Abstract
A better understanding of the underlying mechanisms of angiogenesis and vascular permeability is necessary for the development of therapeutic strategies for ischemic injury. The purpose of this study was to examine the spatial and temporal expression of Src and Src-suppressed C Kinase Substrate (SSeCKS) in brain after middle cerebral artery occlusion (MCAO) and elucidate the relationships among Src, SSeCKS, and the key angiogenic factors present after stroke. Rats were subjected to either MCAO or sham operation. Reverse transcriptase-polymerase chain reaction and Western blotting results revealed that Src gradually increased starting as early as 2 h after MCAO and remained high for 1 day. In contrast, SSeCKS decreased after MCAO. Src expression correlated positively with that of vascular endothelial growth factor and angiopoietin-2, and negatively with that of SSeCKS, angiopoietin-1, and zonula occludens-1. However, SSeCKS had the reverse correlations. Changes in the expression of these factors correlated with the progress of angiogenesis and cerebral edema. Dynamic temporal changes in Src and SSeCKS expression may modulate angiogenesis and cerebral edema formation after focal cerebral ischemia.
Keywords: Src, SSeCKS, angiogenesis, cerebral edema, ischemia
1. Introduction
Cerebral ischemia results in a series of injuries, including cell death (necrosis and apoptosis) and cerebral edema, as well as other cellular reactions such as angiogenesis (Dirnagl et al., 1999) and the reestablishment of functional microvasculature to promote stroke recovery. Therefore, understanding angiogenesis mechanisms and devising ways to modulate them could be crucial to developing therapeutic interventions for the treatment of stroke injury.
One protein known to be involved in angiogenesis is the non-receptor tyrosine kinase Src (Theus et al., 2006; Tang et al., 2007). It is a representative member of the Src kinase family, which is also involved in gene transcription, adhesion regulation (Brown and Cooper, 1996), and cell proliferation. Although it has been reported that Src kinase activity increases dramatically after transient global brain ischemia (Schlessinger, 2000) and that it is associated with vascular endothelial growth factor (VEGF)-mediated vascular permeability (Paul et al., 2001), the expression pattern and function of Src kinase in focal cerebral ischemia has yet to be elucidated. Src-suppressed C kinase substrate (SSeCKS) is a novel protein kinase C substrate that is down-regulated by Src and Ras (Lin et al., 1995). SSeCKS possesses potential tumor suppressor activity and regulates cellular mitogenesis and cytoskeletal architecture. SSeCKS is expressed ubiquitously in a variety of tissues, including brain (Gelman et al., 2000; Gelman, 2002), but its levels are low in the developing rat brain. In vitro data have suggested that SSeCKS regulates angiogenesis and tight junction formation in the blood–brain barrier (BBB) (Lee et al., 2003).
Ischemia-induced angiogenesis is a tightly controlled multistep process. VEGF and angiopoietins are stimulated by ischemia and are crucial for angiogenesis and protection against ischemic injury (Carmeliet, 2003). The up-regulation of VEGF not only promotes angiogenesis, but also increases microvascular permeability (Dvorak et al., 1995). Recently, modifications of angiopoietin (Ang)-1 and Ang-2 expression have been reported in focal cerebral ischemia (Beck et al., 2000; Lin et al., 2001; Zhang et al., 2002; Zhang and Chopp, 2002). Ang-2 is necessary for destabilizing the preexisting vessel and facilitating vascular sprout. In contrast, Ang-1 is a natural antagonist of Ang-2 (Maisonpierre et al., 1997) that promotes stabilization and maturation of neovessels (Papapetropoulos et al., 1999). Unlike VEGF, Ang-1 acts reciprocally as an anti-permeability factor, preventing vessel leakage (Thurston et al., 1999). Although these angiogenic factors are known to be expressed after brain injury, the underlying mechanisms that regulate angiogenesis and vascular leakage remain uncharacterized. Furthermore, the time course of expression of Src, SSeCKS, and other key angiogenic factors has not been established. To further our understanding of their function in ischemic stroke, we characterized their temporal profile at mRNA and protein levels from 2 h to 14 days after middle cerebral artery occlusion (MCAO), compared their expression patterns with the time course of angiogenesis and brain edema formation, and examined the probable correlations among them. Elucidating the function of Src and SSeCKS as regulators in angiogenesis and vascular permeability may provide a novel therapeutic target for treatment of ischemic stroke.
2. Materials and Methods
2.1. Animals and MCAO model
Male Sprague-Dawley rats (250–300 g) were purchased from the Center for Experimental Animals, Harbin Medical University, China and were allowed free access to food and water. All experimental protocols and procedures conformed to the guidelines of the Chinese Council for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Harbin Medical University, China. Adequate measures were taken to ensure minimal pain or discomfort to rats.
