Abstract
Background
Irritable bowel syndrome (IBS) is a chronic and debilitating medical condition with few efficacious pharmacological or psychosocial treatment options available. Evidence suggests that visceral anxiety may be implicated in IBS onset and severity. Thus, cognitive behavioral treatment (CBT) that targets visceral anxiety may alleviate IBS symptoms.
Methods
The current study examined the efficacy of a CBT protocol for the treatment of IBS which directly targeted visceral sensations. Participants (N = 110) were randomized to receive 10 sessions of either: (a) CBT with interoceptive exposure to visceral sensations (IE); (b) stress management (SM); or (c) an attention control (AC), and were assessed at baseline, mid-treatment, post-treatment, and follow-up sessions.
Results
Consistent with hypotheses, the IE group outperformed AC on several indices of outcome, and outperformed SM in some domains. No differences were observed between SM and AC. The results suggest that IE may be a particularly efficacious treatment for IBS.
Conclusions
Implications for research and clinical practice are discussed.
Irritable bowel syndrome (IBS) is a chronic gastrointestinal disorder with no identifiable physiological cause that affects 10-15% of the population (Brandt, et al 2002, Saito, et al 2002). IBS is characterized by abdominal pain or discomfort associated with altered bowel habits (i.e., constipation and/or diarrhea) and is often accompanied by sensations of distention, urgency, or incomplete evacuation (Longstreth, 2005). Also, IBS is associated with significant disability and high cost from both health care utilization and loss of productivity (Longstreth, et al 2003, Pare et al 2006). IBS patients show an increased risk for a variety of psychiatric conditions, primarily anxiety disorders, depression, and somatization disorder (Whitehead, 2002). IBS may be most strongly associated with panic disorder and generalized anxiety disorder (Lydiard, 1992).
Patients with IBS show hypervigilance and hypersensitivity to visceral sensations and increased autonomic arousal to visceral events (Naliboff et al., 1997; Verne et al., 2001; Tillisch et al., 2005). While peripheral GI factors may play a role in subsets of patients with IBS (e.g., post-infectious IBS), converging clinical and neurobiological data suggest that enhanced central stress responsiveness involving anxiety may provide a specific mechanism for enhanced visceral sensitivity (Mayer et al., 2001). GI symptom-specific anxiety may be an especially important variable leading to increased pain sensitivity, hypervigilance, and poor coping responses (Labus et al 2004; Hazlett-Stevens et al., 2003). Consequently, visceral anxiety is seen as a primary affective disturbance in IBS and as the mediating variable between other risk factors (e.g., neuroticism, trait anxiety, worry) and IBS symptom severity (Labus et al., 2005).
Existing psychological treatments for IBS include psychodynamic psychotherapy, hypnotherapy, and cognitive-behavioral stress management therapy. Most of these approaches share the assumption that stress or anxiety is a critical feature that needs to be addressed during treatment, and suggest that stress acts to increase IBS symptoms or that increased stress reactivity (or neuroticism) results in IBS symptoms. For example, multicomponent cognitive-behavioral treatments (Blanchard, Schwarz, Suls, Gerardi, Scharff, Greene, Taylor, Berreman, & Malamood, 1992; Greene & Blanchard, 1994; Payne & Blanchard, 1995; Vollmer & Blanchard, 1998, Drossman et al, 2003, Lackner et al, 2008) aim to increase awareness of the association among stressors, thoughts, and IBS symptoms, identify and modify cognitive appraisals of situations and behaviors, and change depressive and/or anxiety-based schema (Vollmer & Blanchard, 1998). Several reviews and a meta-analysis generally support the efficacy of these interventions for decreasing IBS symptom severity and associated anxiety and depression when compared to no treatment or standard medical care (Lackner et al., 2004; Blanchard & Scharff, 2002; Lackner et al., 2008). However, findings regarding differences from attention control conditions, such as patient education, are mixed (Drossman et al., 2003; Creed et al., 2003). As mentioned, these treatments focus on general stress management, and do not directly address the specific hypervigilance and hypersensitivity to visceral sensations observed in IBS. Outcomes may be improved with such a direct focus of treatment. In support, hypnotherapy for IBS, which has shown successful symptomatic outcomes, focuses on specific suggestions for calming the digestive system and normalizing GI function (Palsson, et al, 2002)
