > .05), MST was correlated significantly with DLMO in DP (r = 0.707, p = 0.033) but not NC, indicating that DLMO and MST tended to covary in DP.
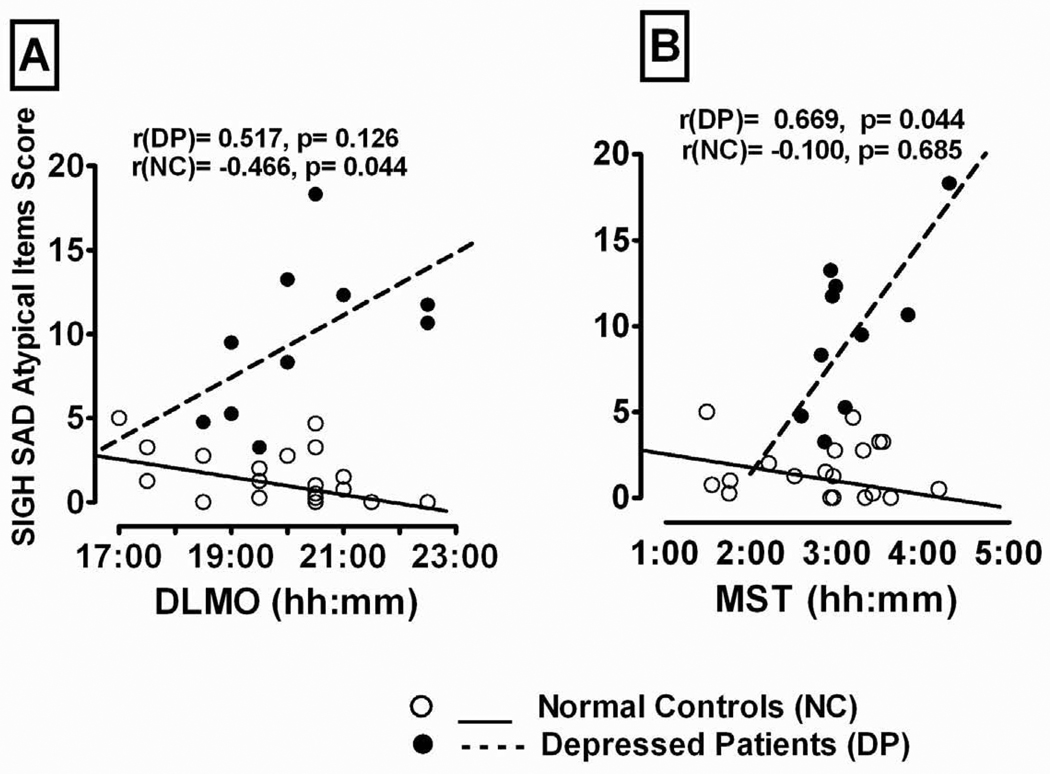
As Table 2 shows, the total of the SIGH-SAD ATD items was related significantly to MEQ score in NC+DP combined, but to none of the other melatonin timing, sleep timing, or PAD measures (all p > 0.05). Furthermore, in the NC group, the correlations of the SIGH-SAD atypical score with the MEQ score, melatonin timing and sleep timing measures were mostly negative and non-significant (p > 0.05); however, in the DP group, the SIGH-SAD ATD score was correlated positively with DLMO (r = 0.517, p = 0.126) and MST (r = 0.669, p = 0.035), and these correlations were significantly greater in DP than NC (Fisher r-to-z transformation) for both DLMO (p = 0.017) and MST (p = 0.044); thus, the DP with longer phase-delays in DLMO and MST had higher ATD scores than those with shorter phase-delays (see Fig. 1). In partial confirmation of this finding, based on a median split on the DLMO and MST scores, ANOVA showed depressed women with DLMOs above the median had significantly higher SIGH-SAD atypical scores than those with DLMOs below the median (mean ± SD = 13.3 ± 3.0 vs. 6.2 ± 2.6; F(1,8) = 15.84, p = 0.004). Similarly, DP with MSTs above the median had somewhat higher SIGH-SAD atypical scores than those with MSTs below the median (mean ± SD = 11.8 ± 4.8 vs. 7.7 ± 4.3), but this difference did not attain statistical significance, F(1,7) = 2.29, p = 0.174 (with Age as covariate in the analysis). Lastly, all correlations between atypical score and the PAD measures were non-significant in both the NC and DP groups; nor were the differences between groups significant (all p > 0.05; data not shown).
Table 2.
Pearson Correlations for SIGH-SAD Atypical Score with Morningness-Eveningness Questionnaire (MEQ) Score, Dim Light Melatonin Onset (DLMO), Dim Light Melatonin Offset (DLMOff), Sleep Onset Time (SOT), Midsleep Time (MST), Sleep End Time (SET), and Phase-angles Differences between DLMOff and Sleep Timing Parameters in Normal Controls (NC), Depressed Patients (DP) and NC+DP combined.
| Correlations with SIGH-SAD Atypical Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin Timing | Sleep Timing | Phase-angle Differences | ||||||||
| MEQ | DLMO | DLMOff | SOT | MST | SET | SOT - DLMOff |
MST - DLMOff |
SET - DLMOff |
BMI | |
| NC+DP | −0.505** | +0.171 | +0.127 | +0.147 | +0.304 | +0.336 | +0.021 | −0.042 | −0.099 | +0.210 |
| NC | −0.078 | −0.466* | −0.312 | −0.198 | −0.100 | +0.028 | −0.120 | −0.207 | −0.264 | −0.071 |
| DP | −0.439 | +0.517 | +0.057 | +0.469 | +0.669* | +0.621 | −0.226 | −0.207 | −0.176 | +0.388 |
| p | n.s. | 0.017 | n.s. | n.s. | 0.044 | n.s. | n.s. | n.s. | n.s. | n.s. |
Asterisks denote significance of correlations: * = p < 0.05.
