Abstract
The ability to adapt behavior in a changing environment is necessary for humans to achieve their goals and can be measured in the lab with tests of rule-based switching. Disease models, such as cocaine addiction, have revealed that alterations in dopamine interfere with adaptive set switching, culminating in perseveration. We explore perseverative behavior in individuals with cocaine use disorders (CUD) and healthy controls (CON) during performance of the Wisconsin Card Sorting Test (WCST) (N = 107 in each group). By examining perseverative errors within each of the 6 blocks of the WCST, we uniquely test two forms of set switching that are differentiated by either the presence (extradimensional set shifting (EDS) – first 3 blocks) or absence (task-set switching – last 3 blocks) of contingency learning. We also explore relationships between perseveration and select cognitive and drug use factors including verbal learning and memory, trait inhibitory control, motivational state, and urine status for cocaine (in CUD). Results indicate greater impairment for CUD than CON on the WCST, even in higher performing CUD who completed all 6 blocks of the WCST. Block by block analysis conducted on completers’ scores indicate a tendency for greater perseveration in CUD than CON but only during the first task-set switch; no such deficits were observed during EDS. This task-set switching impairment was modestly associated with two indices of immediate recall (r = −.32, −.29) and urine status for cocaine [t (134) = 2.3, p <.03]. By distinguishing these two forms of switching on the WCST, the current study reveals a neurocognitive context (i.e. initial stage of task-set switching) implicit in the WCST that possibly relies upon intact dopaminergic function, but that is impaired in CUD, as associated with worse recall and possibly withdrawal from cocaine. Future studies should investigate whether dopaminergically innervated pathways alone, or in combination with other monoamines, underlie this implicit neurocognitive processes in the WCST.
Keywords: perseveration, memory, prefrontal cortex, task switching, cocaine, drug addiction
1. Introduction
The ability to perform two tasks in succession requires several cognitive and emotional processes known as executive functions. For instance, modifying your driving route home as a function of traffic patterns and/or errands requires higher-order functions that encompass planning, sequencing, initiating, sustaining, and updating behavior toward your given goals. Meeting one’s goals requires an appropriate configuration of mental resources to produce a procedural task-set (a configuration of cognitive processes that is actively maintained for subsequent performance of the task) (Monsell, 1996, 2003). Humans apply ‘executive’ control to both select and implement a task-set that is appropriate to obtain their goal and to also inhibit distractions that get in the way of that goal (Monsell, 1996). Each stimulus that is encountered however can produce alternatives (e.g. one could choose to sit in traffic or go to dinner and wait for the traffic to die down, etc.). Switching between task sets therefore depends upon cognitive flexibility. Each time we switch our behavior a switch cost is incurred (e.g. time and/or error).
The Wisconsin Card Sorting Test (WSCT) (Berg, 1948; Heaton, 1999) is a widely used measure of cognitive flexibility that assesses the ability to shift cognitive set from one perceptual attribute of a complex visual stimulus to another through feedback regarding the accuracy of a response received after every trial (Milner, 1963). Specifically, the WCST assesses attentional set shifting in which an individual must learn to switch their behavior based on three sorting rules (cards are sorted by their color, shape, or number, in this sequence). Two types of trials are encountered through contingency learning: those following negative feedback (“wrong”) which require an extradimensional set shift (EDS; shifting responding from a current rule to a new rule) and those following positive feedback (“right”) which require maintaining the current sorting rule (intradimensional shifting). After the three sorting principles have been utilized, the sorting sequence is repeated to the completion of three more sorting sets. Therefore, in the first sequence an individual initially learns to sort according to the three sorting rules, but no new contingency learning is required when they repeat the sequence (i.e., the individual does not need to learn new sorting dimensions/sets). Instead, switching in the second sequence occurs only between previously reinforced rules, and therefore requires reconfiguring (unsuppression) of a previously relevant task set. EDS has been differentiated from task-set switching based on the premise that task-set switching does not involve new learning and therefore different (but sometimes overlapping) neural mechanisms are proposed to underlie each process (see reviews by Robbins, 2007; Sakai, 2008). Based upon this learning distinction, we will refer to switching during the first sequence of the WCST as EDS, and to switching during the second sequence as task-set switching. Both forms of switching (i.e. EDS and task-set switching) require the inhibition of a previous relevant rule and an attentional shift to the relevant features of the stimulus. However, only during task-set switching do previously relevant stimulus features become relevant once again and therefore must be reused for accurate responding. Failures to switch can be perseverative in nature, that is, the individual will continue to sort to a previously relevant rule even after the rule has clearly changed. Perseverative error is considered a marker for prefrontal cortical dysfunction (Strauss, Sherman, & Spreen, 2006).
1.1 Neurotransmission Underlying the WCST
There is some ambiguity as to the specific neural mechanisms that underlie the multiple cognitive processes assessed by set shifting and task switching designs such as the WCST. This ambiguity is reflected in the significant arm of research dedicated to decomposing its neuropsychological processes (for review see Nyhus & Barcelo, 2009). Disease models have proven to be a valuable resource for understanding the neural mechanisms that are associated with cognitive flexibility. For example, there is a reliable association between frontostriatal dopamine and task switching (Cools, Barker, Sahakian, & Robbins, 2003; Robbins, 2007; Stelzel, Basten, Montag, Reuter, & Fiebach, 2010). However, human studies that have utilized modified versions of the WCST as well as studies with animal analogues suggest that other neurotransmitters underlie the multiple cognitive processes in the WCST. For example, EDS has been associated with noradrenaline (for review see Robbins, 2007). Given that cocaine abuse is associated with changes primarily in the corticostriatal dopaminergic circuit (Volkow, Wang et al., 2008), the pattern of perseverative behavior within this population may inform us of neurotransmitter systems that help support both EDS and task-set switching processes within the WCST.
1.2 The Prefrontal Cortex (PFC) and Perseverative Behavior
Humans with substance dependence disorders and/or other dopaminergically-related disorders produce more perseverative errors during set shifting on the WCST as compared to healthy control subjects (CON) (Goldstein et al., 2004; Salo et al., 2005; Verdejo-Garcia & Perez-Garcia, 2007; Woicik et al., 2009). It is hypothesized that these individuals fail to master attentional control to inhibit irrelevant/distracting information, and therefore erroneously apply previous relevant rules even when it becomes inappropriate (i.e., perseveration) (Bishara & Jacoby, 2008; Garavan & Stout, 2005; Hester, Barre, Mattingley, Foxe, & Garavan, 2007; Salo et al., 2005). Human studies of cocaine addiction are supplemented by animal studies showing that repeated cocaine administration impairs learning on WCST analogues (Jentsch, Olausson, De La Garza, & Taylor, 2002). These deficits are attributed to the PFC. For example, animal and human studies show that reductions in the ventrolateral PFC (Hampshire & Owen, 2006) have been reliably associated with EDS. However, the inferior frontal gyrus and its connections to basal ganglia mechanisms have been correlated with task-set switching (Aron, Monsell, Sahakian, & Robbins, 2004; Duncan & Owen, 2000; Konishi et al., 1999). In addition, functional connectivity between the frontal lobes and basal ganglia has been shown to contribute to better performance on the WCST in general, and dopamine has been suggested to modulate this corticostriatal connectivity (Nagano-Saito et al., 2008).