All rats were randomized into two groups: the sham-operated control group and the ischemic stroke model group. For the stroke model, rats were anesthetized with 10% chloral hydrate (400 mg/kg, i.p.) and then underwent permanent MCAO with the filament model as previously described (Longa et al., 1989). Their body temperature was continuously monitored during and after occlusion and maintained at 37.0 ± 0.5°C with a heating pad. Rats in the MCAO group were subdivided into seven subgroups based on duration of ischemia: 2 h, 6 h, 12 h, 1 day, 3 days, 7 days, or 14 days. At each of these time points after MCAO, one subgroup of rats was deeply anesthetized and perfused transcardially with saline followed by 4% paraformaldehyde; brains were quickly removed after decapitation. Sham-operated rats underwent anesthesia and surgery without the filament insertion.
2.2. Reverse transcriptase-polymerase chain reaction
Total RNA from ipsilateral ischemic cerebral cortex (n=6/group) was extracted with TRIzol Reagent (Invitrogen, Carlsbad, CA). The Transcriptor First Stand cDNA Synthesis Kit (Roche, USA) was used to synthesize cDNA from 1 μg of total RNA according to the manufacturer’s instructions. Then 2 μl of the reverse transcription reaction was amplified by polymerase chain reaction (PCR) in a volume of 20 μl with gene-specific primers for Src forward: 5′-AGA GTG CCC TAT CCT GGG AT -3′-, reverse: 5′-AAA GTA GTC TTC CAG GAA GGCC-3′ SSeCKS forward: 5′-AAG TGC TGG CTT CGG AGA AAG-3′ reverse: 5′-TGA CTT CAG GAA CTT CAA GGC TC-3′; VEGF forward: 5′-CTG CTC TCT TGG GTG CACT-3′ reverse: 5′-ATA CAC TAT CTC ATC GGG GTA CT-3′ Ang-1 forward: 5′-GAA AAT TAT ACT CAG TGG CTG GAA AAA-3′ reverse: 5′-TTC TAG GAT TTT ATG CTC TAA TAA ACT-3′ Ang-2 forward: 5′-AAA GAG TAC AAA GAG GGC TTC GGG AGC-3′ reverse: 5′-GTA GTA CCA CTT GAT ACC GTT GAA CTT-3′ zonula occludens-1 (ZO-1) forward: 5′-CAG GAA AAT GAC CGA GTC GC-3′, reverse: 5′-CCA ATG TGA CCT TGG TGG GT-3′ β-actin forward: 5′-CCT CTG AAC CCT AAG GCC AAC-3′, reverse: 5′-TGC CAC AGG ATT CCA TAC CC-3′ PCR was carried out in a thermal cycler (Bio-Rad, USA) with an initial denaturation step of 5 min at 94°C, 40 cycles of amplification (94°C for 30 s, 58°C for 30 s, and 72°C for 40 s), and an extension step of 72°C for 10 min. PCR products were analyzed on 2% agarose gels. Band intensities were evaluated by the Chemidoc XRS imaging densitometer system (Bio-Rad, Hercules, CA) and quantified with Quantity One software.
2.3. Western blot analysis
Protein was extracted from ipsilateral ischemic cerebral cortex (n=6/group) with lysis buffer. Equal amounts of total protein extract were separated by sodium dodecyl sulfate (10%) polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes by electroblotting. After being blocked for 2 h in 5% non-fat dried milk, the membrane was incubated overnight at 4°C with rabbit polyclonal anti-Src (phospho-Y418, 1:600; Abcam, Cambridge, MA), rabbit polyclonal anti-Ang-2 (1:800; Abcam), rabbit polyclonal anti-ZO-1 (1:600; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-VEGF (1:1000; Abcam), goat polyclonal anti-Ang-1 (1:800; Santa Cruz Biotechnology), or sheep polyclonal anti-SSeCKS (1:600; Abcam). The membrane was then washed and incubated with secondary antibodies for 1 h. Glyceraldehyde 3-phosphate dehydrogenase expression was determined as an internal control. Protein bands were visualized by using the Western blot detection system (Amersham Biosciences, Piscataway, NJ), and band intensity was quantified by the NIH ImageJ analysis program. Three independent experiments were duplicated.