P-values in boldface denote significant differences between correlations of NC vs. DP.
Fig. 1.
Relationship of (A) Dim Light Melatonin Onset (DLMO) time and (B) Midsleep Time (MST) to total SIGH-SAD atypical score in depressed patients (DP) and normal control (NC) peri- and post-menopausal women.
Similarly, Table 3 shows that in NC+DP combined as well as in NC subjects alone, BMI was not correlated significantly with measures of melatonin timing, sleep timing, or any of the PAD measures (all p > 0.05). In contrast, in DP alone, BMI was positively correlated significantly with MST (p = 0.044) and SET (p = 0.023), and these correlations proved to be significantly greater in DP than NC (Fisher r-to-z transformation) for both MST (p = 0.031) and SET (p = 0.024); thus, the later the MST and SET, the greater the BMI of depressed, but not NC, women. As with the DLMO, based on a median-split analysis on BMI scores, ANCOVA (with age as covariate) showed that the mean BMI of DP whose MSTs were above the median was significantly greater than the mean of those with MSTs below the median (mean ± SD BMI = 30.6 ± 5.8 vs. 25.8 ± 4.1; F(1,7) = 6.09, p = 0.043). As above, in NC subjects, the difference between those with BMIs above or below the median was not significant (p > 0.05). Thus, DP with delayed MSTs and SETs had greater BMIs than those with earlier MSTs and SETs.
Table 3.
Pearson Correlations for Body Mass Index (BMI) with Morningness-Eveningness Questionnaire (MEQ) Score, Dim Light Melatonin Onset (DLMO), Dim Light Melatonin Offset (DLMOff), Sleep Onset Time (SOT), Midsleep Time (MST), Sleep End Time (SET), and Phase-angles Differences between DLMOff and Sleep Timing Parameters in Normal Control (NC) subjects, Depressed Patients (DP) and NC+DP combined.
| Correlations with Body Mass Index | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Melatonin Timing | Sleep Timing | Phase-angle Differences | |||||||
| MEQ | DLMO | DLMOff | SOT | MST | SET | SOT - DLMOff |
MST - DLMOff |
SET - DLMOff |
|
| NC+DP | −0.353 | −0.188 | −0.228 | +0.004 | +0.053 | 0.078 | −0.199 | −0.236 | −0.239 |
| NC | −0.381 | −0.198 | −0.110 | −0.184 | −0.208 | −0.150 | +0.027 | +0.023 | +0.015 |
| DP | −0.264 | −0.233 | −0.498 | +0.378 | +0.646* | +0.704* | −0.765** | −0.761* | −0.700* |
| p | n.s. | n.s. | n.s. | n.s. | 0.031 | 0.024 | 0.023 | 0.024 | 0.051 |
Asterisks denote significance of correlations: * = p < 0.05;
= p < 0.01.
P-values in boldface denote significant differences between correlations of NC vs. DP.
Finally, the correlations between BMI and the PAD measures (SOT-DLMOFF, MST-DLMOff and SET-DLMOff) were significant in DP but not NC or NC + DP combined (p < 0.05), and were also significantly more negative in DP than NC for SOT-DLMOFF (p = 0.023) and MST-DLMOff (p = 0.023), while approaching significance for SET-DLMOff (p = .051). Thus, in DP but not NC, BMI was related inversely to the length of the time between SOT, MST, SET and DLMOff: the shorter the interval the greater the BMI. In confirmation of this result, based on a median-split analysis, ANOVA showed that the mean BMI of DP with PAD (SOT-DLMOff) below the median was significantly greater than that of those with PADs above the median (mean ± SD BMI = 31.1 ± 4.2 vs. 25.5 ± 4.5, F(1,8) = 5.31, p = 0.050). Similarly, the mean BMI of DP with PAD (MST-DLMOff) below the median was significantly greater than that of those with PADs above the median (mean ± SD BMI = 33.0 ± 3.8 vs. 24.9 ± 4.1; F(1,8) = 12.1, p = 0.008). Further, the distribution of PAD values and the median splits on PAD (MST-DLMOff) and PAD (SET-DLMOff) turned out to be identical, so the results of the ANOVA based on the median splits were identical for both. Thus, DP with shorter time delays between SOT, MST or SET and DLMOff had greater BMIs than those with longer time delays. By comparison, in the NC group the correlations between BMI and these PAD variables were all small and non-significant (all p > 0.05).
3.3. Relationship of individual SIGH-SAD appetite-related and non-appetite-related items to MEQ score, DLMO, MST and BMI in NC vs. DP
Table 4 shows the correlations of MEQ score with the appetite-related and the non-appetite-related SIGH-SAD atypical items. The correlations for the non-appetite-related atypical items (Fatigue, Social Withdrawal, Hypersomnia and Anger) and their mean were small and non-significant in both NC and DP (all p > 0.05). In contrast, for the appetite-related atypical items, the correlations were significantly greater (more negative) in DP than NC for MEQ score with Increased Appetite (p = 0.014) and Increased Eating (p = 0.003); for Weight Gain and the mean of the four appetite-related items the correlations were numerically more negative in DP than NC subjects, but the differences between the correlations were non-significant (p > 0.05); see Fig. 2A. In confirmation of the correlation data, ANOVA with median-split on MEQ scores showed that for women whose MEQ scores were below the median (greater eveningness), DP scores were significantly higher than NC on Increased Appetite, F(1,12) = 9.18, p = 0.010 and Increased Eating F(1,12) = 6.16, p = 0.029, as well as on Carbohydrate Craving F(1,12) = 5.35, p = 0.039, and the mean of the 4 appetite-related items, F(1,12) = 7.45, p = 0.018; but for women with MEQ scores above the median (greater morningness), DP and NC did not differ significantly on their scores on appetite-related items (all p > .05). Thus, among women who endorsed greater eveningness, DP had higher appetite-related atypical scores than NC; but the difference between groups was not significant for subjects endorsing greater morningness.