1.3 Memory Processes, Trait Inhibitory Control, and Motivation
Memory is essential for successful set switching (Asaad, Rainer, & Miller, 1998; Goldman-Rakic, 1990; Levy & Goldman-Rakic, 1999; Mansouri, Matsumoto, & Tanaka, 2006; Pontecorvo, Sahgal, & Steckler, 1996; Rao, Rainer, & Miller, 1997; Sakagami & Niki, 1994; Wallis, Dias, Robbins, & Roberts, 2001; White & Wise, 1999). Indeed, one of the central cognitive components probed by the WCST is working memory, which is necessary for maintaining and updating task-relevant information and goal-directed representations (Strauss et al., 2006). Moreover, it is well established that the PFC has a major role in subserving working memory functions (Goldman-Rakic, 1992, 1994, 1995). For example, neurons in the dorsolateral PFC are considered a correlate for shorter-term memory of the relevant rule and essential for successful performance on the WCST (Mansouri et al., 2006). Similar to task switching and its reported reliance on dopamine, impairment to neural feedback mechanisms subserving memory are considered a result of dopamine dysfunction in the PFC (Goldman-Rakic, 1998a; Seamans & Yang, 2004). Based on previous findings from human studies indicating memory impairment in individuals with cocaine use disorders (CUD) (Goldstein et al., 2004; Woicik et al., 2009), we predict that deficits in memory (i.e., recall) will be correlated with perseverative behavior during switching in CUD.
To achieve a better understanding of the neural mehanisms that support EDS and task-set switching, we examine perseverative error in CUD, a disorder that is marked by dopamine deficiencies. Specifically, we measure perseveration within each of the two sequences of the WCST (i.e. EDS and task-set switching) and compare performances of (gender and education-matched) CUD and CON who successfully completed all six blocks of the WCST. By so doing, we can determine whether perseverative impairment on the WCST reflects an implicit process that may possibly be dopaminergically regulated. We also explore possible cognitive/emotional correlates of perseverative behavior in CUD to determine the impact of select individual differences in perseveration. In addition to memory (i.e., recall), we consider trait inhibitory control and urine status for cocaine as previous studies have documented their effects on neuropsychological dysfunction in drug addiction (Belin, Mar, Dalley, Robbins, & Everitt, 2008; Dolan, Bechara, & Nathan, 2008; Gullo, Jackson, & Dawe, 2010; Verdejo-Garcia, Bechara, Recknor, & Perez-Garcia, 2007; Verdejo-Garcia, Lawrence, & Clark, 2008; Woicik et al., 2009). Alternatively, perseverative responding might result from declines in task interest. Therefore, we also explore the relationship between perseveration and self-reported motivation to perform the neuropsychological (NP) tasks.
2. Materials and Methods
2.1 Subjects and Procedures
All subjects were selected from neuroimaging protocols at Brookhaven National Laboratory which have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. One hundred and seven CUD were matched to 107 CON on gender and years of education. All subjects gave their informed consent prior to their inclusion in the study. Telephone screening and subsequent on-site evaluations by a licensed physician ensured that all subjects were healthy, that cocaine abusers met DSM-IV diagnostic criteria for current cocaine dependence or abuse (reporting cocaine as their preferred drug and with primary use of cocaine by smoked route), and that CON had no history of drug addiction. Exclusionary criteria for all subjects were history of head trauma with a loss of consciousness > 30 minutes and/or a history of past or present psychiatric, neurological, endocrine, or cardiovascular disease, and/or current psychiatric disorders (apart from cocaine, nicotine and alcohol abuse or dependence for the cocaine groups). No subjects were taking any medications at the time of the study.
Subjects were administered a NP battery on a separate random day or as a separate module that was independent of imaging procedures. For the purpose of this study, we use selected tests discussed below (see NP testing). On the morning of NP testing, a triage urine panel tested for the presence of any psychoactive drugs. A positive result for any drug was exclusionary with the exception of the presence of cocaine or its metabolites in the cocaine group. A positive result for cocaine indicated cocaine use within a 72-hour period of testing and was used as an index of short-term withdrawal (Woicik et al., 2009). During medical and psychological evaluations, subjects reported about their history of cocaine (age of onset, lifetime use) and nicotine (number of cigarettes smoked per day) use. Depressive symptoms within the past two weeks were assessed with Beck’s Depression Inventory II (BDI) (Beck, Steer, & Brown, 1996).
The sample was divided into four groups, according to their diagnosis (CUD or CON) and further subdivided according to their ability to complete all six blocks of the WCST (completers [C] versus non-completers [N]) resulting in the following distribution; (CUD-N=54; CON-N=17 CUD-C=53; CON-C=90)].
2.2 NP Testing
Verbal and non-verbal intelligence were estimated with the reading subscale of the Wide Range Achievement Test 3 (WRAT) (Wilkinson, 1993) and the matrix reasoning subscale of the brief Weschler Adult Scale of Intelligence (Wechsler, 1997), respectively (Table 1).
Table 1.
Descriptive Statistics
| Significance test (X2, F or t) | CUD-N < 6 (N =54) | CUD-C = 6 (N =53) | CON-N < 6) (N =17 | CON-C = 6 (N =90) | Contrast t-test significance (All CUD vs. All CON) | ALL CUD (N=107) | ALL CON (N=107) | |
|---|---|---|---|---|---|---|---|---|
| Gender (Female/Male) | 1.7 | 8/46 | 12/41 | 2/15 | 18/72 | 0.0 | 20/87 | 20/87 |
| Race (Caucasian/African American/Other) | 15.6 | 7/45/2 | 10/38/5 | 3/11/3 | 31/49/10 | 11.2** | 17/83/7 | 34/60/13 |
| First language (English/Other) | 4.4 | 54/0 | 53/0 | 16/1 | 88/2 | 7.1 | 107/0 | 104/3 |
| Laterality Quotient (Modified Edinburgh Handedness Inventory) | 0.6 | 0.8 ± 0.5 | 0.8 ± 0.5 | 0.9 ± 0.2 | 0.8 ± 0.5 | −1.3 | 0.8 ± 0.5 | 0.9 ± 0.4 |
| Socioeconomic Status (Hollingshead Index) | 5.8*** | 29.5 ± 11.2d | 33.7 ± 12.0 | 33.7 ± 13.5 | 37.5 ± 10.4a | −2.2 | 31.6 ± 11.7 | 36.9 ± 10.9 |
| Age | 11.9*** | 44.6 ± 5.1d | 42.0 ± 6.3d | 40.1 ± 6.8 | 37.8 ± 8a,b | 3.9*** | 43.3 ± 5.9 | 38.1 ± 7.8 |
| Education (years) | 1.3 | 12.8 ± 2.3 | 13 ± 1.9 | 12.7 ± 2.2 | 13.4 ± 2.0 | −0.5 | 12.9 ± 2.1 | 13.3 ± 2.1 |
| Verbal Intelligence (WRAT-R III – Reading) | 7.9*** | 86.8 ± 14.6b,d | 94.2 ± 12.5a | 95.0 ± 16.4 | 97.9 ± 12.3a | −2.7** | 90.5 ± 14.1 | 97.5 ± 13.0 |
| Non-verbal Intelligence (WASI III-Matrix Reasoning) | 6.3*** | 9.3 ± 3.2d | 10.6 ± 1.8 | 8.9 ± 10.2d | 11.1 ± 2.9a,c | −0.1 | 9.9 ± 2.6 | 10.7 ± 3.0 |
| Depressive Symptoms (BDI) | 11.4*** | 10.2 ± 9.4c,d | 9.4 ± 10.1c,d | 3.9 ± 6.3a,b | 4.0 ± 4.7a,b | 5.3*** | 9.8 ± 9.7 | 4.0 ± 5.0 |
| Cigarette Smoking (Smokers/Non-Smokers) | 89.3*** | 43/11 c,d | 42/11 c,d | 3/14 a,b | 13/77 a,b | 89.3*** | 85/22 | 16/91 |
| Urine Status for Cocaine (Positive/Negative) | 0.8 | 39/15 | 34/19 | - | - | - | - | - |
| Lifetime use (years) of cocaine | 1.1 | 18.3 ± 6.8 | 16.5 ± 6.1 | - | - | - | - | - |
Note: CUD= individuals with cocaine use disorders; CON= control subjects; N = individuals not able to complete 6 WCST categories; C=individuals who completed all six categories of WCST; all values under group categories are either distributions or the mean ± the standard deviation; For analysis among four groups,
mean value significantly differs from CUD-N;
mean value significantly differs from CUD-C;
mean value significantly differs from group CON-N;
mean value significantly differs from group CON-C; Contrast Significance Test= indicate significance t values for planned contrasts testing for differences between all CUD versus all CON; WRAT-R III = Wide Range Achievement Test Revised (3rd Version); WASI III = Wechsler Adult Scales of Intelligence (3rd Version); BDI = Beck’s Depression Inventory;
p < .05,
p < .01,
p < .001.