2.4. Immunohistochemistry
Rats (n=7/group) were perfused transcardially with saline followed by 4% paraformaldehyde, and their brains were removed and post-fixed in 4% paraformaldehyde overnight before being embedded in paraffin. A series of 4-μm-thick sections was cut from the block and used for immunohistochemical staining. Sections were incubated overnight at 4°C with rabbit polyclonal anti-Src (phospho-Y418, 1:100; Abcam), rabbit polyclonal anti-Ang-2 (1:200; Abcam), rabbit polyclonal anti-ZO-1 (1:250; Santa Cruz Biotechnology), mouse monoclonal anti-VEGF (1:200; Abcam), mouse monoclonal anti-GFAP (1:250; Abcam), mouse monoclonal anti-CD31 (1:300; Abcam), goat polyclonal anti-Ang-1 (1:200; Santa Cruz Biotechnology), or sheep polyclonal anti-SSeCKS (1:800; Abcam) and then in the appropriate secondary antibody for 1 h. Staining was visualized with diaminobenzidine (Maixin Bio, China). Sections used as negative controls were processed in parallel but were not exposed to primary antibody.
2.5. Measurement of brain water content
The water content was calculated as the weight difference between wet and dry samples. Animals (n=7/group) were deeply anesthetized and decapitated at different time points after MCAO. The brains were removed and immediately weighed to obtain the wet weight. Then they were dried at 100°C for 48 h and reweighed to obtain the dry weight. The percentage of water in the forebrain was calculated as follows: (wet weight – dry weight)/wet weight × 100%.
2.6. Statistical Analysis
Statistical significance was analyzed by generalized linear model repeated measures ANOVA, Student-Newman-Keuls test, and Pearson Correlation analysis with SPSS 13.0 software. All data are presented as the means ± S.D.; P < 0.05 was considered statistically significant.
3. Results
3.1. Temporal expression of Src and SSeCKS in rat brain after MCAO
To determine whether the cellular mechanisms of angiogenesis and vascular permeability are related to a modulation of Src and SSeCKS, we analyzed the expression time course of Src and SSeCKS. The results revealed that Src mRNA was increased as early as 2 h and peaked at 6 h after MCAO in comparison with controls (P < 0.01). Then it gradually declined and returned to the basal level (Fig. 1A). The Src protein also increased significantly, reaching a maximum at 12 h, and persisting at high levels until 1 day after MCAO (P < 0.05; Fig. 1B). Immunohistochemistry showed Src to be nearly absent in normal adult rat brain; however, its expression became apparent in astrocyte-like and endothelial-like cells at 6 h and 1 d after MCAO, especially in the perifocal region (Fig. 1C).
Fig. 1.
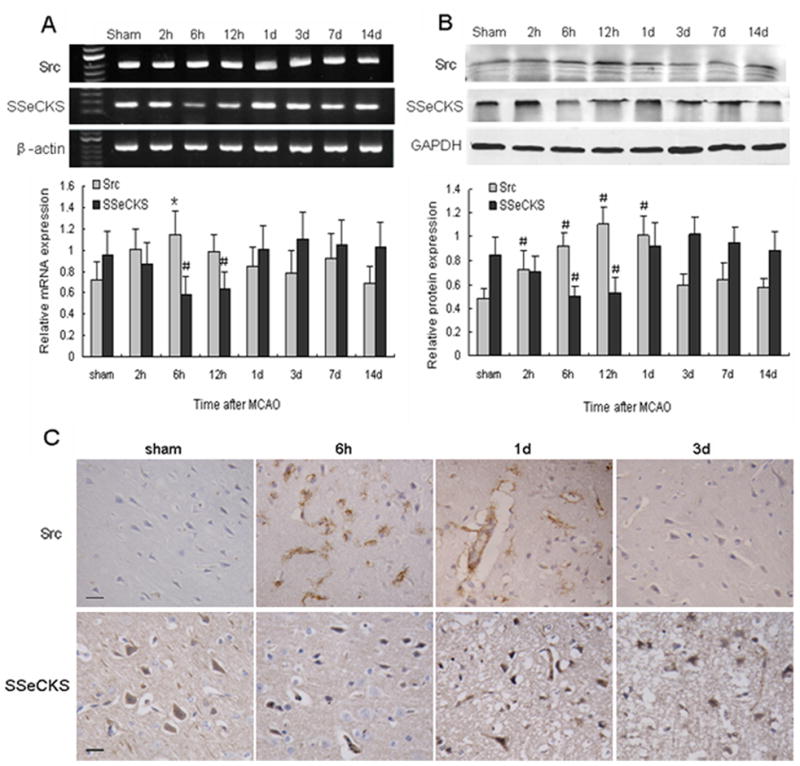
Changes in Src and SSeCKS expression at the mRNA and protein levels after MCAO. (A) (Top) RT-PCR of Src and SSeCKS mRNA at the indicated time points after MCAO; (Bottom) bar graph showing mRNA expression relative to that of β-actin. (B) (Top) Western blot of Src and SSeCKS at the indicated time points after MCAO; (Bottom) bar graph showing protein expression relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are representative of at least three independent experiments and presented as means ± S.D.; n = 6 per group; #P < 0.05, *P < 0.01 compared with the sham group. (C) Immunohistochemical staining for Src and SSeCKS at the indicated time points after MCAO. Scale bar = 20 μm.