Table 4.
Pearson correlations relating Morningness-Eveningness Questionnaire (MEQ) Score, Dim Light Melatonin Onset (DLMO) Time, Midsleep Time (MST) and Body Mass Index (BMI) to Appetite-Related and Non-Appetite-Related SIGH-SAD Atypical Items, in Normal Control (NC) Subjects and Depressed Patients (DP).
| Appetite-Related SIGH-SAD Atypical Items |
Non-Appetite-Related SIGH-SAD Atypical Items |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Increased Appetite |
Increased Eating |
Carbohydrate Craving |
Weight Gain |
Mean of Appetite- related Items |
Fatigue | Social Withdrawal |
Hypersomnia | Anger | Mean of Non- Appetite-related Items |
|
| MEQ | ||||||||||
| r(NC) | +0.238 | +0.466* | −0.419 | −0.046 | −0.030 | −0.090 | +0.185 | −0.099 | +0.058 | −0.001 |
| r(DP) | −0.705* | −0.562 | −0.380 | −0.566 | −0.601 | −0.113 | +0.040 | −0.207 | +0.072 | −0.051 |
| p | 0.014 | 0.003 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| DLMO | ||||||||||
| r(NC) | −0.389 | −0.338 | −0.225 | −0.525 | −0.426 | −0.039 | −0.307 | −0.260 | −0.512 | −0.378 |
| r(DP) | +0.785** | +0.685* | +0.616 | +0.498 | +0.715* | +0.038 | +0.222 | +0.109 | −0.158 | +0.078 |
| p | 0.001 | 0.002 | 0.036 | 0.003 | 0.001 | n.s. | n.s. | n.s. | n.s. | n.s. |
| MST | ||||||||||
| r(NC) | −0.470 | −0.598* | 0.034 | −0.344 | −0.234 | 0.097 | +0.211 | −0.188 | −0.022 | 0.054 |
| r(DP) | +0.574 | +0.549 | +0.596 | +0.767** | .+0.666* | −0.267 | +0.455 | +0.713* | +0.512 | +0.463 |
| p | 0.010 | 0.004 | n.s. | 0.002 | 0.021 | n.s. | n.s. | 0.017 | n.s. | n.s. |
| BMI | ||||||||||
| r(NC) | −0.032 | −0.125 | −0.008 | +0.057 | −0.226 | −0.197 | +0.054 | −0.176 | −0.239 | −0.226 |
| r(DP) | +0.764 | +0.596 | +0.587 | +0.642 | +0.742 | −0.136 | +0.001 | +0.561 | +0.494 | +0.276 |
| p | 0.022 | n.s. | n.s. | n.s. | 0.009 | n.s. | n.s. | n.s. | n.s. | n.s. |
Significant correlations are identified with asterisks: * = p < 0.05;
= p <0.01.
P-values denoting significant differences between the correlations in NC and DP are highlighted in boldface.
N.B.: The correlations between BMI and SIGH-SAD items are first-order/partial correlations, controlling for DLMO.
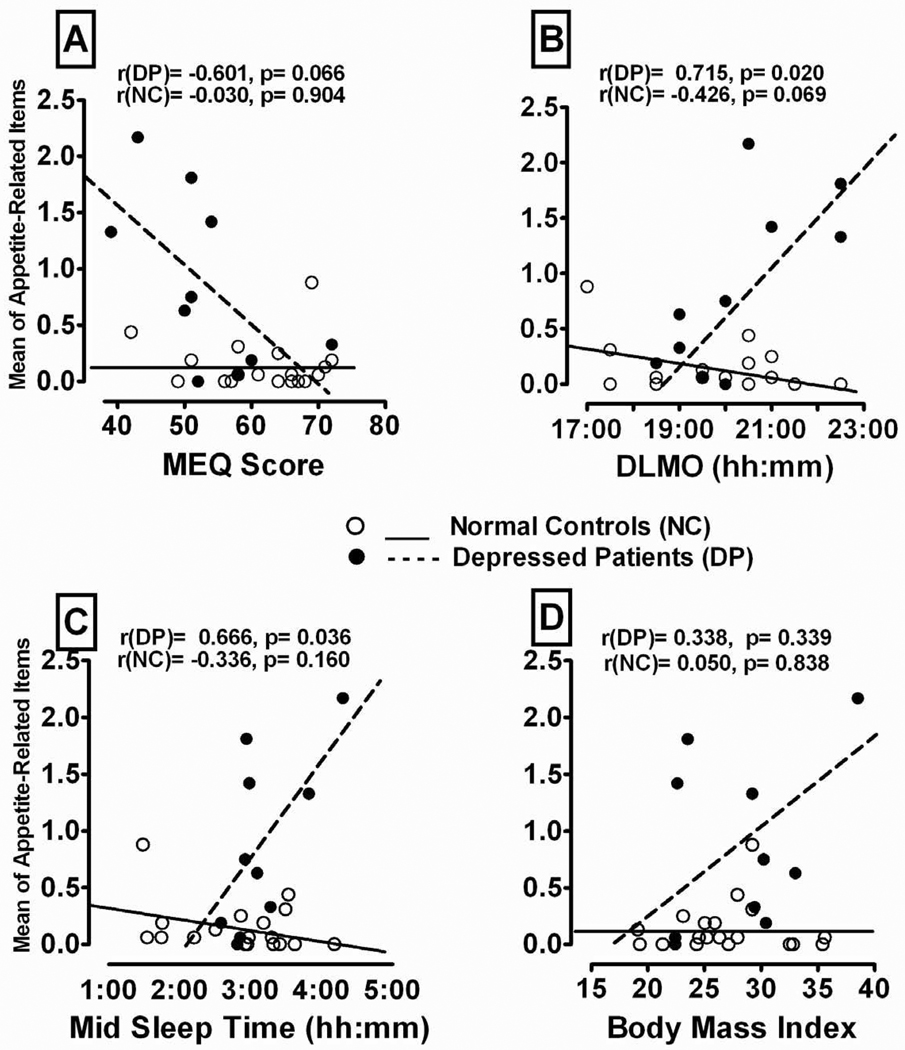
Fig. 2.