Cognitive flexibility was assessed with a computerized version of the WSCT (Heaton, 1999). This version presents stimulus cards one at a time on a computer screen to which respondents must learn a sorting strategy based on minimal feedback (a recorded voice indicating whether the response was “right” or “wrong”). The task consists of six blocks of trials: The first three blocks require novel discrimination learning in which the respondent must learn to sort stimulus cards on color first, then on geometric shape, and then on the number of objects on the card (sequence 1- EDS). Blocks 4–6 represent blocks in which the respondent is required to sort on the three previously reinforced sorting rules (sequence 2 - task-set switching). The WCST permits an examination of the patterns of errors made by subjects to determine if they are perseverative or random in nature. Successful performance of the WCST requires a number of intact cognitive functions and traditional performance indices include: Establishing set which is inferred by the participant’s ability to understand the instructions and utilize computer feedback to establish the first sorting principle. The ability to maintain set is demonstrated by the ability to continue sorting to the correct principle once it is discovered (intradimensional shifting). Set-shifting ability is evaluated when, after a series of 10 correct responses, and without warning, the computer changes the sorting criterion so that previously correct responses are no longer correct. Thus, a participant must be able to recognize that the principle has changed and again use the feedback to determine the new sorting principle. The following performance scores are yielded and reported in the current study: Number of categories completed (representing the number of blocks with 10 consecutive correct matches), perseverative responses (continuing to choose no-longer correct cards), non-perseverative responses, percent perseverative error (the concentration of perseverative errors in relation to overall test performance), failures to maintain set (after 5 consecutive correct matches an incorrect match is made before the block is successfully completed), and percent of conceptual level responses (consecutive correct matches occurring in runs of three).
Recall was assessed by the California Verbal Learning Test II (CVLT) (Delis, Kaplan, Kramer, & Ober, 2000). The CVLT-II is a popular measure of list learning, in which a 16-item word list (List A) is read to the subject over five learning trials. The words comprising this list are equally divided into four semantic categories (vegetables, furniture, ways of traveling, and animals). Immediate free recall is assessed with free recall of list A over five consecutive trials. Thus, these trials are an index of overall auditory attention and verbal learning skills (Delis et al., 2000), a process necessary for acquiring memory (Okano, Hirano, & Balaban, 2000). In previous studies by our group, CUD subjects exhibited impairment on this task as compared to matched CON (Goldstein et al., 2004; Woicik et al., 2009).
Trait inhibitory control was assessed by the control subscale of the Multidimensional Personality Questionnaire (MPQ) which possesses good reliability and validity (Tellegen & Waller, 1997). To test whether perseverative error might be driven by declines in task interest/motivation, subjects were asked to rate the extent they felt “motivated” on a likert scale ranging from 0 to 10 (“Not at all” to “Very much”) before the start and at the close of the NP battery. The mean of these two scores was computed for use in the current analyses.
2.3 Statistical Analyses
One-way analyses of variance (ANOVA) with planned contrasts were conducted on demographic and NP variables to examine between-subject main effects among the four groups (i.e CUD-N, CUD-C, CON-N, and CON-C) and to test two nested between-subject factors [i.e., subject diagnostic status (all CUD versus all CON) and WCST completion status (all C versus all N)]. Post-hoc follow-up analyses were conducted using Tukey t-tests (Klockars, Hancock, & McAweeney, 1995). Chi-square analyses were used for categorical variables (Tables 1 and 2).1 2 A secondary analysis was conducted in the sub sample of subjects that completed all six blocks of the WCST. A mixed 2 (sequence: 1st versus 2nd) X 3 (stimulus block: blocks 1–3) X 2 (subject status: CUD-C vs. CON-C) ANOVA was conducted on indices of perseverative3 and non-perseverative error. Post hoc independent and paired-sample t-tests were conducted in these analyses.
Table 2.
Results from ANOVAs with Planned Contrasts on WCST and other Neuropsychological Tests
| Significance test (X2, F or t) | CUD-N < 6 (N =54) | CUD-C = 6 (N =53) | CON-N < 6 (N =17) | CON-C = 6 (N =90) | Contrast t-test significance (All CUD vs. All CON) | ALL CUD (N=107) | ALL CON (N=107) | |
|---|---|---|---|---|---|---|---|---|
| WCST Variables | ||||||||
| Categories Completed | 129.9*** | 3.0 ± 1.4 b, d | 6.0 ± 0.0 a, c | 2.9 ± 1.4 b, d | 6.0 ± 0.0 a, c | −0.7 | 4.4 ± 2.0 | 5.5 ± 1.3 |
| Cards Completed | 139.1*** | 128.0 b, d | 89.6 ± 13.9 a, c | 128.0 b, d | 90.2 ±16.6 a, c | −0.5 | 109.0 ± 21.6 | 96.2 ± 20.6 |
| % Correct | 139.6*** | 55.4 ± 11.8 b, d | 80.7 ± 5.6 a, c | 59.6 ± 9.4 b, d | 81.5 ± 6.7 a, c | −1.8 | 68.3 ± 15.4 | 78.3 ± 10.6 |
| % Errors | 146.6*** | 44.5 ± 11.8 b, d | 18.9 ± 5.1 a, c | 40.3 ± 9.4 b, d | 18.5 ± 6.7 a, c | 1.7 | 31.8 ± 15.8 | 22.0 ± 10.8 |
| % Perseverative Responses | 91.7*** | 24.9 ± 10.7 b, d | 10.0 ± 3.9 a, c | 23.4 ± 9.0 b, d | 9.7 ± 3.4 a, c | 0.6 | 17.5 ±11.0 | 11.8 ± 6.9 |
| % Perseverative Errors | 99.6*** | 22.2 ± 8.3 b, d | 9.5 ± 3.4 a, c | 21.2 ± 7.2 b, d | 9.2 ± 2.9 a, c | 0.5 | 15.9 ± 9.0 | 11.1 ± 5.8 |
| % Non-Perseverative Errors | 71.3*** | 22.5 ± 8.5 b, d | 9.4 ± 3.0 a, c | 19.2 ± 6.3 b, d | 9.4 ± 4.9 a, c | 1.7 | 16.0 ± 9.1 | 10.9 ± 6.3 |
| % Conceptual Level Responses | 167.6*** | 41.0 ± 15.7 b, d | 77.8 ± 6.7 a, c | 44.9 ± 13.7 b, d | 77.8 ± 8.9 a, c | −1.1 | 59.3 ± 22.1 | 72.5 ± 15.5 |
| Trials to Complete 1st Category | 12.9*** | 31.4 ± 27.0 b, d | 14.7 ± 6.6 a | 22.6 ± 17.5 | 16.3 ± 9.0 a | 1.4 | 23.1 ± 21.4 | 17.3 ± 10.9 |
| Failure to Maintain Set | 11.8*** | 1.8 ± 1.7 b, d | 0.7 ± 0.9 a, c | 2.1 ± 1.6 b, d | 0.8 ± 1.0 a, c | −0.9 | 1.3 ± 1.5 | 1.0 ± 1.2 |
| Perseverative Error (% Change Sequence 2–1) | 0.3 | -- | 0.0 – 0.3 | -- | −0.1 ± 0.3 | 0.7 | -- | -- |