The temporal expression of SSeCKS mRNA was reverse that of Src. Indeed, SSeCKS mRNA decreased to a minimum level at 6 h (P < 0.05) after ischemia and then gradually increased (Fig. 1A). SSeCKS protein underwent a similar course (Fig. 1B). The immunostaining results showed SSeCKS to be widespread in adult rat brain. However, the expression of SSeCKS was weakened at 6 h after ischemia. After 1 day, the number of SSeCKS-positive cells gradually increased in the ischemic boundary zone. SSeCKS was primarily expressed in astrocyte-like, neuron-like, and endothelial-like cells and was diffusely distributed throughout most of the cytoplasm of the labeled cell bodies (Fig. 1C).
3.2. The spatial distribution of VEGF and angiopoietins after MCAO
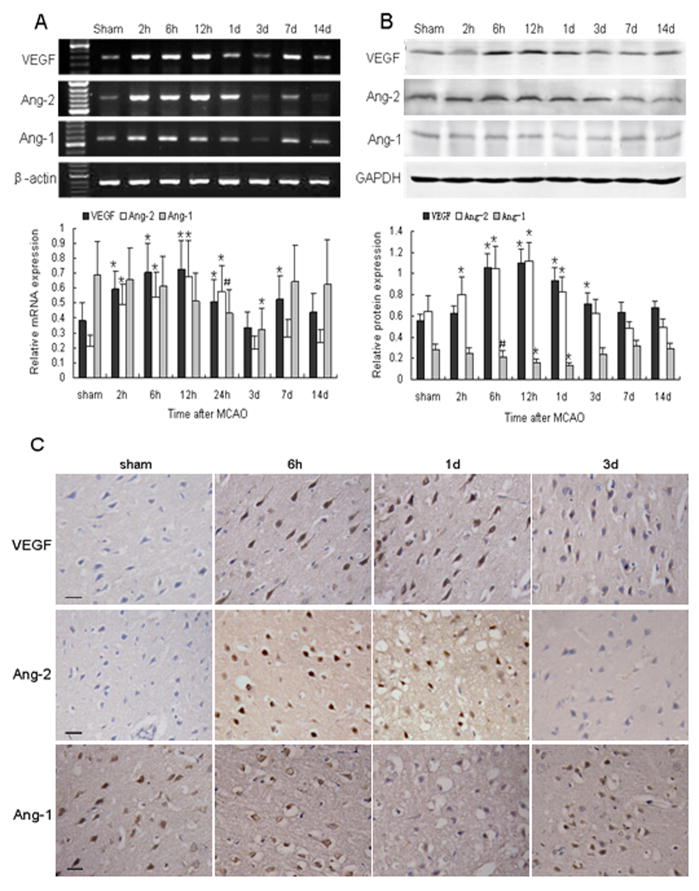
To examine the contribution of VEGF and angiopoietins after cerebral ischemia, we investigated the expression of these factors after MCAO. The results show that the change in VEGF mRNA level was biphasic. It was markedly increased at 2 h, had an initial peak at 12 h, decreased, and then had a second peak at 7 days (P < 0.01; Fig. 2A). However, Western blot results indicated that VEGF protein underwent a marked increase at 6 h, reached a peak level at 12 h, and remained high until 1 day after MCAO, when it began to decrease gradually (P < 0.01; Fig. 2B). The VEGF-immunoreactive cells were primarily located in the ischemic perifocal region. Strong VEGF-positive staining was visible in the whole cell body of neuron-like and glial-like cells (Fig. 2C).
Fig. 2.
Changes in expression of VEGF, Ang-1, and Ang-2 after MCAO. (A) (Top) RT-PCR of VEGF, Ang-1, and Ang-2 mRNA at the indicated time points after MCAO; (Bottom) bar graph showing mRNA expression relative to that of β-actin. (B) (Top) Western blot of VEGF, Ang-1, and Ang-2 at the indicated time points after MCAO; (Bottom) bar graph showing protein expression relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are presented as means ± S.D.; n = 6/group; #P < 0.05, *P < 0.01. (C) Immunohistochemical staining revealed VEGF and Ang-2 immunoreactivity in neuron-like and glial-like cells in the ischemic boundary area after MCAO. Ang-1 immunostaining was especially weak at 1 day after ischemia. Scale bar = 20 μm.