The hypersensitivity and hypervigilance to gut sensations observed in IBS is analogous to the sensitivity to bodily sensations observed in panic disorder, in which anxiety becomes acutely focused on somatic sensations associated with panic attacks. For example, individuals with panic disorder become anxious during procedures that elicit sensations similar to ones experienced during panic attacks, such as hyperventilation (Antony, Ledley, Liss, & Swinson, 2006; Gorman et al., 1994; Perna et al., 1995), fear signals that ostensibly reflect heightened arousal in false physiological feedback paradigms (Craske, Lang, Rowe et al., 2002; Ehlers, Margraf, Roth et al., 1988), and preferentially attend to heartbeat stimuli and panic-related verbal stimuli (Kroeze & van den Hout, 2000; Pauli et al., 2005; Teachman, Smith-Janik, & Saporito, 2007). This sensitivity to bodily sensations has been attributed to conditional fear of internal cues (such as elevated heart rate) due to their association with intense fear/distress (Razran, 1961; Bouton et al., 2001) and catastrophic appraisals of bodily sensations as causing physical or mental harm (Clark, 1986; Clark et al., 1988). Consequently, low-level bodily sensations produce anxiety (via conditioning and/or catastrophic appraisals) and the resultant anxiety-induced autonomic arousal intensifies the sensations that are feared, thus creating a reciprocating cycle of fear and sensations that builds into a panic attack (Barlow, 1988, 2002). In turn, anxiety increases the likelihood of panic attacks, by directly increasing the availability of, and/or attentional vigilance to, sensations that have become cues for panic. Finally, avoidance behaviors are believed to maintain catastrophic beliefs about bodily sensations and interrupt natural extinction of conditional fear of bodily sensations.
The corresponding treatment involves cognitive skills for misappraisals of bodily sensations and repeated exposure to feared bodily sensations and situations where panic attacks are expected to occur, in order to extinguish conditional fear responding and provide further evidence to dispute catastrophic misappraisals (e.g., Barlow & Craske, 1994). This treatment has proven to be highly effective for panic disorder (e.g., Barlow et al., 1989; Craske et al., 1997). This model and treatment of panic disorder can be easily translated to a model of treatment for IBS. That is, the fear of gut sensations may contribute to pain intensity and acute IBS episodes. The anxiety about future IBS symptoms and associated vigilance to visceral sensations are likely to provide low level somatic cues that elicit a conditional distress/pain response due to prior experiences; and avoidance of visceral cues is likely to maintain anxiety about them.
The primary aim of the current study was to evaluate the efficacy of a treatment for IBS that directly targets hypervigilance and hypersensitivity to visceral sensations, modeled on the methods used for the treatment of panic disorder. The CBT approach for panic disorder was modified to target IBS by addressing threat-laden appraisals of visceral sensations and anxiety about IBS sensations through cognitive (e.g., cognitive restructuring) and behavioral (i.e., interoceptive and in vivo exposure) exercises. It was hypothesized that those receiving CBT focused on interoceptive cues (IE) would show significantly greater reductions in pain, pain vigilance, and bowel symptoms, and greater improvement in quality of life compared to CBT focused on stress management (SM) and an attention control (AC), with SM outperforming AC.
Methods
Design
Eligible participants completed a baseline screening/pre-treatment assessment and were then randomized to 10 weekly sessions of AC, SM, or IE. Participants completed a mid-treatment, post-treatment, and a follow-up assessment three months after the end of treatment. Mid, post and follow-up assessments were completed by independent, blinded assessors. Therapists were blind to these assessments.