Pearson correlations between the mean of the Appetite-Related SIGH-SAD atypical item scores and the (A) Morningness-Eveningness Questionnaire (MEQ) score, (B) Dim Light Melatonin Onset (DLMO) time, (C) Midsleep Time (MST), and (D) Body Mass Index in Depressed Patients (DP) vs. Normal Control (NC) peri- and post-menopausal women.
Similarly, Table 4 shows that the correlations between DLMO and non-appetite-related items were small and non-significant in both NC and DP; but the correlations between DLMO and the appetite-related SIGH-SAD atypical scores were significantly more positive in DP than NC for each of the four atypical appetite-related items and their mean (all p < 0.05, at least). In partial confirmation of this finding, based on a median split between higher and lower DLMO scores, ANOVA showed that depressed women with DLMOs above the median had significantly higher SIGH-SAD atypical scores than those with DLMOs below the median (mean ± SD = 13.3 ± 3.0 vs. 6.2 ± 2.6; F(1,8) = 15.84, p = 0.004).
Confirming the correlational data, ANOVA on the median-split on DLMO scores showed that for women with DLMOs above the median, DP had significantly higher scores than NC subjects on Increased Appetite, F(1,12) = 12.28, p = 0.001, Increased Eating F(1,12) = 13.01, p = 0.007, Carbohydrate Craving F(1,12) = 30.61, p = 0.0001, Weight Gain F(1,12) = 6.44, p = 0.035 and the mean of the appetite-related items, F(1,12) = 22.66, p = 0.001; but for women with DLMOs below the median, DP and NC subjects did not differ significantly on appetite-related items (all p > 0.05). Thus, among women with phase-delayed DLMOs, DP had higher appetite-related atypical scores than NC subjects, but this group difference was not significant in participants with earlier DLMOs. Thus, DP with longer DLMO phase-delays had significantly higher scores on appetite-related atypical items than their NC counterparts with comparably longer phase-delays in DLMO (see Fig. 2B).
Correlations were also significantly more positive in DP than NC for MST with the appetite-related items, except for “Carbohydrate Craving” (p > 0.05); the DP with greater MST phase-delays had significantly higher appetite-related atypical scores than the DP with shorter MST phase-delays (see Fig. 2C). Confirming the correlation data, ANOVA with median-split on MST scores showed that DP women with more phase-delayed MSTs (above the median) had significantly higher scores than NC on Increased Appetite, F(1,12) = 22.46, p = 0.0005, Increased Eating, F(1,12) = 12.23, p = 0.004, Carbohydrate Craving, F(1,12) = 16.18, p 0.002, Weight Gain, F(1,12) = 7.03, p = 0.021 and the mean of these four items, F(1,12) = 18.86, p =0 .001; but for the women with earlier MSTs (below the median), DP and NC did not differ significantly on appetite-related items (all p > 0.05). Thus, DP with later MSTs had higher appetite-related atypical scores than NC, but this difference was not significant among women with earlier MSTs.
As a point of particular interest, the correlations of MST with non-appetite-related items were non-significant in both DP and NC with one important exception: A significantly higher correlation between MST and hypersomnia in DP than NC (p = 0.017); DP with later MSTs reported greater hypersomnia than those with earlier MSTs – a relationship absent in NC subjects. ANOVA confirmed that DP reported significantly greater hypersomnia than NC when MST was above the median (mean ± SD = 0.97 ± 0.23 vs. 0.25 ± 0.12, F(1,12) = 4.81, p = 0.049), but DP and NC did not differ in hypersomnia when MST was below the median (p >0.05). However, of particular relevance to this study was our finding that, contrary to expectation, the TSTs of the DP did not differ significantly from those of the NC subjects (Table 1). Furthermore, ANOVA after a median split on total SIGH-SAD atypical score showed the groups with ATD scores below and above the median (mean ± SD = 310.7 ± 91.7 vs. 307.5 ± 96.7 min) did not differ significantly from each other, F(1,26) = 0.009, p = 0.926). Furthermore, the correlations between the participants’ SIGH-SAD hypersomnia scores and their TSTs also were non-significant in NC (r = 0.044, p = 0.857), DP (r = −0.195, p = 0.588) and NC+DP combined (r = −0.109, p = 0.574).
Our initial analyses showed the Pearson correlations between BMI and appetite-related atypical scores were numerically more positive in DP than NC, but the correlations in both diagnostic groups and the differences between the group correlations were non-significant (all p > 0.05) (see Fig. 2D). However, as shown in Table 4, when DLMO was controlled for using partial correlation, the first-order correlations of BMI with the appetite-related items were small and mostly negative in NC, but were positive and moderately large in DP. In fact, the correlations of BMI with Appetite Increase (partial r = 0.764 p = 0.017), and with the mean of the appetite items (partial r = 0.742, p = 0.022) were significant, as were the differences between these correlations in NC and DP (Fisher r-to-z transformation, p < .05). Partial confirmation for this conclusion comes from ANCOVA following a median-split analysis on BMI, with DLMO as a covariate, showing that the Increased Appetite scores were higher in DP (but not NC) when BMIs were above the mean than when they were below the mean, F(1,7) = 6.69, p = 0.041.
