Abstract
Tissue engineering aims at constructing biological substitutes to repair damaged tissues. Three-dimensional (3D) porous scaffolds are commonly utilized to define the 3D geometry of tissue engineering constructs and provide adequate pore space and surface to support cell attachment, migration, proliferation, differentiation and neo tissue genesis. Biomimetic 3D scaffolds provide synthetic microenvironments that mimic the natural regeneration microenvironments and promote tissue regeneration process. While nano-fibrous (NF) scaffolds are constructed to mimic the architecture of NF extracellular matrix, controlled-release growth factors are incorporated to modulate the regeneration process. The present article summarizes current advances in methods to fabricate NF polymer scaffolds and the technologies to incorporate controlled growth factor delivery systems into 3D scaffolds, followed by examples of accelerated regeneration when the scaffolds with growth factor releasing capacity are applied in animal models.
Keywords: drug delivery, growth factor, nano-fibrous, polymer scaffold, tissue engineering
Introduction
Tissue engineering aims at constructing biological substitutes to repair damaged tissues (1). Porous three-dimensional (3D) scaffolds are commonly utilized to define the 3D geometry of regenerating tissues and to provide interconnected pore network and adequate pore surface to support cell attachment, migration, proliferation, differentiation and neo tissue genesis. Biomimetic 3D scaffolds provide synthetic microenvironments that mimic the natural regeneration microenvironments and promote tissue regeneration process (2–4). A synthetic biomimetic niche can provide the local microenvironment to control stem cell recruitment and fate (5). Therefore, a biomimetic scaffold can be designed to have certain artificial features (such as porosity and interconnected pore network) to accommodate tissue growth, to resemble certain features such as nano-fibrous (NF) architecture of the natural extra-cellular matrix (ECM), and to deliver bioactive molecules in a temporally and spatially controlled fashion (2). A variety of natural and synthetic materials have been exploited to fabricate 3D scaffolds and drug delivery systems. Among these, synthetic biodegradable polymers have been extensively studied (6–8) due to the flexibility of adjusting the composition and structure to meet specific needs of various tissue regeneration applications. The present article summarizes current advances in methods to fabricate synthetic biodegradable NF polymer scaffolds and the technologies to incorporate controlled-release growth factor delivery system into the 3D scaffolds.
Fabricaion of Synthetc NF Biodegradable Polymer Scaffods
Collagen is the major ECM component of many tissues and often has a fibrous architecture with fiber bundle diameters ranging from 50 to 500 nm (9). Collagen and its derived materials (such as gelatin) have been widely used to fabricate 3D scaffolds (10–13). Synthetic polymer scaffolds mimicking the architecture of collagen fibers have been developed with several techniques.
Electrospinning
Electrospinning technique has been recently utilized to fabricate tissue engineering scaffolds (14–16), although this technique was initially applied to produce industrial and household non-woven fabric products. The electrospinning system includes a polymer solution or melt reservoir, a high voltage electric field and a grounded target collector. When high voltage is applied to overcome the surface tension of polymer solution or melt, a charged jet is generated toward the collector. The solvent evaporates or the melt solidifies while arriving at the grounded collector. In this way, a non-woven fibrous mat is fabricated. By adjusting the electrospinning conditions, fiber diameters ranging from nanometer to micrometer scales can be generated. The major advantage of the electrospinning technique is that it can be used to fabricate NF scaffolds from a large variety of materials, including natural macromolecules, such as collagen, chitosan and silk fibroin (14,17–19); synthetic biodegradable polymers, such as poly(glycolic acid) (PGA), poly(lactic acid) (PLA), poly(lactic acid-co-glycolic acid) (PLGA) and poly(ε-caprolactone) (PCL) (15,16,20–22); or a blend of these materials (23–26). Although electrospun scaffolds have been utilized to regenerate multiple types of tissues (27–31), it remains a great challenge to incorporate macro-pore network structure that allows deep cell penetration (32–34), limiting their applications in regenerating large-sized tissues. Another disadvantage of this technique is that complex 3D shapes are hard to achieve.