Participants
Participants were recruited from a digestive disease clinic at a large university in California, and from community advertisements. To be eligible, participants had to be diagnosed with IBS based on the Rome II diagnostic criteria. The Rome criteria are a consensus based symptom criteria system for functional gastrointestinal disorders analogous to the DSM for psychiatric disorders. IBS is diagnosed based on at least 12 weeks (which need not be consecutive) of abdominal pain or discomfort in the preceding 12 months with at least two of the following three features: (1) Relieved with defecation; (2) Onset associated with a change in frequency of stool; and (3) Onset associated with a change in form (appearance) of stool (Thompson, 1994). Participants were excluded if they (a) had another significant chronic pain condition; (b) had a major mental illness such as schizophrenia, biopolar disorder or substance abuse (anxiety disorders and depression without suicidal ideation were not exclusions); or (c) were taking narcotic pain medication. Participants who took IBS symptomatic medications (e.g. anti-diarrheal medications) or antidepressants were asked to maintain a stable dose throughout the study.
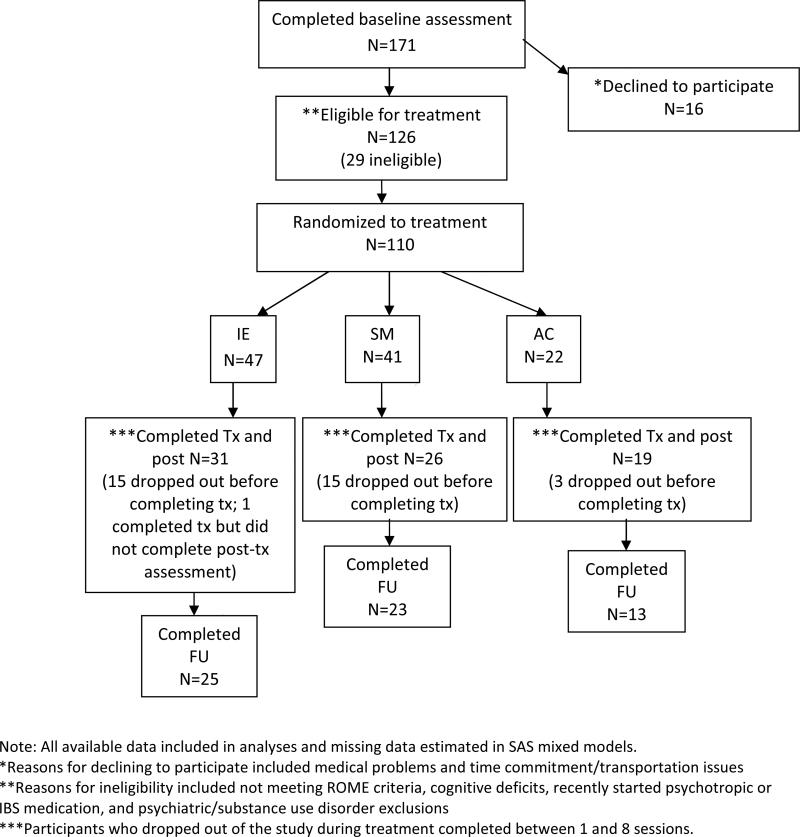
171 participants completed initial screening. Sixty were deemed ineligible and one dropped out before randomization, leaving 110 participants who were randomized. A random number generator was used to create separate random lists for males and females and opaque envelopes with group assignment for each consecutive entering participant were prepared in advance by personnel not directly involved in the study. Randomization was set a priori to result in twice as many participants in IE and SM compared to AC, based on the assumption that the effect sizes between the active interventions would be smaller than either group compared to the control condition. Although previous studies using active control conditions have shown mixed results with regard to specific IBS symptoms, the combined literature with CBT suggests there should be larger differences between the active and control groups than between two CBT treatments. A pair-wise proportional power analysis with an assumption of 30% response in the control group and 70% in the active treatment yielded a minimal enrollment of 20 participants in the control condition and 40 in the active treatment (p = .05, power =.77). After randomization, 11 participants (10%) declined to participate, withdrawing before beginning treatment. An additional 23 participants were treatment dropouts (23% of 99 who began treatment), defined as not completing at least 8 of 10 sessions. Thus, 76 participants were treatment completers. All of the treatment completers and 10 of the dropout participants completed the post-treatment assessment. Follow-up data were collected for 61 of the treatment completers. All those participants who were randomized to treatment (i.e., dropouts and completers, N = 110) were included in the ITT analyses. Only those defined as completers (i.e., completed at least 8 sessions and a post-treatment assessment, N = 76) were included in completer analyses.