Ang-2 mRNA and protein significantly increased as early as 2 h after MCAO, peaked at 12 h, and persisted at high levels until 1 day (P < 0.01). The levels then decreased rapidly and remained at baseline from 3 days until the end of the experimental period (Fig. 2). Ang-2 was mainly expressed in neuron-like and glial-like cells in the ipsilateral hemisphere (Fig. 2C). Ang-1 mRNA was significantly decreased from baseline at 24 h after MCAO (P < 0.05) and remained depressed for up to 3 days (P < 0.01). It had returned to control levels by 7 days post-MCAO (Fig. 2A). Ang-1 protein also decreased after MCAO, reaching a minimum at 1 day (P < 0.01; Fig. 2B). Ang-1 immunoreactivity was found primarily in neuron-like, glial-like, and endothelial-like cells. Some immunostaining was observed around the nucleus of certain individual neuron-like cells (Fig. 2C).
3.3. Induction of angiogenesis in response to focal cerebral ischemia
To detect the angiogenesis that occurs in response to ischemia in rat brain, we used immunohistochemistry to label microvessels with antibody against CD31 at different time points after MCAO. The capillaries in the perifocal area were counted at high magnification. As shown in Figure 3, cerebral ischemia did not stimulate an increase in capillary density during the early ischemic period. Neovessels began to be observed in the ischemic boundary area at 12 h and around 1 day (P < 0.01; Fig. 3). However, some were tortuous and enlarged. The capillary number remained significantly increased for up to 14 days (P < 0.01) after MCAO and may contribute to the recovery from ischemic injury.
Fig. 3.
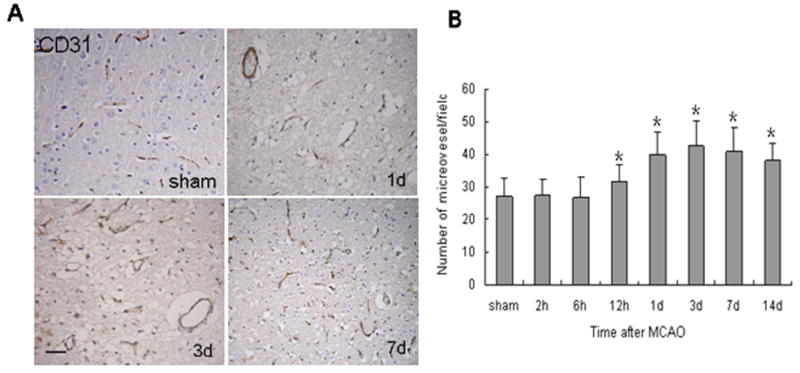
Neovascularization in rat brain after MCAO. (A) Brain sections were immunostained for CD31 to identify capillaries. Scale bar = 50 μm. (B) The bar graph shows microvessel counts at different time points after MCAO. Microvessels were counted from 10 fields of the ischemic penumbra area under a microscope at 400× magnification. Data are means ± S.D.; n = 7 per group; *P < 0.01 compared with the sham group.
3.4. Cerebral edema following focal cerebral ischemia
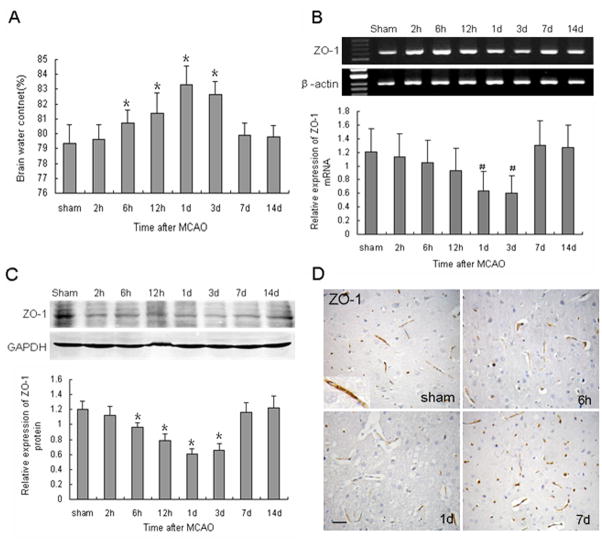
Changes in brain water content over time are shown in Figure 4A. Brain edema was detected beginning at 6 h, peaked at 1 day, and remained significantly higher than baseline until 3 days after MCAO (P < 0.01). It then sharply receded to pre-ischemia levels by 7 days.
Fig. 4.
Effect of MCAO on brain water content and ZO-1 expression. (A) Brain water content was calculated as the weight difference between wet and dry samples. Data are means ± S.D.; n = 7 per group; *P < 0.01 compared with sham group. (B) Northern blot of ZO-1 mRNA at the indicated time points after MCAO. β-actin was used as a loading control. (C) Western blot of ZO-1 protein after MCAO. GAPDH was used as a loading control. Data are presented as means ± S.D.; n = 6 per group, #P < 0.05, *P < 0.01 compared with the sham group. (D) ZO-1 immunoreactivity was observed around the vessels, especially at sites of endothelial cell–cell contact in control brain. ZO-1 was weakly expressed at 1 day after MCAO, and strongly expressed at 7 days. Scale bar = 50 μm.