Thermally Induced Phase Separation
Thermally induced phase separation (TIPS) of a variety of polymer solutions has been exploited in our laboratory to fabricate 3D scaffolds (35–38). Under certain temperature conditions, a homogeneous polymer solution becomes thermodynamically unstable and separates into a polymer-rich phase and a polymer-lean phase. After removal of the solvent, the polymer-lean domains become pores, while the solidified polymer-rich phase forms a 3D structure. Depending on the type of polymer and phase separation parameters (e.g. solvent and temperature) applied, various pore structure and scaffold architecture can be generated. When a PLLA solution with a suitable solvent is subjected to TIPS at the right conditions, NF architecture develops (35,36). The fiber diameters range from 50–500 nm, resembling the architecture of natural collagen fiber bundles. To overcome the limitation of cell penetration into NF matrices, a pre-designed interconnected spherical macro-pore network has been incorporated into the NF structure during the fabrication process (36,37) (Fig. 1). Furthermore, to control the overall 3D shape of the scaffold, a reverse solid free-form fabrication technique has been developed based on reversed images of computed tomography scans or histological sections of human anatomical parts (39,40). In this way, the 3D NF scaffold has hierarchical structures: the wall matrix resembles the architecture of natural ECM; the interconnected macro-pore network supports cell penetration and tissue growth; the gross geometry resembles the patient anatomical shape of defects. The NF architecture leads to significant changes in physical properties of the scaffolds. The NF scaffolds have surface areas about two orders of magnitude higher than those of the conventional solid-walled (SW) scaffolds (36). The high surface area of the NF architecture substantially accelerates the hydrolytic degradation rate of the scaffolds, making the NF scaffolds more suitable for tissue engineering applications (41). The higher surface area also greatly increased the amount of serum proteins adsorbed on the NF scaffolds (42), which have a beneficial effect on the cell attachment, growth, and tissue regeneration (42–44). The NF scaffolds have been demonstrated to promote the differentiation of multiple cell types and regeneration of various tissues (43,45–48).
Fig. 1.

Scanning electron micrographs of a macro-porous NF PLLA scaffold prepared from sugar sphere template leaching and TIPS, with (A) macro-pore network structure and (B) NF wall matrix architecture (37). Reproduced with permission from John Wiley & Sons.
Delivery of Growth Factors on 3D NF Scaffolds
Growth factors are important bioactive molecules that play a critical role in the regeneration microenvironment. Incorporation of appropriate growth factors into the scaffolds can greatly accelerate the regeneration process. As proteins and peptides, growth factors usually have very short half-lives in vivo, while the target cells are often only responsive to a growth factor when the concentration is above a certain threshold level. Therefore, controlled-release systems are desired for the delivery of growth factors in 3D scaffolds (49–53). One of the major challenges in this strategy is that the growth factors may denature during the incorporation into the delivery vehicles or the incorporation of the delivery vehicles into the 3D scaffolds due to inappropriate exposure to organic solvents or heat treatments. Another challenge is that they may be eluted either partially or entirely out of the vehicles during the fabrication, for example when an aqueous solution is used to remove the porogens used to generate macro-pores. In addition, incorporated drug delivery systems may negatively affect the structure, mechanical properties or degradation properties of the scaffolds (49,54). Therefore, optimal delivery systems and appropriate incorporation techniques are needed to achieve the growth factor delivery in a temporally and spatially controlled fashion while maintaining the necessary properties of the designed scaffolds.
Incorporation of Growth Factors Inside the 3D NF Scaffolds Wall Matrices
Several methods have been developed to encapsulate the growth factors directly inside the scaffolds during the scaffold fabrication process. In an emulsion freeze-drying method, an emulsion containing proteins and PLGA was subjected to the freeze-drying treatment to form porous structures and encapsulate the proteins inside the pore walls simultaneously (49). Encapsulated bone morphogenetic protein-2 promoted ectopic bone formation in rats (55). However, the small pore size of the scaffolds did not support effective host cells penetration and caused a void in the central part of the implants. Similar polymer-protein emulsions were electrospun into protein-encapsulated fibers (56–58). In an alternative method, coaxial electrospinning technique was developed to simultaneously spin two different polymer solutions and generate a core-shell structure (59). While the proteins were mainly incorporated and distributed in the core part, burst release was significantly reduced, and the release duration was prolonged (60,61). High bioactivity was preserved when aqueous solution was used to dissolve growth factors and core materials (62). Incorporation of porogens in the shell materials was utilized to further tune the release profile (63).
The major shortcomings associated with the direct encapsulation methods include 1) the structure, mechanical properties and/or degradation properties of the scaffolds are altered during the incorporation process; 2) the releasing profile of incorporated proteins is dependent on the bulk degradation of the scaffolds, which may not be appropriate for many tissue regeneration applications that need slower degradation of scaffolds to provide a longer mechanical support. Therefore, strategies capable of separating the scaffold fabrication process and delivery system incorporation process may provide more versatile solutions.
Incorporation of Growth Factors on the 3D NF Scaffold Wall Matrices
Immersing the scaffolds in protein solution and thereby adsorbing the proteins on the inner surface of porous scaffolds may be the easiest way to deliver growth factors (64). NF scaffolds have been demonstrated to be able to adsorb a much higher amount of serum proteins than SW scaffolds, due to the significantly larger surface areas (42). This characteristic can be utilized to load a higher amount of growth factors. However, the ability to control release kinetics is poor for this passive adsorption method, with a short release duration that may not be sufficient to achieve the desired biological effects (64). Furthermore, the adsorption of proteins on solid-phase materials may change the conformation and result in loss of the bioactivity of proteins. Alternatively, in an emulsion coating process, the growth factors were coated on the surface of inner pores of pre-fabricated porous scaffolds, with a protective polymer layer (51,65). However, the availability of solvent types is limited, and the macro-pore structure is substantially altered by the coating process.