| Perseverative Error (Trial 4 – Trial 1) | 2.3* | -- | 1.4 ± 2.7 | -- | 0.4 ± 2.3 | 1.1 | -- | -- |
| CVLT-Trial 1 | 2.5 | 5.8 ± 2.3 | 6.0 ± 1.7 | 6.4 ± 1.3 | 6.6 ± 2.0 | −1.9 | 5.9 ± 2.0 | 6.6 ± 1.9 |
| CVLT-Trial 5 | 4.7*** | 10.5 ± 2.5 d | 10.6 ± 2.4 d | 10.87 ± 1.9 | 11.9 ± 2.5 a,b | −2.0* | 10.5 ± 2.4 | 11.7 ± 2.4 |
| CVLT (% Change Trial 5 – 1) | −0.5 | -- | 0.8 ± 0.5 | 0.9 ± 0.6 | 1.2 | -- | -- | |
| Trait Inhibitory Control (MPQ-Control) | 4.2*** | 14.7 ± 4.8 d | 15.1 ± 4.8 d | 17.0 ±5.1 | 17.6 ± 4.9 a,b | −2.6** | 14.9 ± 4.8 | 17.5 ± 4.9 |
| State Motivation | 0.1 | 6.7 ± 2.2 | 6.7 ± 1.9 | 6.9 ± 2.4 | 6.7 ± 2.2 | −0.3 | 6.7 ± 2.0 | 6.7 ± 2.2 |
Note: CUD= individuals with cocaine use disorders; CON= control subjects; N = individuals not able to complete 6 WCST categories; C=individuals who completed all six categories of WCST; CVLT =; MPQ=Multidimensional Personality Questionnaire; all values under group categories are either distributions or the mean ± the standard deviation;
mean value significantly differs from CUD-N;
mean value significantly differs from CUD-C;
mean value significantly differs from CON-N;
mean value significantly differs from CON-C; Contrast Significance Test= indicate significance t values for planned contrasts testing for differences between all CUD versus all CON;
p < .05,
p < .01,
p < .001.
To directly contrast perseveration during EDS and task-set switching, we calculated two percentage change scores in this sub sample of completers; the rate of increased perseverative error from sequence 1 (EDS blocks that require sorting to novel sorting rules) to sequence 2 (task-set switch blocks that require sorting to a previously relevant rule) and from block 1 (first block of the test in which the individual must learn to sort by color) to block 4 (the first block to require sorting to a previously relevant rule). The percentage change from sequence 1 to sequence 2 would account for performances on all 3 blocks within a sequence and would supplement the other change score given that, unlike all other blocks in the WCST, block 1 does not require a set shift (Heaton, 1999). In addition, we calculated a percentage change score representing the rate of list learning from trial 1 to trial 5 on the CVLT. We reasoned that this would estimate a learning “snapshot” of immediate free recall. Pearson correlations were conducted to test associations between perseverative error, immediate free recall, trait inhibitory control, and state motivation.
The potential impact of all demographic variables that differed between the study groups (Table 1) was examined. If significantly associated with the dependent variables, the demographic variable was entered as a covariate one at a time (Tabachnick & Fidel, 1983). To protect against type I error in all analyses we applied a significance level of p < .01 and corrected for the number of comparisons by performing Tukey t-tests in ANOVAs (Klockars et al., 1995). We also report WCST trends that reached a significance level of p < .05.
3. Results
3.1 Descriptives
One-way ANOVAs and chi-square analyses revealed that there were no differences between the four study groups in distributions of gender and years of education in accordance with matching. Groups also did not differ on first language, race, or handedness (note however there was a significantly different distribution of African Americans when CUD groups were collapsed and compared to all CON). We did observe differences in socioeconomic status (SES), age, verbal (WRAT- reading) and non-verbal (matrix reasoning) intelligence, depressive symptoms, and smoking status (Table 1). Table 1 provides results from the one-way ANOVA, Tukey t-tests, and planned contrasts between all CUD and all CON. These tests indicated a significant main effect for diagnostic status on age (CON-C>CUD-N = CUD-C), verbal intelligence (CUD-N< CUD-C = CON-C), and depressive symptoms (CUD-N=CUD-C > CON-N=CON-C). In addition, there was a main effect for WCST completion status on scores of non-verbal intelligence (t (210) = −3.8, p < .001) and a trend for this factor on SES (CUD-N< CON-C). CUD groups were comprised of more smokers than CON groups.
Within CUD, severity of cocaine use (as measured by lifetime years of use) and urine status for cocaine did not differ between C and N subgroups (Table 1). Based on the above results, SES, age, verbal and non verbal intelligence, depressive symptoms, race, and smoking status were considered for covariate analyses in all subsequent analysis.
3.2 Wisconsin Card Sorting Test Performance
Chi-square analysis indicated that CUD were associated with greater inability to complete all six blocks of the WCST (N = 54 CUD versus 17 CON, X2 =28.9, p < .001). One way ANOVAs yielded significant main effects for group across all WCST indices. Table 2 presents results of the one-way ANOVA on all WCST indices computed for the study. Planned contrasts indicated a main effect of WCST completion status (and not diagnostic category) on these scores (C>N) indicating that N groups in general (including both CUD and CON) performed more poorly than C groups across all WCST indices (CUD-C=CON-C>CUD-N=CON-N; Tukey t scores ranged from 4.0 – 29.5, all p < .001).
3.3 Secondary Analysis with Completers
No main effects were found in the mixed ANOVA conducted on perseverative errors but we observed a significant sequence × block interaction [F (2, 133) = 24.7, p < .0001] and a trend for a sequence × block × group interaction [F (2, 133) = 3.5, p < .05]. Post hoc analyses revealed greater perseverative errors for all C on the second block in sequence 1 as compared to the second block in sequence 2 [(t (134) = 5.7, p < .0001]. The trend for the three-way interaction suggested greater perseverative error for CUD-C as compared to CON-C on the initial block requiring respondents to sort on a previously relevant rule, that is the first task-set switch (block 4) [t = 2.2, p < .05 (Figure 1)]. CUD-C showed an increase in perseverative errors on the initial block requiring respondents to sort on a previous rule (block 4 > block 1, t (52) = −2.7, p < .01), followed by a decrease in perseverative errors in the following blocks [block 2 > block 5: t (52) = 3.7, p < .001; and block 3 > block 6: t (52) = 2.1, p < .05]. In contrast, the scores of CON-C reflected a trend for a smooth learning curve (with a decrease in perseverative errors documented when comparing block 5 with block 2: t (89) = 4.5, p < .001).
Figure 1.