Finally, the correlations between PAD scores and the appetite-related and non-appetite related items were all non-significant in both NC and DP, as were the differences between the correlations of the separate diagnostic groups (all p > 0.05; data not shown).
4. Discussion
The main findings of the present study of depressed peri- and post-menopausal women were: (1) Symptoms of atypical depression were more severe in depressed women who were more evening-types (lower MEQ scores) and whose DLMOs and MSTs occurred later in time, than in those who were more morning-types (higher MEQ scores) and whose DLMOs and MSTs occurred earlier; (2) With regard to individual SIGH-SAD atypical items, scores on items relating to appetite were greater (a) in more evening-types than in more morning-types and (b) in women whose DLMOs and MSTs occurred later, rather than earlier, in time; (3) BMIs were greater in depressed women with (a) later MSTs and SETs than in those with earlier MSTs and SETs, (b) those in whom the PAD time interval separating SOT, MST and SET from DLMOff was shorter, rather than longer, and (c) those with higher, rather than lower scores on the SIGH-SAD Increased Appetite atypical item and the mean of the appetite-related items; (4) self-reported hypersomnia in DP was related to MST, but was not correlated with objectively-measured TST.
4.1. Relationship of MEQ score to melatonin, sleep timing, and years past menopause
Women who endorsed greater eveningness (lower MEQ scores) had DLMOs and MSTs that were phase-delayed relative to those endorsing greater morningness, irrespective of diagnosis. Sleep onset and melatonin offset times were not significantly related to morning vs. evening preference. Thus, our results provide confirmation in peri- and post-menopausal women of the earlier findings (Goulet et al., 2007); Burgess and Eastman (2006), Martin and Eastman (2002), that morning-types have significantly earlier DLMOs and SETs than evening-types, and that phase-delayed melatonin onset was associated with delayed sleep end time.
We found that eveningness decreased with YPM, as did atypical item scores. Thus, severity of atypical depressive symptoms tended to resolve as morningness increased over time, following the onset of the menopausal transition. In contrast to Drennan et al. (1991), we did not find that age was significantly correlated with depression severity in our subjects, suggesting that depression in this cohort of peri- and post-menopausal women might have been related more specifically to hormonal alterations after menopause, rather than to age, per se.
4.2. Relationship of SIGH-SAD atypical depression score to DLMO and MST
Atypical symptom severity was related reliably to DLMO and MST: Depressed women with phase-delayed DLMOs and MSTs had higher total SIGH-SAD ATD scores than those with earlier DLMOs and MSTs; notably, phase-delays in DLMO and MST were unrelated to ATD symptom severity in non-depressed women. Furthermore, analyses of individual SIGH-SAD atypical items in the depressed women showed that the correlations of DLMO and MST with atypical symptoms were limited essentially to self-reported increases in items relating to appetite, increased eating, carbohydrate craving, and weight gain, rather than to anger, fatigue, social withdrawal or hypersomnia (with the exception of MST and hypersomnia).
4.3. Relationship of SIGH-SAD atypical depression score to MEQ score
Although the MEQ score was correlated with total SIGH-SAD atypical score in NC + DP combined, it was not significantly correlated with the total atypical score in DP alone (Table 2). Nevertheless, when the atypical items were examined separately, (Table 4 plus confirmatory ANOVAs), depressed women who endorsed greater eveningness (lower MEQ score) had more severe appetite-related atypical symptoms than those who endorsed greater morningness (higher MEQ score). Morningness-eveningness score was unrelated to appetite-related symptom severity in NC, or to the atypical symptoms that were not appetite-related, in either NC or DP.
Taken together, these results show that the depressed women who endorsed greater eveningness had higher scores on appetite-related items than those who endorsed greater morningness. Non-depressed women who endorsed greater eveningness did not display corresponding increments in their scores on appetite-related items. Thus, depressed women who were more morning-types reported smaller increases in appetite than those who were more evening-types; among NC, the more morning and more evening preferences did not differ in SIGH-SAD appetite-related scores. Thus, the MEQ score appeared to differentiate women who experienced increased severity of appetite-related atypical features from those who did not. This result is consistent with the conclusions of Drennen (1991) and Gaspar-Barba (2009), that morningness may be “protective” against SIGH-SAD atypical depression. Our data and those of others suggest that vulnerability to ATD in DP, but not NC women, is related to phase-delays in DLMO and MST.
4.4. Relationship of DLMO and MST to SIGH-SAD atypical depression score
That the DLMO was significantly correlated with MEQ score in both NC subjects and DP, as well as in NC + DP combined, shows that women who endorsed greater eveningness tended to have later DLMOs, irrespective of whether they had ATD symptoms. That is, phase-delayed DLMO was associated with atypical symptom severity in DP, but was without mood consequence in NC. Similarly, MST was correlated negatively with MEQ score in NC+DP combined, but MST was significantly correlated with the SIGH-SAD atypical score only in DP, suggesting that phase-delays in DLMO and MST were not, by themselves, reliable markers of atypical symptom severity. Rather, phase-delayed DLMO and MST were associated with increased vulnerability to ATD only in the clinically depressed women we studied; symptoms were mild or absent in the NC women with comparable DLMO and MST phase-delays.