A novel method developed in our lab immobilizes the microspheres (MS) or nanospheres (NS), encapsulating growth factors onto the inner pore surface of prefabricated NF scaffolds without altering the overall architecture of the 3D scaffolds (52,53). In this way, the pore architecture and the properties of the 3D scaffolds are separated from the controlled delivery profiles of growth factors that are primarily determined by the MS or NS formulation. Therefore, this approach is more versatile to meet the specific requirements for various combinations of scaffold properties and release profiles to regenerate various tissues. Polymeric MS and NS have been widely utilized to effectively encapsulate bioactive molecules, protect unstable substance from denaturing and degradation, and control the release profile in a temporally controlled fashion (7,66). A variety of methods of fabricating MS/NS have been addressed by others in details (67,68). In a typical double emulsion protocol, aqueous protein solution is first emulsified into a PLGA solution to form primary water-in-oil emulsion, followed by adding the emulsion into an aqueous solution under stirring or sonication to generate a water-in-oil-in-water double emulsion. After the removal of organic solvent, spherical particles with diameters ranging from nanometers to micrometers are generated, depending on the concentration of surfactant and the emulsion strength applied (69). The release of encapsulated proteins is controlled by the diffusion in the first stage and polymer degradation in the second stage. By adjusting the ratio of LA/GA or the molecular weight of the PLGA copolymers, various release profiles can be achieved with release duration from several days to months.
It has been verified that the high bioactivities of both encapsulated parathyroid hormone and platelet-derived growth factor (PDGF) were preserved after being released from the spheres, with the ability to stimulate cellular cyclic adenosine monophosphate (cAMP) level (69) or human gingival fibroblast proliferation (52), respectively. The spheres were then immobilized onto the inner surface of porous NF scaffolds (Fig. 2). Although the size and related surface-to-volume ratios of PLGA MS/NS can be used to tune release profile (70), to achieve high efficiency of post-seeding inside the 3D scaffolds, the MS/NS with diameters less than 1 μm were selected. The PLGA MS/NS suspension in hexane was seeded into the 3D scaffolds, followed by solvent evaporation, and a mixture of hexane/THF treatment to immobilize the spheres. MS or NS were attached on the pore wall surface and evenly distributed in the porous NF scaffolds, without altering the macro-porous network structure. The dose of growth factors to be delivered can be adjusted either by the amount of encapsulated proteins in the spheres or the amount of spheres immobilized on the porous scaffolds. The release profile was tuned mainly by adjusting the ratio of LA/GA or the molecular weight of PLGA copolymer (Fig. 3). Compared to the free spheres, the immobilization of spheres on 3D scaffolds reduced the burst release and prolonged the release duration (52), which are desired for most regeneration applications involving growth factors. While the temporally controlled delivery was mainly tuned by the chemical composition of polymeric spheres, the localization of spheres inside 3D scaffolds could be used to control the spatial distribution of delivered growth factors (unpublished data).
Fig. 2.
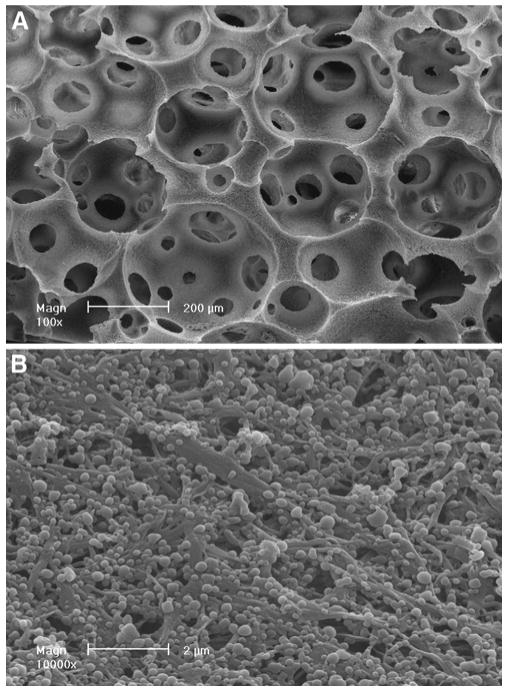
Scanning electron micrographs of a PLGA NS immobilized PLLA NF scaffold: (A) macro-pore network structure of the NS-containing scaffold and (B) NS immobilized on the NF wall matrices (53). Reproduced with permission from Elsevier.