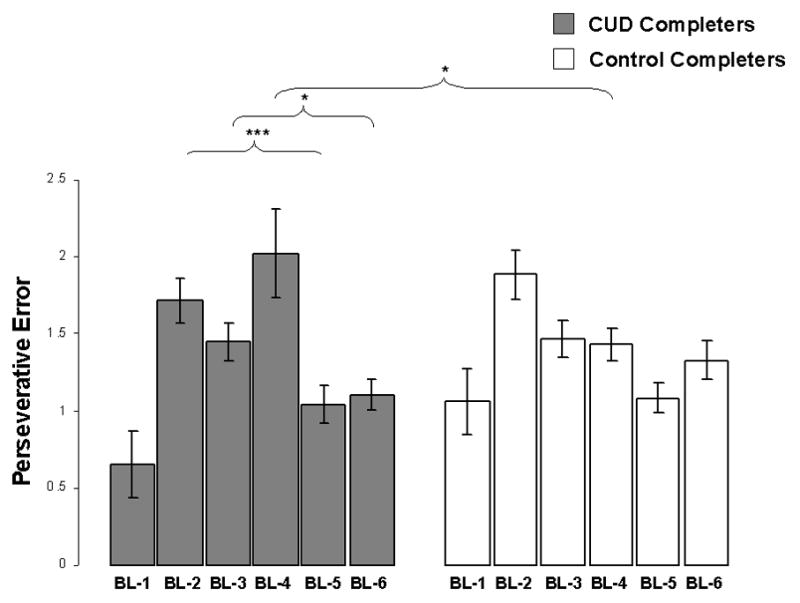
Perseverative error for each of the 6 blocks on the WCST. Error bars are standard error means; BL=Block on the WCST; Sequence × Block × Group interaction, F = 3.5, p < .05; *p < .05; ** p < .01; ***p < .001
The analysis conducted on non-perseverative errors revealed main effects for sequence [F (2,134) = 18.7, p < .0001 (sequence 1 > sequence 2)] and block [F (2,134) = 11.8, p < .0001 (block 1 > block 2)]. In addition, there was a sequence X block interaction [(F (2,133) = 12.6, p < .0001] indicating steep learning in both groups between blocks 1 and 2 [t (48) =3.7 and t (85) = 4.6, for CUD-C and CON-C, respectively, p < .001] (Figure 2). A group main effect or interaction with group were not significant.
Figure 2.

Non- perseverative error for each of the 6 blocks on the WCST. Error bars are standard error means; BL=Block on the WCST; Main effects for sequence, F (2,133) = 18.7, p < .0001, block, F (2,133) = 11.8, p < .0001, and a sequence X block interaction, (F (2,133) = 12.6, p < .0001; *p < .05; ** p < .01; ***p < .001
3.4 Correlations between Memory, Trait Inhibitory Control, and Motivation with Perseveration
Table 2 reports mean scores and standard deviations for immediate free recall, trait inhibitory control, and motivational state for the entire sample. There were no main effects for group on scores measuring the level of state motivation or recall as measured by trial 1 of the CVLT. However, main effects for group were found for the number of words recalled on trial 5 of the CVLT as well as trait inhibitory control as measured by MPQ-control. Post hoc tests indicated that both CUD groups had worse recall on trial 5 and reported lower control on the MPQ as compared to CON-C.
The correlations between change in perseverative errors (sequence 2 minus 1 and trial 4 minus 1) and percentage change in immediate free recall (CVLT trial 5 – 1) approached significance (r = −.32 and .29, respectively, p < .05) exclusively in CUD-C (as compared to CON-C in which r = 0.1 and −0.1, p > .10) (Figure 3). This correlation shows that the lower the increase in immediate free recall, the greater the perseverative behavior during sequence 2 (i.e., task-set switching). Trait inhibitory control and motivational state were not correlated with either of the WCST change scores (rs ranged from −.21 − .22 for CUD and −.01 − .23 in CON, all p > .05).
Figure 3.
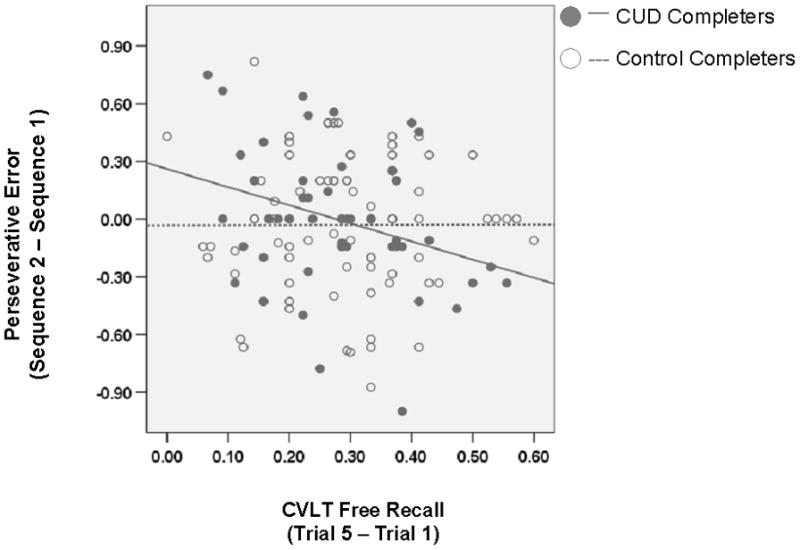
Correlation between percentage change in perseverative error from sequence 1 to sequence 2 and CVLT immediate free recall; CUD r = −.32, p < .05; CON r =.01, p > .10
3.5 Cocaine Use and WCST performance
Urine status and lifetime use of cocaine were not associated with any of the standard WCST indices. In our secondary analyses of completers, we found a trend for a main effect of urine status; CUD-C with a urine negative status for cocaine committed more perseverative error on block 4 in relation to block 1 [t (47) = 2.1, p <.05].
3.6 Effects of Potential Covariates on WCST Performance
BDI scores did not correlate with any of the WCST indices. However, age, intelligence (WRAT-reading and matrix reasoning scores), smoking status, race, and SES were associated with some of the WCST indices and therefore were entered singly as covariates in analyses conducted on the entire sample. All of the main effects remained significant (F values ranged from 6.6 – 210.0, all p < .01).
In the full sample, both intelligence scores (matrix reasoning and WRAT-reading) as well as race and smoking status were associated with immediate recall on trial 5 of the CVLT. The main effect for group in this analysis was reduced to a trend when each (except race) was controlled [F values ranged from = 2.3 – 3.2, all p < .10]. Smoking status was associated with MPQ-control such that after controlling for its impact the main effect for WCST group failed to reach significance[F (3, 173) = 1.4, p > .10].
Importantly, in the secondary analysis of completers only, we found that non-verbal intelligence (matrix reasoning) was associated with perseverative errors: when entered as a covariate, the sequence × block interaction failed to reach significance [F (2,134) = 0.3, p > .10)] but the trend for the three way interaction was maintained [F (2, 134) = 3.6, p < .05]. In the analysis of non-perseverative error we found that SES was associated with errors: when entered as a covariate, the main effect for sequence and the sequence × block interaction were reduced to trends [F (2,134) = 3.1 and 2.7, respectively, p < .10] and the main effect for block did not reach significance. None of the descriptive variables were correlated with the dependent variables in correlation analyses.
4. Discussion
The current study explored perseveration during performance of the WCST in healthy individuals and CUD. In a large matched sample of CUD, we observed greater error (perseverative and non-perseverative) and greater inability to complete the test as compared to CON. The design of the WCST allowed us to compare performances of a group of higher functioning CUD (CUD-C, those able to complete the WCST) who had comparable scores to the CON-C group on all traditional WCST indices. By uniquely examining the block-by-block performance on the task, we were able to detect trends that suggested impairment in otherwise normally performing CUD-C. Indeed, we observed that CUD-C exhibited more perseveration when they were initially required to sort by a previous sorting rule (the initial task-set switch). In contrast, CON-C exhibited generally less perseveration as they progressed through blocks. The current study also points to deficits in recall and possibly withdrawal from cocaine (crudely indexed by urine status for cocaine), but not self reported motivation and inhibitory control, as factors that may impact task-set switching.