If DLMO and MST are causally related to ATD, then NC subjects appear to be protected from the effects of phase-delayed DLMO and MST on atypical symptoms. Of course, it is not possible to establish causality from the present correlational results. On the one hand, phase-delayed DLMO and MST in DP could exacerbate atypical symptom severity. On the other hand, increased appetite and hypersomnia could shift DLMO and MST later via the effects of tryptophan and serotonin (5-HT), a melatonin precursor, on circadian rhythm regulation and sleep. Decreased 5-HT levels are associated with carbohydrate cravings in patients with various forms of depression including Seasonal Affective Disorder and Premenstrual Syndrome (Sandyk, 1996). Increased appetite may be a signal for compensatory carbohydrate consumption, thereby increasing insulin levels and facilitating tryptophan uptake into the central nervous system where it is converted to serotonin in the raphe nuclei, and eventually into melatonin in the pineal (Wurtman and Wurtman, 1989). Leu-Semenescu (2010) found 5-HT metabolism and melatonin profiles were blunted in a patient with hypersomnia, and supplementation with the serotonin precursor 5-hydroxytryptophan restored melatonin profiles while normalizing 5-HT metabolism. Decreased levels of plasma tryptophan and brain serotonin decrease nocturnal melatonin secretion (Zimmermann et al., 1993) and alter sleep architecture (e.g. delay REM sleep onset) (Arnulf et al., 2002).
4.5. Relationship of BMI to SIGH-SAD atypical items and PAD scores
Contrary to expectation and some earlier reports (Parry et al., 2008; Schubert and Randler, 2008), NC and DP groups did not differ significantly in BMI (Table 1), and BMI was not significantly correlated with SIGH-SAD atypical score in NC, DP or the combined groups (Table 2) in our initial analyses. However, when we controlled for the contribution of DLMO, the BMI of depressed women was correlated significantly and positively with the self-reported appetite increase and the mean of the appetite-related SIGH-SAD atypical appetite items (Table 4), suggesting that BMI was associated more closely with the appetite-related SIGH-SAD atypical items than with the total SIGH-SAD atypical score.
That BMI was also correlated significantly with two sleep timing measures, MST and SET (Table 3), suggests that BMI in DP was related more to the phase-delay in sleep timing parameters than to the phase-delay in melatonin timing. We also suspect that the significant correlations between the PAD values for SOT-DLMOff, MST-DLMOff and SET-DLMOff may not reflect unique relationships between the phase-angle differences and BMI, but simply represent the algebraic sum of the significant phase-delays in sleep timing (for MST and SET) in combination with the non-significant phase-timing effects of DLMOff on BMI. That is, these significant correlations of PAD values with BMI are more a reflection of the phase-delays in sleep than of circadian relationships to DLMOff.
4.6. Relationship of hypersomnia score to midsleep and total sleep times
Hypersomnia, a defining characteristic of atypical, rather than melancholic depression, was reported to be associated with a relative risk of 2.9 for developing major depression (Breslau et al., 1996). In the present study, we noted a significant correlation between MST and scores on the SIGH-SAD hypersomnia score in DP, but we found no significant differences between NC and DP in TST (as measured by PSG), or between participants who scored high or low on the total SIGH-SAD atypical scale. And the correlations of TST with total SIGH-SAD ATD score and with the individual atypical items – including hypersomnia – were non-significant in both NC subjects and DP. We also performed linear regression analyses and found that the correlation between MEQ score and atypical depression score was not affected significantly by the addition of TST (p = .431), sleep efficiency (p = .319) or wake after sleep onset (p = .214) to the model. The same pattern of outcomes was found for the HRSD and SIGH-SAD total score. Together with our finding that the score on the hypersomnia item was not significantly correlated with TST, these findings suggest that depressed women’s subjective evaluations of their excessive sleep times may more accurately reflect their perceptions, confirmed by PSG, that their MSTs and times of awakening are phase-delayed, rather than that their TSTs are increased. Thus, despite the fact that hypersomnia is considered to be a defining feature of ATD (Peterson and Benca, 2006), we found no objective evidence of prolonged sleep times in this sample of atypically depressed women at menopause. Objective and subjective sleep measures are often dissociated, especially in women with mood disorders (Meliska et al., 2010).
Knutson et al. (2007) reviewed literature showing that sleep loss is associated with diabetes and/or obesity, as well as increased appetite. We found increased BMIs among DP with later midsleep and sleep end times, but no relationship of BMI to TST or other PSG-derived sleep quality measures, consistent with the observation that sleep timing and duration are largely independent (Roenneberg et al., 2007). Furthermore, the relationship of sleep to BMI may be nonlinear. For example, Chaput et al. (2008) found that increases occurring over time in body weight, waist circumference and percent body fat were greater in long and short sleepers than in average sleepers.
4.7. Clinical implications
Drennan et al. (1991) and Gaspar-Barba (2009) proposed that greater eveningness may be a marker for increased depression vulnerability, while the morning chronotype could be protective against depression. Our results extend these earlier findings by suggesting that phase advances in DLMO and MST also may provide protection against atypical symptoms in DP; or, conversely, vulnerability to ATD may be increased in DP whose DLMOs and MSTs are phase-delayed. A possible mechanism for this association might be that morning-types, with their earlier DLMOs and SETs, are also exposed to more morning light than evening-types, as reported by Goulet et al., (2007); Emens et al., (2009); Staples et al., (2009). To the extent that morning light exposure advances DLMO at the same time that it benefits mood, this finding is consistent with evidence showing that morning-types are less depressed than evening-types. In light of these results, our finding that MEQ score was negatively correlated with ATD severity, melatonin onset time and sleep end time supports the hypothesis that some manifestations of clinical depression may be related causally to alterations in chronobiologic indices such as melatonin and sleep timing parameters (Parry et al., 1997; Wirz-Justice, 2003; Parry et al., 2008). Furthermore, therapeutic interventions that alter these parameters (e.g., sleep deprivation, exposure to bright light) can advance melatonin onset (Burgess and Eastman, 2006); Lewy et al., 2006), and may thereby bring about improvements in sleep, alertness, and mood (Cajochen et al., 2003; Wirz-Justice et al., 2004; Parry et al., 2006a, b).