Fig. 3.
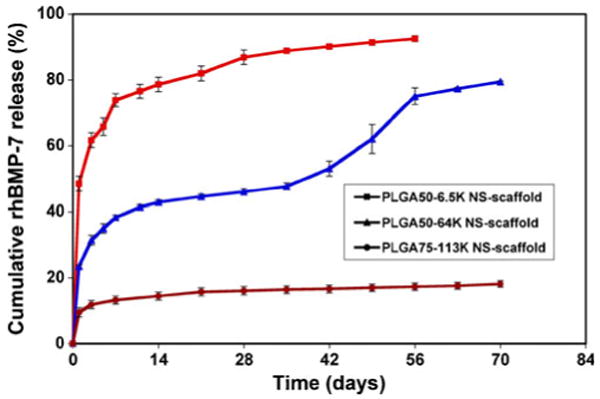
In vitro release kinetics of BMP-7 from NS-immobilized scaffolds. Three distinct release profiles were achieved with NS composed of different LG/GA ratios (50/50 or 75/25) or molecular weights (6.5 K, 64 K or 113 K) (53). Reproduced with permission from Elsevier.
The MS/NS-containing scaffolds have been evaluated for promoting angiogenesis and osteogenesis in animal models. In one study, bone morphogenetic protein-7 (BMP-7) was incorporated into the NS and immobilized onto the 3D NF scaffolds (53). Bone morphogenetic proteins have been shown to promote neo-bone-formation both at orthotopic and ectopic sites in various animal models (71–75). After subcutaneously implanted in rats, BMP-7 NS-containing scaffolds significantly induced ectopic bone formation (Fig. 4). In contrast, passive adsorption of the same amount of BMP-7 on the scaffolds failed to elicit osteogenesis, probably due to the loss of bioactivity and insufficient release duration. In another study, PDGF was incorporated into the 3D NF scaffolds the (52). Recombinant human PDGF has been approved by the US Food and Drug Administration to treat diabetic foot ulcers, due to its ability to promote angiogenesis and wound healing (76). After subcutaneously implanted in rats, the PDGF MS-containing scaffolds promoted tissue growth and angiogenesis, compared to the control group without PDGF. The extent of effect was not only dependent on the dosage delivered, but was also dependent on the release profile. Notably, angiogenesis was significantly increased in the scaffold that released PDGF relatively slowly than in the scaffold that released PDGF rapidly (Fig. 5). Further study found that the PDGF-induced gene expression profiles of several chemokine family members were affected by the release profile (77), which may contribute to the observed various extents of angiogenesis and tissue ingrowth. While in these cases the biological effect of single growth factor delivery is illustrated, this method can be readily adopted to deliver multiple growth factors with distinct release profiles (78).
Fig. 4.

BMP-7 NS-containing scaffolds induced ectopic bone formation after subcutaneously implanted in rats for six weeks: (A) fibrous tissue growth in the NS-containing scaffolds without BMP-7, (B) fibrous tissue growth in the NS-containing scaffolds adsorbed with 5 μg BMP-7/scaffold, and (C) bone formation in the NS-containing scaffolds encapsulated with 5 μg BMP-7/scaffold (H&E staining) (53). Reproduced with permission from Elsevier.
Fig. 5.

MS-scaffolds encapsulating PDGF promoted angiogenesis in a release profile-dependant way, after subcutaneously implanted in rats for one week. Left panel is at a lower magnification (10×), right panel is at a higher magnification (40×). (A), (D) blank scaffolds, (B), (E) fast-releasing MS-scaffolds encapsulating 25 μg PDGF/scaffold, and (C), (F) slow-releasing MS-scaffolds encapsulating 25 μg PDGF/scaffold (von Willebrand factor immunohistochemical staining) (77).
Due to limited pore size, post-seeding of MS/NS is limited for most electrospun NF scaffolds. Different methods have been developed to incorporate MS/NS drug delivery system into scaffolds during the electrospinning process. In a dual-electrospinning method, a PCL jet and a poly(ethylene oxide) (PEO) jet with pre-fabricated MS were electrospun onto a common mandrel (54). After removal of PEO, the MS were entrapped between the electrospun PCL nano-fibers. The stiffness and modulus of the composite scaffold were not altered with the incorporation of MS. In another method combining electrosprying and electrospinning, insulin-like growth factor was encapsulated into MS by coaxial electrosprying and entrapped between electrospun PLGA nano-fibers (79) sequentially. The composite scaffolds were found to promote the in vitro growth of mesenchymal stem cells.