3.5. Expression of ZO-1 after MCAO
To examine the structural basis for the formation of edema, we examined the distribution of ZO-1. As shown in Figure 4B, ZO-1 mRNA gradually decreased to a minimum 3 days after MCAO (P < 0.05). ZO-1 protein also decreased beginning at 2 h and reached a minimum at 1 day (P < 0.01). It persisted at a low level until 3 days, and then increased rapidly to a level higher than that of control (Fig. 4C). The results demonstrate that the decrease in ZO-1 expression was in accordance with the extent of cerebral edema. Immunostaining revealed that ZO-1 was especially expressed in endothelial-like cells. (Fig. 4D), indicating that ZO-1 is important for maintaining the integrity of the BBB.
3.6. Src and SSeCKS modulate ischemia-induced cerebral edema
Our results indicate that after ischemia, Src and SSeCKS may modulate VEGF and angiopoietins, the levels of which were closely correlated with brain edema formation. VEGF and Ang-2 were up-regulated during the formation of cerebral edema, whereas Ang-1 was down-regulated. Src levels correlated positively with those of VEGF and Ang-2, but negatively with those of SSeCKS and Ang-1 (Tables 1 and 2). The relationships between SSeCKS and those angiogenic factors were exactly opposite to those of Src. We further analyzed the relationships between Src, SSeCKS, angiogenic factors, and ZO-1. The results showed that Src, VEGF, and Ang-2 have a negative correlation with ZO-1, whereas SSeCKS and Ang-1 have a positive correlation (Tables 1 and 2). The decrease in Ang-1 paralleled that of ZO-1. Src and SSeCKS may play opposite roles in cerebral edema formation by modulating VEGF and angiopoietins.
Table 1.
The correlation of Src, SSeCKS, and angiogenic factors at the mRNA level after cerebral ischemia.
| Factor | Ang-2 | Src | SSeCKS | VEGF | ZO-1 |
|---|---|---|---|---|---|
| Ang-1 | −.267* | −.238* | .118* | −.226* | .248** |
| Ang-2 | .220* | −.271** | .344** | −.164* | |
| Src | −.245** | .486** | −.335* | ||
| SSeCKS | −.524** | .310* | |||
| VEGF | −.341* |
Ang-1, angiopoietin-1; Ang-2, angiopoietin-2; SSeCKS, Src-suppressed C kinase substrate; VEGF, vascular endothelial growth factor; ZO-1, zonula occludens-1. Pearson correlation was used to analyze the correlations among the proteins.
P < 0.05;
P < 0.01.
Table 2.
The correlation of Src, SSeCKS, and angiogenic factors at the protein level after cerebral ischemia.
| Factor | Ang-2 | Src | SSeCKS | VEGF | ZO-1 |
|---|---|---|---|---|---|
| Ang-1 | −.501** | −.500** | .365** | −.519** | .474** |
| Ang-2 | .585** | −.522** | .602** | −.354** | |
| μ | |||||
| Src | −.407** | .727** | −.464** | ||
| SSeCKS | −.468** | .261* | |||
| VEGF | −.535** |
Ang-1, angiopoietin-1; Ang-2, angiopoietin-2; SSeCKS, Src-suppressed C kinase substrate; VEGF, vascular endothelial growth factor; ZO-1, zonula occludens-1. Pearson correlation was used to analyze the correlations among the proteins.
P < 0.05;
P < 0.01.
4. Discussion
Angiogenesis induced by cerebral ischemia contributes to the recovery from ischemic brain injury and is correlated with longer survival in humans (Krupinski et al., 1994). However, vascular permeability complicated by angiogenesis is inevitable and can exacerbate the subsequent injury. Therefore, identifying the potential mechanisms that regulate angiogenesis and vascular permeability following stroke may provide new insights into the pathogenesis and future therapy.
Based on previous reports that an Src inhibitor efficiently reduced VEGF-induced vascular permeability and infarct volume (Paul et al., 2001) and protected animals from brain edema and injury (Akiyama et al., 2004; Lennmyr et al., 2004), we suggest that Src might modulate angiogenic factors after focal cerebral ischemia. Here we found that Src immunoreactivity was preferentially increased in the astrocyte-like cells surrounding the capillaries, suggesting that Src has a close association with capillaries in cerebral ischemia. Astrocytes, which produce Src, perform an indispensable function in the process of ischemic injury. Src increased during the formation of edema and decreased as the edema receded. Src protein increased significantly in comparison with its mRNA. The results indicate that the increased Src kinase activity was not due solely to the increased Src mRNA expression levels. These findings support the importance of Src kinase activity in ischemic stroke injury.