Our results are consistent with numerous human studies showing impaired executive function (and recall) in CUD (K. Bolla et al., 2004; K. I. Bolla, Funderburk, & Cadet, 2000; Goldstein et al., 2004; Jovanovski, Erb, & Zakzanis, 2005; Verdejo-Garcia, Bechara, Recknor, & Perez-Garcia, 2006; Verdejo-Garcia & Perez-Garcia, 2007; Woicik et al., 2009). The current study shows that, in otherwise normally performing CUD, deficits in set shifting may only be evident in a specific neurocognitive context (i.e., when for the first time an individual must sort on a previous sorting rule). Given equivalent performance to CON-C on all other WCST indices, this deficit, and possibly its association with impairment to recall, may help identify more specific neurocognitive processes underlying task-set switching. Indeed, chronic cocaine use is associated with impaired dopaminergic neurotransmission in a frontostrial loop (Goldstein & Volkow, 2002), and therefore impairment in this circuit may account for interference with this particular aspect of task-set switching in the WCST. The fact that perseveration at this juncture was higher for CUD in a more protracted withdrawal state emphasizes this point, given that dopamine neurotransmission is down regulated during withdrawal (Volkow, Fowler, Wang, Baler, & Telang, 2008). We speculate about the precise mechanism underlying this sepcific deficit in section 4.2 below.
4.1 Deficits in Task-Set Switching Among Higher Functioning CUD
A substantial amount of research has been dedicated to parcelling the multiple cognitive processes implicit in the WCST (Rogers, Andrews, Grasby, Brooks, & Robbins, 2000). With this in mind, the current results show a selective impairment in CUD. In our sample of higher functioning CUD-C, the exaggerated perseveration isolated to a single task-set switch is starkly contrasted by equivalent behavior to CON-C on all other blocks. Therefore, our results raise the question of why CUD-C experienced more failures than CON-C at this particular juncture of the task. Perseverative errors result from interference from either an attention bias or a strong stimulus-reinforcement association formed for the previous sorting rule (Robbins, 2007). However, CUD-C were capable of overcoming both forms of interference on all other blocks and therefore the unique features of the fourth block may reveal a specific cognitive hurdle for CUD-C. Indeed, in the fourth block, for the first time no new learning is involved; instead, a respondent must reconfigure a previous task set (i.e., sorting by color) for which there was no interference from previously reinforced sorting rules. Subsequently, compared to all other task blocks, the switch cost is the largest on the fourth block. Task set switching involves the interactions between three factors: task-set inertia (the persistence of activation and/or inibition from previous trials; Allport & Wylie, 1999; Monsell, 2003; Yeung and Monsell 2003), exogenous activation (activation of a task set induced by stimulus features, particularly on switch trials; Lhermite, 1983; Rogers and Monsell 1995), and endogenous control [a top-down input that biases a task set by directing attention to the relevant stimulus feature to overcome both the inertia on a switch trial and an irrelevant but activated task set (Norman & Shallice, 1986; Yeung and Monsell 2003)]. Therefore, it might be speculated that the larger switch cost observed in the fourth block in which for the first time an individual must reconfigure a previous task set reflected impairment to endogenous control such that top down input was insufficient for the reactivation of the relevant task set and the inhibition of all irrelevant but activated task sets.. Note that it is unlikely that the observed deficit was a result of the type of stimulus (color) since CUD-C perfomances were comparable to CON on the first block which also required sorting to color.
Large switch costs also result from failures in working memory processes including the retrieval of goal states (what to do) and condition-action rules (how to do it) (Monsell, 2003). Working memory processes including recall are also essential for performance of the WCST for facilitating inihibition, response selection, and holding stimuli representations “online” (Goldman-Rakic, 1998b). It is possible that the relationship we found between perseveration and CVLT recall [previously found to be compromised in CUD (Goldstein et al., 2004; Woicik et al., 2009)] may have a role in reducing the ability to hold stimulus representations ‘online’ at this particular junction of the test (i.e. at sequence 2). The notion that recall is necessary for acquiring memory for later retrieval is consistent with previous research (Okano et al., 2000) and suggests impairment to recall may promote perseverative error by increasing inhibition of the current sorting rule and/or activating transient carryover effects of the preceeding relevant rule. What might clarify the nature of the observed impairment would be to determine whether at this junction of the WCST carryover effects slow down a correct response selection in CUD or increase attention to the previously formed task sets when conflict is detected (Monsell, 2003). Nevertheless, our results suggest a particular cognitive context in which cognitive flexibility is challenged in a dopaminergically deficient population.
4.2 Dopaminergic Links to Task Switching
Results from human and animal studies show that alterations of different monamines elicit differential impairment in discrimination learning: Dopamine with task-set switching, noradrenaline (and in some studies dopamine) with EDS, and serotonin with reversal learning (Clarke, Dalley, Crofts, Robbins, & Roberts, 2004; Clarke, Walker, Dalley, Robbins, & Roberts, 2007; Crofts et al., 2001; Evers et al., 2005; Leber, Turk-Browne, & Chun, 2008; Oades, 1985; Robbins, 2007; Roberts, Loh, Baker, & Vickers, 1994; R. D. Rogers et al., 2003) (Cools, Barker, Sahakian, & Robbins, 2001; Funahashi, Bruce, & Goldman-Rakic, 1989; Mehta, Goodyer, & Sahakian, 2004). The absence of error differences between CUD-C and CON-C in the first sequence (involving EDS) is consistent with the above literature to the extent that EDS is associated with noradrenaline while impairment in CUD has been linked primarily to dopamine. Similarly, we cannot rule out a deficit in reversal learning but reversal learning is primarily associated with serotonin and typically engaged by tasks that simply reverse stimulus reinforcer associations (Dias, Robbins, & Roberts, 1997; Robbins, 2007; R. D. Rogers et al., 2000) which does not occur on the WCST. Instead, our focus on task-set shifting is consistent with results in Parkinson’s patients, another dopamine deficient population, where a similar impairment (in task switching) has been remediated by L-Dopa (Cools et al., 2003). More recent evidence suggests that increased D2 receptor density and not decreased density is associated with higher switch costs (Stelzel et al., 2010; Thoma, Wiebel, & Daum, 2007) consistent with the effects of suprastimulation of D2 receptors in inducing perseveration during a set shift (Haluk & Floresco, 2009). These seemingly conflicting results have been reconciled through the suggestion that the relationship between dopamine and task switching effort can be represented as an inverted U-shape such that extreme alterations in dopaminergic function in either direction may have a negative impact on task switching (Arnsten & Li, 2005).
Our behavioral results are also similar to results from earlier animal studies conducted with non-human primates who performed a WCST analogue or other EDS tasks. Following cocaine administration, primates exhibited intact acquisition of novel discriminations (i.e., learning to respond to a new sorting strategy), equivalent to sequence 1 in the current study, but they perseverated on blocks that required the inhibition of a previously reinforced association (Jentsch et al., 2002), equivalent to sequence 2 in the current study. Similarly, striatal-dopamine-depleted marmosets had equivalent performance to healthy control primates on EDS (novel set shifting) and intradimensional shifting (IDS) except they committed more errors at the end of the EDS/IDS series when faced with the choice of selecting between one of two previously learned/relevant reinforced task sets (Crofts et al., 2001), that is, when the switching deficit was isolated to an interpolated novel shift. Dopamine depletion in this striatal region (as well as the dorsolateral PFC) was also found to be correlated with working memory deficits in these animals (Collins et al., 1998).