Delivery of Other Bioactive Molecules on NF Scaffolds
While growth factor delivery is discussed here, the above-mentioned methods can be easily adapted to deliver other bioactive molecules. DNA has been incorporated into other 3D scaffolds with several methods (80,81) and has been recently delivered from electropsun NF scaffolds (82–84). Antibiotics (85,86) and chemotherapeutics (87,88) were also reported to be delivered from electrospun or TIPS NF scaffolds. For instance, doxycycline was recently incorporated into the 3D NF scaffolds in a similar way (86). Doxycline, a broad-spectrum antibiotic against both Gram-positive and Gram-negative bacteria, has been applied clinically to treat periodontal disease (89,90). The in vitro released doxycycline from NS immobilized on NF PLLA Scaffolds significantly inhibited the in vitro growth of common bacteria including S. aureus and E. coli, which may have a beneficial effect in preventing infection when the scaffolds are used to repair periodontal defects.
Conclusions
Tissue engineering has experienced tremendous growth in the past two decades. Enormous advances have been achieved in fabricating novel biomimetic scaffolds, which resemble the natural regeneration microenvironment at multiple levels. Sophisticated technologies are being developed to more precisely control temporal and spatial delivery of bioactive molecules in tissue engineering scaffolds. This review focused on the delivery of growth factors in porous NF synthetic biodegradable polymer scaffolds. Readers can find a comprehensive review on the delivery of growth factors from hydrogels (91). We hope that the contents have illustrated the principles of growth factor deliveries from 3D NF scaffolds and that readers will be able to find detailed information from the cited references and related literature.
Acknowledgments
The authors gratefully acknowledge the past and present grant support to our research in biologic delivery and biomimetic tissue engineering scaffolds from the NIH (DE014755, DE015384, GM075840, DE017689).
Abbreviations
- 3D
three-dimensional
- BMP-7
bone morphogenetic protein-7
- ECM
extra-cellular matrix
- MS
microspheres
- NF
nano-fibrous
- NS
nanospheres
- PCL
poly(ε-caprolactone)
- PDGF
platelet-derived growth factor
- PEO
poly(ethylene oxide)
- PGA
poly(glycolic acid)
- PLA
poly(lactic acid)
- PLGA
poly(lactic acid-co-glycolic acid)
- PLLA
poly(L-lactic acid)
- SW
solid-walled
- TIPS
thermally induced phase separation
Contributor Information
Jiang Hu, Department of Biologic and Materials Sciences, The University of Michigan, 1011 North University Ave., Room 2211, Ann Arbor, Michigan 48109-1078, USA.
Peter X. Ma, Email: mapx@umich.edu, Department of Biologic and Materials Sciences, The University of Michigan, 1011 North University Ave., Room 2211, Ann Arbor, Michigan 48109-1078, USA; Department of Biomedical Engineering, The University of Michigan, Ann Arbor, Michigan 48109, USA; Macromolecular Science and Engineering Center, The University of Michigan, Ann Arbor, Michigan 48109, USA; Department of Materials Science and Engineering, The University of Michigan, Ann Arbor, Michigan 48109, USA.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–98. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 4.Fisher OZ, Khademhosseini A, Langer R, Peppas NA. Bioinspired materials for controlling stem cell fate. Acc Chem Res. 2010;43:419–28. doi: 10.1021/ar900226q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peerani R, Zandstra PW. Enabling stem cell therapies through synthetic stem cell-niche engineering. J Clin Invest. 2010;120:60–70. doi: 10.1172/JCI41158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediatr Surg. 1988;23:3–9. doi: 10.1016/s0022-3468(88)80529-3. [DOI] [PubMed] [Google Scholar]
- 7.Langer R. New methods of drug delivery. Science. 1990;249:1527–33. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 8.Ma PX. Tissue Engineering. In: Kroschwitz JI, editor. Encyclopedia of polymer science and technology. Vol. 12. Hoboken: Wiley; 2005. pp. 261–91. [Google Scholar]
- 9.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–37. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavallaro JF, Kemp PD, Kraus KH. Collagen fabrics as biomaterials. Biotechnol Bioeng. 1994;43:781–91. doi: 10.1002/bit.260430813. [DOI] [PubMed] [Google Scholar]