SSeCKS, an Src-suppressed C kinase substrate, was recently identified and reported to regulate the expression of both VEGF and Ang-1 in astrocytes (Lee et al., 2003). One study has indicated that activated Src and SSeCKS control similar sets of genes in both human and mouse fibroblasts and epithelial cells (Liu et al., 2006). In this study we observed that SSeCKS was widespread in normal rat brain and dominantly expressed in astrocyte-like, neuron-like, and endothelial-like cells. We further showed that the temporospatial expression of SSeCKS is reverse that of Src. Indeed, SSeCKS decreased after ischemia and then increased gradually, returning to control level at 1 day, the time at which neovessels were detected. The results indicate that the expression of SSeCKS was markedly decreased by ischemia. It has been reported that SSeCKS can repress angiogenesis and induce maturation and stabilization of permeable vessels by enhancing the expression of tight junction protein in vitro (Lee et al., 2003).
Angiogenesis is a step-wise process. Necessary steps include destabilization of vessels, sprouting and branching of destabilized vessels, proliferation and migration of endothelial cells, and stabilization of neomicrovessels (Conway et al., 2001; Folkman and D’Amore, 1996; Risau, 1997). Many angiogenic factors are involved in this progression, and VEGF, Ang-2, and Ang-1 may play essential roles (Jain, 2003). Our results show that all these angiogenic factors were highly expressed in neuron-like and glial-like cells in the ischemic penumbra. VEGF and Ang-2 increased significantly in the early period after cerebral ischemia during the formation of brain edema. Ang-2 may stimulate microvessel sprouting and angiogenesis in the presence of VEGF (Zhu et al., 2005; Lobov et al., 2002). These results indicate that Ang-2 acts synergistically with VEGF to promote brain angiogenesis and vascular permeability after cerebral ischemia. Ang-1 is a strong anti-permeability factor that can reduce vascular leakage. In this study, we observed that Ang-1 immunoreactivity decreased in the ischemia boundary zone, supporting the leakage-resistant role attributed to Ang-1 (Thurston et al., 1999). The Ang-1 protein was significantly decreased at 1 day, when cerebral edema was at its greatest. This dynamic expression may explain the progression of cerebral edema after MCAO and reflect the fact that Ang-1 acts at a later stage of vascular stabilization and maturation (Wakui et al., 2006).
Angiogenesis has been suggested to contribute to brain repair in stroke (Manoonkitiwongsa et al., 2001; Wei et al., 2001; Valable et al., 2005). Our study described the temporal evolution of vascular remodeling in relation to the expression of Src, SSeCKS, and primary angiogenic factors. Neovessels became clearly apparent in the ischemic boundary area at 1 day after MCAO. However, some of them were dilated and tortuous. With the maturation of neovessels during the later period of ischemia, the cerebral edema decreased significantly. These results suggest that the newly formed microvessels are functional and promote the recovery of ischemic injury.
Although the increase in vascular permeability that accompanies angiogenesis is inevitable after cerebral ischemia (Lee et al., 2004; Harrigan et al., 2002), a minor increase in vascular permeability may be necessary for angiogenesis. The increase in edema is likely due, at least in part, to a decrease in the tight junction protein ZO-1 (Dvorak et al., 1999; Wang et al., 2001; Fischer et al., 2002). It has been shown that absence of ZO-1 results in vascular leakage. In this study we found that ZO-1 was expressed around the vessels, especially at sites of endothelial cell–cell contact, suggesting that ZO-1 preserves the integrity of the BBB. We further observed that the expression of ZO-1 was in accordance with that of Ang-1. The decrease in ZO-1 coincided with increases in the extent of cerebral edema.
Our results also showed that the expression of Src and SSeCKS correlates with that of angiogenic factors and the tight junction protein ZO-1 after ischemia. Src correlated positively with VEGF and Ang-2 but negatively with SSeCKS, Ang-1, and ZO-1. The opposite was true of SSeCKS. Src, VEGF, and Ang-2 were increased, whereas SSeCKS, Ang-1, and ZO-1 were decreased during the formation of cerebral edema. These data demonstrate that Src and SSeCKS have opposite effects on angiogenesis and brain edema formation. We further infer that Src inhibits SSeCKS and regulates angiogenesis and vascular permeability by modulating angiogenic factors and tight junction proteins after cerebral ischemia. Src–SSeCKS may be a new signal pathway involved in ischemic injury.