Taken together, these studies suggest that the striatum, dorsolateral PFC (DLPFC) and their dopaminergic innervation are important in mediating shifts between previously established task sets (task-set switching). Recent human evidence revealed that the left DLPFC is involved in processing the negative feedback that is necessary for a set shift to occur on card sorting tests (Petrides, 2000; Monchi, Petrides, Petre, Worsley, & Dagher, 2001). Specificity to the left DLPFC has been suggested by its transient disruption with a continuous theta burst stimulation which impaired card sorting task performance and dopamine release in the striatum (Ko and colleagues (2008). To summarize, given that cocaine addiction is associated with lower dopamine D2 receptor density in the striatum as well as reduced regional activity in the DLPFC (Volkow, 2006), this mechanism may underlie the impairment reported in the current study.
4.3 Greater Perseveration and Negative Urine Status
The relationship between negative urine status and greater perseverative error suggests a possible effect of withdrawal on task-set switching. That perseveration at this juncture was higher for CUD in a more protracted withdrawal state emphasizes the role of dopamine neurotransmission in task-set switching given that dopamine is down regulated during withdrawal (Volkow, Fowler, Wang, Baler, & Telang, 2008). However, all monoamine neurotransmission (dopamine, serotonin, and noradrenaline) may be altered during withdrawal from cocaine (McDougle et al., 1994; Parsons, Koob, & Weiss, 1995; Rudoy & Van Bockstaele, 2005; Volkow, Fowler et al., 2008). Deficiencies in these neural chemical pathways, especially within PFC systems, may therefore differentially contribute to reduce an individual’s initial flexibility to appropriately switch to a previous task set (Monchi et al., 2001).
4.4 Study Limitations and Future Investigation
Some limitations of the current study raise questions for future investigation. First, CUD-C were significantly older than CON-C in the current study, and this may account for the observed differences in set shifting/switching. Indeed, as age increases so does perseveration on the WCST, and this age effect has been attributed to declines in processing speed, temporal processing, and deficits in working memory, all of which are mediated by decreased prefrontal cortical volume (Head, Kennedy, Rodriguez, & Raz, 2009). Although we controlled for age in all relevant analyses, similar testing in a sample that is also matched on age would help validate the current findings.
Second, with the exception of 19 urine samples in CUD-C, all others tested positive for cocaine indicating that cocaine had been consumed within 72 hours of the test. Therefore, the low number of subjects testing negative for cocaine may have reduced power in the analysis of urine status, and therefore conclusions on how compromised neurotransmission (i.e., withdrawal which was crudely indexed by urine status) may be related to this specific deficit should be cautiously interpreted (in either direction). Moreover, we tried to address other factors associated with drug use and withdrawal such as smoking status and depressive symptoms (i.e., BDI scores) as these factors may have also influenced our results.
It is well established that the PFC contributes to the performance of the WCST (Goldman-Rakic, 1987). Of particular interest to the current study is the precise PFC circuitry that mediates EDS and task-set switching. While the lateral PFC (Dias, Robbins, & Roberts, 1996; Dias et al., 1997) [including ventrolateral (Hampshire & Owen, 2006) and dorsolateral PFC (Petrides, 2000; Monchi, Petrides, Petre, Worsley, & Dagher, 2001)] have been reliably associated with EDS, the inferior frontal gyrus and its connections to basal ganglia mechanisms have been correlated with task-set switching (Aron et al., 2004; Duncan & Owen, 2000; Konishi et al., 1999). Alternatively, reversal shifting is mediated by the orbitofrontal cortex, striatal and caudate regions (Robbins, 2007; Stalnaker, Takahashi, Roesch, & Schoenbaum, 2009). The observed impairment in CUD may reflect dysfunction of one or all of these PFC regions and their associated circuitry. Since many of these PFC regions have been shown to be dysregulated in human CUD, future neuroimaging investigations should examine what monoaminergic pathways (e.g., dopaminergically innervated PFC pathways in combination with other monoamines) underlie the observed deficit.
Future investigations should also explore the extent that this deficit explains the compulsive behavior and relapsing nature associated with drug addiction. For example, it is possible that CUD may fail to use previously learned and possibly less salient strategies (e.g., a cognitive strategy to resist cues) and therefore persist in drug use. The current findings in higher functioning CUD has significant relevance given studies indicating that the higher the cognitive function in drug addicted individuals the better adherence to treatment (Aharonovich et al., 2006; Aharonovich, Nunes, & Hasin, 2003).
In summary, the current study compared the pattern of perseverative behavior in CON and CUD, a dopamine deficient population. In otherwise normally functioning CUD, perseverative deficits were observed but were isolated to task-set switch trials rather than EDS. By distinguishing these two forms of switching on the WCST, the current study reveals a neurocognitive context (i.e. initial stage of task-set switching) implicit in the WCST, which possibly relies upon intact dopaminergic function and its DLPFC innervation, and where impairments are associated with worse recall and withdrawal from cocaine. Future studies should investigate whether dopaminergically innervated pathways alone, or in combination with other monoamines, underlie this implicit neurocognitive process in the WCST.
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (to RZG: 1R01DA023579; R21DA02062) and General Clinical Research Center (5-MO1-RR-10710).
Notice: This manuscript has been authored by Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CHI-886 with the U.S. Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes.
Footnotes
Scores for WCST perseverative responses, perseverative errors, non perseverative errors, and failure to maintain set were non-normally distributed for the entire sample (skewness > 1) and therefore were transformed using a log transformation.
Transformed values for BDI scores were computed by adding a numerical constant of 2 to each score, and subsequently, taking a log of that resultant value. Note the F-value for BDI (reported in Table 1) was computed using transformed BDI scores but means and standard deviations are raw scores.
In this analyses N= 135, CUD-C = 49 and CON-C = 86 [4 subjects from each group who had extreme scores (> 3 standard deviations from the mean) on perseverative error trials 1 through 4 were removed from this analysis)].