- 11.Nathan A, Nugent MA, Edelman ER. Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc Natl Acad Sci USA. 1995;92:8130–4. doi: 10.1073/pnas.92.18.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Ouyang H, Lim CT, Ramakrishna S, Huang ZM. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res B Appl Biomater. 2005;72:156–65. doi: 10.1002/jbm.b.30128. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Ma PX. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials. 2009;30:4094–103. doi: 10.1016/j.biomaterials.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–8. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 15.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–82. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 17.Bhattarai N, Edmondson D, Veiseh O, Matsen FA, Zhang M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials. 2005;26:6176–84. doi: 10.1016/j.biomaterials.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori silk with poly(ethylene oxide) Biomacromolecules. 2002;3:1233–9. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 19.Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25:1289–97. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nano-fibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67:1105–14. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 21.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–10. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 22.Li WJ, Cooper JA, Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377–85. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Mo XM, Xu CY, Kotaki M, Ramakrishna S. Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25:1883–90. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 24.Park KE, Kang HK, Lee SJ, Min BM, Park WH. Biomimetic nanofibrous scaffolds: preparation and characterization of PGA/chitin blend nanofibers. Biomacromolecules. 2006;7:635–43. doi: 10.1021/bm0509265. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Mondrinos MJ, Chen X, Gandhi MR, Ko FK, Lelkes PI. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. J Biomed Mater Res A. 2006;79:963–73. doi: 10.1002/jbm.a.30833. [DOI] [PubMed] [Google Scholar]
- 26.Chen F, Li X, Mo X, He C, Wang H, Ikada Y. Electrospun chitosan-P(LLA-CL) nanofibers for biomimetic extracellular matrix. J Biomater Sci Polym Ed. 2008;19:677–91. doi: 10.1163/156856208784089661. [DOI] [PubMed] [Google Scholar]
- 27.Shin M, Yoshimoto H, Vacanti JP. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng. 2004;10:33–41. doi: 10.1089/107632704322791673. [DOI] [PubMed] [Google Scholar]
- 28.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25:877–86. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 29.Janjanin S, Li WJ, Morgan MT, Shanti RM, Tuan RS. Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J Surg Res. 2008;149:47–56. doi: 10.1016/j.jss.2007.12.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieponice A, Soletti L, Guan J, Hong Y, Gharaibeh B, Maul TM, et al. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng A. 2010;16:1215–23. doi: 10.1089/ten.tea.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pritchard CD, Arner KM, Neal RA, Neeley WL, Bojo P, Bachelder E, et al. The use of surface modified poly(glycerol-co-sebacic acid) in retinal transplantation. Biomaterials. 2010;31:2153–62. doi: 10.1016/j.biomaterials.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telemeco TA, Ayres C, Bowlin GL, Wnek GE, Boland ED, Cohen N, et al. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005;1:377–85. doi: 10.1016/j.actbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Balguid A, Mol A, van Marion MH, Bank RA, Bouten CV, Baaijens FP. Tailoring fiber diameter in electrospun poly(epsilon-caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng A. 2009;15:437–44. doi: 10.1089/ten.tea.2007.0294. [DOI] [PubMed] [Google Scholar]
- 34.Guimaraes A, Martins A, Pinho ED, Faria S, Reis RL, Neves NM. Solving cell infiltration limitations of electrospun nanofiber meshes for tissue engineering applications. Nanomedicine (Lond) 2010;5:539–54. doi: 10.2217/nnm.10.31. [DOI] [PubMed] [Google Scholar]
- 35.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46:60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 36.Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25:2065–73. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 37.Wei G, Ma PX. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J Biomed Mater Res A. 2006;78:306–15. doi: 10.1002/jbm.a.30704. [DOI] [PubMed] [Google Scholar]
- 38.Wei G, Ma PX. Partially nanofibrous architecture of 3D tissue engineering scaffolds. Biomaterials. 2009;30:6426–34. doi: 10.1016/j.biomaterials.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27:3973–9. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 40.Wang P, Hu J, Ma PX. The engineering of patient-specific, anatomically shaped, digits. Biomaterials. 2009;30:2735–40. doi: 10.1016/j.biomaterials.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen VJ, Ma PX. The effect of surface area on the degradation rate of nano-fibrous poly(L-lactic acid) foams. Biomaterials. 2006;27:3708–15. doi: 10.1016/j.biomaterials.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res A. 2003;67:531–7. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 43.Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28:335–43. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Woo KM, Chen VJ, Jung HM, Kim TI, Shin HI, Baek JH, et al. Comparative evaluation of nanofibrous scaffolding for bone regeneration in critical-size calvarial defects. Tissue Eng A. 2009;15:2155–62. doi: 10.1089/ten.tea.2008.