Of additional interest is the report of Selvi et al. (2007) who studied the effect of partial and total sleep deprivation on mood in normal/non-depressed subjects. They found that the response to sleep-deprivation (wake therapy) was related to the morningness-eveningness preference of the individuals studied: Total (but not partial) sleep deprivation worsened mood in morning-types, while decreasing POMS depression subscale scores in the evening-types. In another study, Reinink et al. (1990) showed that diurnal variation in mood predicted positive treatment response to total sleep deprivation; patients who reported feeling better in the evening derived greater benefit from sleep deprivation than those who felt better in the morning. In that regard, morning light advances while evening light phase-delays circadian rhythms, and seasonal affective disorder patients with phase-delayed rhythms respond more to morning than to evening light (Burgess et al., 2004; Lewy et al., 2006). Further research may clarify the degree to which the morningness-eveningness will prove useful in identifying individuals whose depressive symptoms have a strong chronotypical component, thereby making them susceptible to chronobiological alterations. The Munich Chronotype Questionnaire (MCTQ), an alternative instrument for chronotype studies which has been shown to correlate with the MEQ (Zavada et al., 2005; Roenneberg et al., 2007) has the valuable feature of assessing sleeping habits separately on work and free days. Future work could profit from testing whether results reported here and elsewhere using the MEQ are replicable with the MCTQ.
4.8. Limitations
Although the conclusions of this study are based on inferences from conservative, two-tailed parametric statistical tests, larger Ns than those we used (NC = 19, DP = 10) would engender greater confidence in the conclusions drawn. While our data also suggest that under the conditions of our study, the intensity of the 30 lux dim light we used caused no apparent suppression or phase-delay in melatonin onset/DLMO, some contemporary researchers advocate using lower dim light intensities than those that we used (Eastman et al., 2000; Revell et al., 2006).
Acknowledgements
This work was supported by NIH grant R01 MH080159-01A1 and NIH Clinical Research Center (CRC) Grant M01 RR00827. We thank Alan Turken B.S. for his excellent work in performing the melatonin assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DC, Hopper BR, Lasley BL, Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28:179–196. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition - Text Revision (DSM-IV-TR) Washington, D. C.: American Psychiatric Association; 2000. [Google Scholar]
- Arnulf I, Quintin P, Alvarez JC, Vigil L, Touitou Y, Lebre AS, Bellenger A, Varoquaux O, Derenne JP, Allilaire JF, Benkelfat C, Leboyer M. Mid-morning tryptophan depletion delays REM sleep onset in healthy subjects. Neuropsychopharmacology. 2002;27:843–851. doi: 10.1016/S0893-133X(02)00358-5. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Burger HG. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition--an analysis of FSH, oestradiol and inhibin. Eur J Endocrinol. 1994;130:38–42. doi: 10.1530/eje.0.1300038. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. A late wake time phase delays the human dim light melatonin rhythm. Neurosci Lett. 2006;395:191–195. doi: 10.1016/j.neulet.2005.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Fogg LF, Young MA, Eastman CI. Bright light therapy for winter depression--is phase advancing beneficial? Chronobiol Int. 2004;21:759–775. doi: 10.1081/cbi-200025979. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Jewett ME, Dijk DJ. Human circadian melatonin rhythm phase delay during a fixed sleep-wake schedule interspersed with nights of sleep deprivation. J Pineal Res. 2003;35:149–157. doi: 10.1034/j.1600-079x.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–523. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan MD, Klauber MR, Kripke DF, Goyette LM. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. J Affect Disord. 1991;23:93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Martin SK, Hebert M. Failure of extraocular light to facilitate circadian rhythm reentrainment in humans. Chronobiol Int. 2000;17:807–826. doi: 10.1081/cbi-100102116. [DOI] [PubMed] [Google Scholar]
- Emens JS, Yuhas K, Rough J, Kochar N, Peters D, Lewy AJ. Phase angle of entrainment in morning- and evening-types under naturalistic conditions. Chronobiol Int. 2009;26:474–493. doi: 10.1080/07420520902821077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Biometerics Research Dept. New York: New York State Psychiatric Institute; 1995. Structured Clinical Interview for DSM-IV Axis I Disorders- research version. [Google Scholar]
- Gaspar-Barba E, Calati R, Cruz-Fuentes CS, Ontiveros-Uribe MP, Natale V, De Ronchi D, Serretti A. Depressive symptomatology is influenced by chronotypes. J Affect Disord. 2009;119:100–106. doi: 10.1016/j.jad.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Goulet G, Mongrain V, Desrosiers C, Paquet J, Dumont M. Daily light exposure in morning-type and evening-type individuals. J Biol Rhythms. 2007;22:151–158. doi: 10.1177/0748730406297780. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19:247–255. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hirata FC, Lima MC, de Bruin VM, Nobrega PR, Wenceslau GP, de Bruin PF. Depression in medical school: the influence of morningness-eveningness. Chronobiol Int. 2007;24:939–946. doi: 10.1080/07420520701657730. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Youngstedt SD, Elliott JA, Tuunainen A, Rex KM, Hauger RL, Marler MR. Circadian phase in adults of contrasting ages. Chronobiol Int. 2005;22:1–15. doi: 10.1080/07420520500180439. [DOI] [PubMed] [Google Scholar]