Disclosure/Conflicts of Interest
The authors declare that, except for income received from their primary employer, no finanancial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71(2):207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport DA, Wylie GR. Task switching, stimulus response bindings, and negative priming. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance. XVIII. Cambridge, MA: MIT Press; 2000. pp. 35–70. [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127(Pt 7):1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21(6):1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320(5881):1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Bishara AJ, Jacoby LL. Aging, spaced retrieval, and inflexible memory performance. Psychon Bull Rev. 2008;15(1):52–57. doi: 10.3758/pbr.15.1.52. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16(4):456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Funderburk FR, Cadet JL. Differential effects of cocaine and cocaine alcohol on neurocognitive performance. Neurology. 2000;54(12):2285–2292. doi: 10.1212/wnl.54.12.2285. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304(5672):878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17(1):18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and strategy in a novel spatial self-ordered sequencing task for non human primates: effects of excititoxic lesions and dopamine depletions of the prefrontal cortex. Journal of Cognitive Neuroscience. 1998;10:332–354. doi: 10.1162/089892998562771. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11(12):1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41(11):1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11(11):1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J, Ober B. California Verbal Learning Test-II. San Antonio, Tx: The Psychological Corporation; 2000. [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17(23):9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SL, Bechara A, Nathan PE. Executive dysfunction as a risk marker for substance abuse: the role of impulsive personality traits. Behav Sci Law. 2008;26(6):799–822. doi: 10.1002/bsl.845. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Evers EA, Cools R, Clark L, van der Veen FM, Jolles J, Sahakian BJ, et al. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005;30(6):1138–1147. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9(4):195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58(3):601–622. [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. discussion 335–326. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory and the mind. Sci Am. 1992;267(3):110–117. doi: 10.1038/scientificamerican0992-110. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6(4):348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998a;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Oxford: Oxford University Press; 1998b. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42(11):1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullo MJ, Jackson CJ, Dawe S. Impulsivity and reversal learning in hazardous alcohol use. Personality and Individual Differences. 2010;48:123–127. [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatum dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16(12):1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Head D, Kennedy KM, Rodrigue KM, Raz N. Age differences in perseveration: cognitive and neuroanatomical mediators of performance on the Wisconsin Card Sorting Test. Neuropsychologia. 2009;47(4):1200–1203. doi: 10.1016/j.neuropsychologia.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test: computer version 3 for Windows research edition: Psychological Assessment Resource. 1999. [Google Scholar]
- Hester R, Barre N, Mattingley JB, Foxe JJ, Garavan H. Avoiding another mistake: error and posterror neural activity associated with adaptive posterror behavior change. Cogn Affect Behav Neurosci. 2007;7(4):317–326. doi: 10.3758/cabn.7.4.317. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26(2):183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27(2):189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Klockars AJ, Hancock GR, McAweeney MJ. Power of unweighted and weighted versions of simultaneous and sequential multiple-comparison procedures. Psychological Bulletin. 1995;118:300–307. [Google Scholar]
- Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP. Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine releaseduring a set shifting task – a TMS- [11C] raclopride PET study. Eur J of Sci. 2008;28(10):2147–2155. doi: 10.1111/j.1460-9568.2008.06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Kawazu M, Uchida I, Kikyo H, Asakura I, Miyashita Y. Contribution of working memory to transient activation in human inferior prefrontal cortex during performance of the Wisconsin Card Sorting Test. Cereb Cortex. 1999;9(7):745–753. doi: 10.1093/cercor/9.7.745. [DOI] [PubMed] [Google Scholar]
- Leber AB, Turk-Browne NB, Chun MM. Neural predictors of moment-to-moment fluctuations in cognitive flexibility. Proc Natl Acad Sci U S A. 2008;105(36):13592–13597. doi: 10.1073/pnas.0805423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Association of storage and processing functions in the dorsolateral prefrontal cortex of the nonhuman primate. J Neurosci. 1999;19(12):5149–5158. doi: 10.1523/JNEUROSCI.19-12-05149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhermite F. “Utilization behavior” and its relation to lesions of the frontal lobes. Brain. 1983:237–255. doi: 10.1093/brain/106.2.237. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Matsumoto K, Tanaka K. Prefrontal cell activities related to monkeys’ success and failure in adapting to rule changes in a Wisconsin Card Sorting Test analog. J Neurosci. 2006;26(10):2745–2756. doi: 10.1523/JNEUROSCI.5238-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle CJ, Black JE, Malison RT, Zimmermann RC, Kosten TR, Heninger GR, et al. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch Gen Psychiatry. 1994;51(9):713–719. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45(2):293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Archives of Neurology. 1963;9:90–100. [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21(19):7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S. Unsolved Mysteries of the Mind: Tutorial Essays in Cognition. Erlbaum; 1996. Control of mental processes; pp. 93–148. [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7(3):134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28(14):3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman D, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson R, Schwartz RG, Shapiro D, editors. Consciousness and self regulation: Advances in research and theory. New York: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Nyhus E, Barcelo F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. 2009;71(3):437–451. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Oades RD. The role of noradrenaline in tuning and dopamine in switching between signals in the CNS. Neurosci Biobehav Rev. 1985;9(2):261–282. doi: 10.1016/0149-7634(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Okano H, Hirano T, Balaban E. Learning and memory. Proc Natl Acad Sci U S A. 2000;97(23):12403–12404. doi: 10.1073/pnas.210381897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. J Pharmacol Exp Ther. 1995;274(3):1182–1191. [PubMed] [Google Scholar]
- Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133:44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- Pontecorvo MJ, Sahgal A, Steckler T. Further developments in the measurement of working memory in rodents. Brain Res Cogn Brain Res. 1996;3(3–4):205–213. doi: 10.1016/0926-6410(96)00007-9. [DOI] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276(5313):821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: Monoaminergic Modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Baker G, Vickers G. Lesions of central serotonin systems affect responding on a progressive ratio schedule reinforced either by intravenous cocaine or by food. Pharmacol Biochem Behav. 1994;49(1):177–182. doi: 10.1016/0091-3057(94)90473-1. [DOI] [PubMed] [Google Scholar]
- Rogers R, Monsell S. Costs of predictable switch between simple cognitgive tasks. Journal of Experimental Psychology:General. 1995;124:207–231. [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12(1):142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28(1):153–162. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- Rudoy CA, Van Bockstaele EJ. Cocaine effects on norepinephrine in the amygdala: Cocaine withdrawal-related anxiety and stress related relapse. Cellscience Reviews. 2005;2(2):193–212. [Google Scholar]
- Sakagami M, Niki H. Encoding of behavioral significance of visual stimuli by primate prefrontal neurons: relation to relevant task conditions. Exp Brain Res. 1994;97(3):423–436. doi: 10.1007/BF00241536. [DOI] [PubMed] [Google Scholar]
- Sakai K. Task Set and Prefrontal Cortex. Annual Review of Neuroscience. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Moore C, Waters C, Natsuaki Y, Galloway GP, et al. A dissociation in attentional control: evidence from methamphetamine dependence. Biol Psychiatry. 2005;57(3):310–313. doi: 10.1016/j.biopsych.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74(1):1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on genetic differences in D2 receptor density. J Neurosci. 2010;30(42):14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman S, Spreen O. A compendium of neuropsychological tests. 3. New York: Oxford; 2006. [Google Scholar]
- Tabachnick BG, Fidel LS. Using Multivariate Statistics. New York: Harper Row; 1983. [Google Scholar]
- Tellegen A, Waller NG. Exploring personality through test construction: development of the multidimensional personality questionnaire. In: Briggs SR, Cheek JM, editors. Personality measures: development and evaluation. Vol. 1. Greenwich: JAI Press; 1997. [Google Scholar]
- Thoma P, Wiebel B, Daum I. Response inhibition and cognitive flexibility in schizophrenia with and without comorbid substance use disorder. Schizophr Res. 2007;92(1–3):168–180. doi: 10.1016/j.schres.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12(3):405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Negative emotion-driven impulsivity predicts substance dependence problems. Drug Alcohol Depend. 2007;91(2–3):213–219. doi: 10.1016/j.drugalcdep.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology (Berl) 2007;190(4):517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39(3):1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Dias R, Robbins TW, Roberts AC. Dissociable contributions of the orbitofrontal and lateral prefrontal cortex of the marmoset to performance on a detour reaching task. Eur J Neurosci. 2001;13(9):1797–1808. doi: 10.1046/j.0953-816x.2001.01546.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- White IM, Wise SP. Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res. 1999;126(3):315–335. doi: 10.1007/s002210050740. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. The Wide-Range Achievement Test 3 - Administration Manual. Wilmington, DE: Wide Range Inc; 1993. [Google Scholar]
- Wilkosc M, Hauser J, Tomaszewska M, Dmitrzak-Weglarz M, Skibinska M, Szczepankiewicz A, et al. Influence of dopaminergic and serotoninergic genes on working memory in healthy subjects. Acta Neurobiol Exp (Wars) 2010;70(1):86–94. doi: 10.55782/ane-2010-1777. [DOI] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, et al. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychologia. 2009;34(5):1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Monsell S. Switching between tasks of unequal familiarity: role of stimulus-attribute and response-set selection. J Exp Psychol Hum Percept Perform. 2003;29(2):455–469. doi: 10.1037/0096-1523.29.2.455. [DOI] [PubMed] [Google Scholar]