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu J, Feng K, Liu X, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30:5061–7. doi: 10.1016/j.biomaterials.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith LA, Liu X, Hu J, Wang P, Ma PX. Enhancing osteogenic differentiation of mouse embryonic stem cells by nanofibers. Tissue Eng A. 2009;15:1855–64. doi: 10.1089/ten.tea.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith LA, Liu X, Hu J, Ma PX. The influence of three-dimensional nanofibrous scaffolds on the osteogenic differentiation of embryonic stem cells. Biomaterials. 2009;30:2516–22. doi: 10.1016/j.biomaterials.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun H, Feng K, Hu J, Soker S, Atala A, Ma PX. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 2010;31:1133–9. doi: 10.1016/j.biomaterials.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whang K, Goldstick TK, Healy KE. A biodegradable polymer scaffold for delivery of osteotropic factors. Biomaterials. 2000;21:2545–51. doi: 10.1016/s0142-9612(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 50.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 51.Sohier J, Haan RE, de Groot K, Bezemer JM. A novel method to obtain protein release from porous polymer scaffolds: emulsion coating. J Control Release. 2003;87:57–68. doi: 10.1016/s0168-3659(02)00350-4. [DOI] [PubMed] [Google Scholar]
- 52.Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112:103–10. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–96. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ionescu LC, Lee GC, Sennett BJ, Burdick JA, Mauck RL. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials. 2010;31:4113–20. doi: 10.1016/j.biomaterials.2010.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whang K, Tsai DC, Nam EK, Aitken M, Sprague SM, Patel PK, et al. Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. J Biomed Mater Res. 1998;42:491–9. doi: 10.1002/(sici)1097-4636(19981215)42:4<491::aid-jbm3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 56.Valmikinathan CM, Defroda S, Yu X. Polycaprolactone and bovine serum albumin based nanofibers for controlled release of nerve growth factor. Biomacromolecules. 2009;10:1084–9. doi: 10.1021/bm8012499. [DOI] [PubMed] [Google Scholar]
- 57.Yan S, Xiaoqiang L, Shuiping L, Xiumei M, Ramakrishna S. Controlled release of dual drugs from emulsion electrospun nanofibrous mats. Colloids Surf B Biointerfaces. 2009;73:376–81. doi: 10.1016/j.colsurfb.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Li X, Su Y, Liu S, Tan L, Mo X, Ramakrishna S. Encapsulation of proteins in poly(L-lactide-co-caprolactone) fibers by emulsion electrospinning. Colloids Surf B Biointerfaces. 2010;75:418–24. doi: 10.1016/j.colsurfb.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 59.McCann JT, Li D, Xia YN. Electrospinning of nanofibers with core-sheath, hollow, or porous structures. J Mater Chem. 2005;15:735–8. [Google Scholar]
- 60.Jiang H, Hu Y, Li Y, Zhao P, Zhu K, Chen W. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. J Control Release. 2005;108:237–43. doi: 10.1016/j.jconrel.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Zhang YZ, Wang X, Feng Y, Li J, Lim CT, Ramakrishna S. Coaxial electrospinning of (fluorescein isothiocyanate-conjugated bovine serum albumin)-encapsulated poly(epsilon-caprolactone) nanofibers for sustained release. Biomacromolecules. 2006;7:1049–57. doi: 10.1021/bm050743i. [DOI] [PubMed] [Google Scholar]
- 62.Liao IC, Chew SY, Leong KW. Aligned core-shell nanofibers delivering bioactive proteins. Nanomedicine (Lond) 2006;1:465–71. doi: 10.2217/17435889.1.4.465. [DOI] [PubMed] [Google Scholar]
- 63.Jiang H, Hu Y, Zhao P, Li Y, Zhu K. Modulation of protein release from biodegradable core-shell structured fibers prepared by coaxial electrospinning. J Biomed Mater Res B Appl Biomater. 2006;79:50–7. doi: 10.1002/jbm.b.30510. [DOI] [PubMed] [Google Scholar]
- 64.Ziegler J, Mayr-Wohlfart U, Kessler S, Breitig D, Gunther KP. Adsorption and release properties of growth factors from biodegradable implants. J Biomed Mater Res. 2002;59:422–8. doi: 10.1002/jbm.1258. [DOI] [PubMed] [Google Scholar]
- 65.Sohier J, Vlugt TJ, Cabrol N, Van Blitterswijk C, de Groot K, Bezemer JM. Dual release of proteins from porous polymeric scaffolds. J Control Release. 2006;111:95–106. doi: 10.1016/j.jconrel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 66.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–33. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 2009;26:1025–58. doi: 10.1007/s11095-008-9800-3. [DOI] [PubMed] [Google Scholar]