- Leu-Semenescu S, Arnulf I, Decaix C, Moussa F, Clot F, Boniol C, Touitou Y, Levy R, Vidailhet M, Roze E. Sleep and rhythm consequences of a genetically induced loss of serotonin. Sleep. 2010;33:307–314. doi: 10.1093/sleep/33.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Martinez LF, Parry BL. Sleep-endocrine relationships in depressed women across the reproductive cycle. In: Pandi-Perumal SR, Kramer M, editors. Sleep and Mental Illness. New York: Cambridge University Press; 2010. [Google Scholar]
- Parry BL, Martinez LF, Maurer EL, Lopez AM, Sorenson DL, Meliska CJ. Sleep rhythms and women's mood. Part I: Menstrual cycle, pregnancy and postpartum. Sleep Med Rev. 2006a;10:129–144. doi: 10.1016/j.smrv.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Parry BL, Martinez LF, Maurer EL, Lopez AM, Sorenson DL, Meliska CJ. Sleep, rhythms and women's mood. Part II: Menopause. Sleep Med Rev. 2006b;10:197–208. doi: 10.1016/j.smrv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Parry BL, Meliska CJ, Sorenson DL, Lopez AM, Martinez LF, Nowakowski S, Hauger RL, Elliott JA. Increased melatonin and delayed offset in menopausal depression: role of years past menopause, follicle-stimulating hormone, sleep end time, and body mass index. J Clin Endocrinol Metab. 2008;93:54–60. doi: 10.1210/jc.2006-2853. PMC2190736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL, Udell C, Elliott JA, Berga SL, Klauber MR, Mostofi N, LeVeau B, Gillin JC. Blunted phase-shift responses to morning bright light in premenstrual dysphoric disorder. J Biol Rhythms. 1997;12:443–456. doi: 10.1177/074873049701200506. [DOI] [PubMed] [Google Scholar]
- Peterson MJ, Benca RM. Sleep in mood disorders. Psychiatr Clin North Am. 2006;29:1009–1032. doi: 10.1016/j.psc.2006.09.003. abstract ix. [DOI] [PubMed] [Google Scholar]
- Quitkin FM. Depression With Atypical Features: Diagnostic Validity, Prevalence, and Treatment. Prim Care Companion J Clin Psychiatry. 2002;4:94–99. doi: 10.4088/pcc.v04n0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales AA. Superintendent of Documents. Washington, D. C.: US Government Printing Office; 1968. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- Reinink E, Bouhuys N, Wirz-Justice A, van den Hoofdakker R. Prediction of the antidepressant response to total sleep deprivation by diurnal variation of mood. Psychiatry Res. 1990;32:113–124. doi: 10.1016/0165-1781(90)90077-i. [DOI] [PubMed] [Google Scholar]
- Revell VL, Arendt J, Fogg LF, Skene DJ. Alerting effects of light are sensitive to very short wavelengths. Neurosci Lett. 2006;399:96–100. doi: 10.1016/j.neulet.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Sandyk R. Treatment with weak electromagnetic fields attenuates carbohydrate craving in a patients with multiple sclerosis. Int J Neurosci. 1996;86:67–77. doi: 10.3109/00207459608986699. [DOI] [PubMed] [Google Scholar]
- Schubert E, Randler C. Association between chronotype and the constructs of the Three-Factor-Eating-Questionnaire. Appetite. 2008;51:501–505. doi: 10.1016/j.appet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Selvi Y, Gulec M, Agargun MY, Besiroglu L. Mood changes after sleep deprivation in morningness-eveningness chronotypes in healthy individuals. J Sleep Res. 2007;16:241–244. doi: 10.1111/j.1365-2869.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- Staples VS, Archer SN, Arber S, Skene DJ. Daily light exposure profiles in older non-resident extreme morning and evening types. J Sleep Res. 2009;18:466–471. doi: 10.1111/j.1365-2869.2009.00762.x. [DOI] [PubMed] [Google Scholar]
- Williams JB, Link MJ, Rosenthal NE, Amira L, Terman M. Seasonal Affective Disorders Version (SIGH-SAD) New York: New York Psychiatric Institute; 1994. Structured Interview Guide for the Hamilton Depression Rating Scale. revised edition. [Google Scholar]
- Wirz-Justice A. Chronobiology and mood disorders. Dialogues Clin Neurosci. 2003;5:315–325. doi: 10.31887/DCNS.2003.5.4/awirzjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, Terman M, Oren DA, Goodwin FK, Kripke DF, Whybrow PC, Wisner KL, Wu JC, Lam RW, Berger M, Danilenko KV, Kasper S, Smeraldi E, Takahashi K, Thompson C, van den Hoofdakker RH. Brightening depression. Science. 2004;303:467–469. doi: 10.1126/science.303.5657.467c. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Wurtman JJ. Carbohydrates and depression. Sci Am. 1989;260:68–75. doi: 10.1038/scientificamerican0189-68. [DOI] [PubMed] [Google Scholar]
- Yoon IY, Kripke DF, Elliott JA, Langer RD. Naps and circadian rhythms in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2004;59:844–848. doi: 10.1093/gerona/59.8.m844. [DOI] [PubMed] [Google Scholar]
- Zavada A, Gordijn MC, Beersma DG, Daan S, Roenneberg T. Comparison of the Munich Chronotype Questionnaire with the Horne-Ostberg's Morningness-Eveningness Score. Chronobiol Int. 2005;22:267–278. doi: 10.1081/cbi-200053536. [DOI] [PubMed] [Google Scholar]
- Zimmermann RC, McDougle CJ, Schumacher M, Olcese J, Mason JW, Heninger GR, Price LH. Effects of acute tryptophan depletion on nocturnal melatonin secretion in humans. J Clin Endocrinol Metab. 1993;76:1160–1164. doi: 10.1210/jcem.76.5.8496306. [DOI] [PubMed] [Google Scholar]