The present study indicates that Src and SSeCKS may play opposite roles in angiogenesis and vascular permeability after focal cerebral ischemia. Angiogenic factors that serve as downstream mediators participate in the process. A detailed understanding of the modulation of angiogenesis and vascular leakage by the Src–SSeCKS pathway will be helpful in the development of therapeutics for focal cerebral ischemia.
Acknowledgments
This work was supported by NSFC (30973106), the Ph.D. Program Foundation of the Ministry of Education of China (200802260011), the Science Innovation Foundation of Harbin Medical University (HCXB2010013), the Science Research Foundation of the First Affiliated Hospital of Harbin Medical University, AHA (09BGIA2080137), and NIH (K01AG031926). We thank Claire Levine, Mali Wiederkehr, and Jialan Shi for assistance with this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama C, Yuguchi T, Nishio M, Tomishima T, Fujinaka T, Taniguchi M, Nakajima Y, Kohmura E, Yoshimine T. Src family kinase inhibitor PP1 reduces secondary damage after spinal cord compression in rats. J Neurotrauma. 2004;21:923–931. doi: 10.1089/0897715041526230. [DOI] [PubMed] [Google Scholar]
- Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol. 2000;157:1473–1483. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- Gelman IH. The role of SSeCKS/gravin/AKAP12 scaffolding proteins in the spaciotemporal control of signaling pathways in oncogenesis and development. Front Biosci. 2002;7:1782–1797. doi: 10.2741/A879. [DOI] [PubMed] [Google Scholar]
- Gelman IH, Tombler E, Vargas J., Jr A role for SSeCKS, a major protein kinase C substrate with tumour suppressor activity, in cytoskeletal architecture, formation of migratory processes, and cell migration during embryogenesis. Histochem J. 2000;32:13–26. doi: 10.1023/a:1003950027529. [DOI] [PubMed] [Google Scholar]
- Harrigan MR, Ennis SR, Masada T, Keep RF. Intraventricular infusion of vascular endothelial growth factor promotes cerebral angiogenesis with minimal brain edema. Neurosurgery. 2002;50:589–598. doi: 10.1097/00006123-200203000-00030. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang GY, Young WL. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke. 2004;35:1715–1719. doi: 10.1161/01.STR.0000129334.05181.b6. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- Lennmyr F, Ericsson A, Gerwins P, Akterin S, Ahlstrom H, Terent A. Src family kinase-inhibitor PP2 reduces focal ischemic brain injury. Acta Neurol Scand. 2004;110:175–179. doi: 10.1111/j.1600-0404.2004.00306.x. [DOI] [PubMed] [Google Scholar]
- Lin TN, Nian GM, Chen SF, Cheung WM, Chang C, Lin WC, Hsu CY. Induction of Tie-1 and Tie-2 receptor protein expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2001;21:690–701. doi: 10.1097/00004647-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Lin X, Nelson PJ, Frankfort B, Tombler E, Johnson R, Gelman IH. Isolation and characterization of a novel mitogenic regulatory gene, 322, which is transcriptionally suppressed in cells transformed by src and ras. Mol Cell Biol. 1995;15:2754–2762. doi: 10.1128/mcb.15.5.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gao L, Gelman IH. SSeCKS/Gravin/AKAP12 attenuates expression of proliferative and angiogenic genes during suppression of v-Src-induced oncogenesis. BMC Cancer. 2006;6:105. doi: 10.1186/1471-2407-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab. 2001;21:1223–1231. doi: 10.1097/00004647-200110000-00011. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79:213–223. [PubMed] [Google Scholar]
- Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7:222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293–296. doi: 10.1016/s0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- Tang X, Feng Y, Ye K. Src-family tyrosine kinase fyn phosphorylates phosphatidylinositol 3-kinase enhancer-activating Akt, preventing its apoptotic cleavage and promoting cell survival. Cell Death Differ. 2007;14:368–377. doi: 10.1038/sj.cdd.4402011. [DOI] [PubMed] [Google Scholar]
- Theus MH, Wei L, Francis K, Yu SP. Critical roles of Src family tyrosine kinases in excitatory neuronal differentiation of cultured embryonic stem cells. Exp Cell Res. 2006;312:3096–3107. doi: 10.1016/j.yexcr.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S, Petit E. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, Hano H, Endou H, Kanai Y. Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab Invest. 2006;86:1172–1184. doi: 10.1038/labinvest.3700476. [DOI] [PubMed] [Google Scholar]
- Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol. 2001;280:H434–440. doi: 10.1152/ajpheart.2001.280.1.H434. [DOI] [PubMed] [Google Scholar]
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12:62–66. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Lee C, Shen F, Du R, Young WL, Yang GY. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke. 2005;36:1533–1537. doi: 10.1161/01.STR.0000170712.46106.2e. [DOI] [PubMed] [Google Scholar]