- 68.Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. J Control Release. 2003;90:261–80. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 69.Wei G, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25:345–52. doi: 10.1016/s0142-9612(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 70.Kalaji N, Deloge A, Sheibat-Othman N, Boyron O, About I, Fessi H. Controlled release carriers of growth factors FGF-2 and TGFbeta1: synthesis, characterization and kinetic modelling. J Biomed Nanotechnol. 2010;6:106–16. doi: 10.1166/jbn.2010.1102. [DOI] [PubMed] [Google Scholar]
- 71.Ripamonti U, Van Den Heever B, Sampath TK, Tucker MM, Rueger DC, Reddi AH. Complete regeneration of bone in the baboon by recombinant human osteogenic protein-1 (hOP-1, bone morphogenetic protein-7) Growth Factors. 1996;13:273–89. doi: 10.3109/08977199609003228. color plates III-VIII,pre bk. [DOI] [PubMed] [Google Scholar]
- 72.Zegzula HD, Buck DC, Brekke J, Wozney JM, Hollinger JO. Bone formation with use of rhBMP-2 (recombinant human bone morphogenetic protein-2) J Bone Joint Surg Am. 1997;79:1778–90. doi: 10.2106/00004623-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998:26–37. [PubMed] [Google Scholar]
- 74.Welch RD, Jones AL, Bucholz RW, Reinert CM, Tjia JS, Pierce WA, et al. Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goat tibial fracture model. J Bone Miner Res. 1998;13:1483–90. doi: 10.1359/jbmr.1998.13.9.1483. [DOI] [PubMed] [Google Scholar]
- 75.Cook SD. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics. 1999;22:669–71. [PubMed] [Google Scholar]
- 76.Robson MC, Mustoe TA, Hunt TK. The future of recombinant growth factors in wound healing. Am J Surg. 1998;176:80S–2. doi: 10.1016/s0002-9610(98)00186-x. [DOI] [PubMed] [Google Scholar]
- 77.Jin Q, Wei G, Lin Z, Sugai JV, Lynch SE, Ma PX, et al. Nanofibrous scaffolds incorporating PDGF-BB microspheres induce chemokine expression and tissue neogenesis in vivo. PLoS ONE. 2008;3:e1729. doi: 10.1371/journal.pone.0001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei G. Growth factor-delivering nano-fibrous scaffolds for tissue regeneration, PhD Dissertation, Department of Biomedical Engineering. The University of Michigan; Ann Arbor, MI: 2006. [Google Scholar]
- 79.Wang F, Li Z, Tamama K, Sen CK, Guan J. Fabrication and characterization of prosurvival growth factor releasing, anisotropic scaffolds for enhanced mesenchymal stem cell survival/growth and orientation. Biomacromolecules. 2009;10:2609–18. doi: 10.1021/bm900541u. [DOI] [PubMed] [Google Scholar]
- 80.Chun KW, Cho KC, Kim SH, Jeong JH, Park TG. Controlled release of plasmid DNA from biodegradable scaffolds fabricated using a thermally-induced phase-separation method. J Biomater Sci Polym Ed. 2004;15:1341–53. doi: 10.1163/1568562042368103. [DOI] [PubMed] [Google Scholar]
- 81.Jang JH, Shea LD. Controllable delivery of non-viral DNA from porous scaffolds. J Control Release. 2003;86:157–68. doi: 10.1016/s0168-3659(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 82.Luu YK, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J Control Release. 2003;89:341–53. doi: 10.1016/s0168-3659(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 83.Liang D, Luu YK, Kim K, Hsiao BS, Hadjiargyrou M, Chu B. In vitro non-viral gene delivery with nanofibrous scaffolds. Nucleic Acids Res. 2005;33:e170. doi: 10.1093/nar/gni171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saraf A, Baggett LS, Raphael RM, Kasper FK, Mikos AG. Regulated non-viral gene delivery from coaxial electrospun fiber mesh scaffolds. J Control Release. 2010;143:95–103. doi: 10.1016/j.jconrel.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kriegel C, Kit KM, McClements DJ, Weiss J. Nanofibers as carrier systems for antimicrobial microemulsions. Part I: fabrication and characterization. Langmuir. 2009;25:1154–61. doi: 10.1021/la803058c. [DOI] [PubMed] [Google Scholar]
- 86.Feng K, Sun H, Bradley MA, Dupler EJ, Giannobile WV, Ma PX. Novel antibacterial nanofibrous PLLA scaffolds. J Control Release. 2010;146:363–9. doi: 10.1016/j.jconrel.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu X, Yang L, Wang X, Chen X, Liang Q, Zeng J, et al. Ultrafine medicated fibers electrospun from W/O emulsions. J Control Release. 2005;108:33–42. doi: 10.1016/j.jconrel.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 88.Wolinsky JB, Liu R, Walpole J, Chirieac LR, Colson YL, Grinstaff MW. Prevention of in vivo lung tumor growth by prolonged local delivery of hydroxycamptothecin using poly(ester-carbonate)-collagen composites. J Control Release. 2010;144:280–7. doi: 10.1016/j.jconrel.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 89.Mandell RL, Socransky SS. Microbiological and clinical effects of surgery plus doxycycline on juvenile periodontitis. J Periodontol. 1988;59:373–9. doi: 10.1902/jop.1988.59.6.373. [DOI] [PubMed] [Google Scholar]
- 90.Heimdahl A, Nord CE. Antimicrobial agents in the treatment of periodontal diseases: special aspects on tetracycline and doxycycline. Scand J Infect Dis Suppl. 1988;53:35–45. [PubMed] [Google Scholar]
- 91.Tessmar JK, Gopferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:274–91. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]