See Figure 1 for participant flow through the study. The total randomized sample (including dropouts) was primarily female (74.3%) and had at least a college degree (67.6%). The mean age was 39.47 (SD = 13.50). The sample was primarily Caucasian (72.3%), with 9% African-American, 9.7% Asian-American/Pacific Islander, 3.9% Native-American, 1.9% Hispanic, and 3.1% other race/ethnicity. With regard to bowel habit, 34.1% reported constipation, 36.0% reported diarrhea, and 29.9% reported mixed constipation/diarrhea based on proposed ROME II criteria. Of those randomized, 2.7% reported mild bowel symptom severity, 45% reported moderate bowel symptom severity, 44.1% reported severe bowel symptom severity, and 8.1% reported very severe bowel symptom severity at baseline. Also, 8.1% met the DSM-IV diagnostic criteria for panic disorder, as assessed by the Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV; Brown, diNardo, and Barlow, 1994). Additionally, 8.1% of randomized participants were prescribed benzodiazepines and 13.5% were prescribed antidepressant medication (i.e., SSRIs, SNRIs, or tricyclics).
1.
Flow of Participants through the study
Measures
Outcome Measures
Primary Outcome Measures
Bowel Symptom Composite Score
We previously validated the use of 21 point numerical ratings scales for individual IBS symptoms, including overall symptom severity (Spiegel et al 2008). Herein, a composite bowel symptom severity index (BSS) was created from standardized scores from individual symptom ratings of overall gastrointestinal symptoms, lower abdominal pain, lower abdominal bloating, and lower abdominal discomfort.1 Each scale was anchored from (0) no symptoms to (20) most intense symptoms imaginable, and referred to the past two weeks. The average of the four standardized scores comprised the BSS value. This measure was administered at pre, post, and follow-up assessment.
Visceral Sensitivity Index (VSI; Labus et al., 2004)
Anxiety related specifically to IBS symptoms and misappraisal of them was assessed using this 15-item scale. Participants rated how much they agreed with statements such as “No matter what I eat, I will probably feel uncomfortable” on a 6-point Likert scale. This scale shows good internal consistency (Cronbach's α = .93), as well as good convergent, discriminant, content, and predictive validity (Labus et al., 2004; Labus et al., 2007). This measure was administered at pre-, mid-, post-, and follow-up assessments.
Secondary Outcome Measures
Pain Vigilance and Awareness Questionnaire (PVAQ; McCracken, 1997)
This 16-item measure assessed pain awareness on a 0-5 point scale, tapping into constructs of awareness, vigilance, preoccupation, and observation of pain. Participants rated a number of statements from 0 (never) to 5 (always), such as “I keep track of my pain level”. The measure shows good internal consistency (Cronbach's α = .86), test-retest reliability (r = .80), and convergent and discriminant validity. This measure was administered at the same four assessment periods.
IBS-Quality of Life (Patrick et al., 1998)
The IBS-Qol is a 34-item measure of the degree to which IBS symptoms affect lives (5-point scale). The measure shows excellent internal consistency (Cronbach's α = .95), good test-retest reliability (r = .86), and convergent validity. We examined two of the eight IBS-QOL subscales, Interference (e.g., “I feel I get less done because of my bowel problems”) and Food Avoidance (e.g., “I have to watch the kind of food I eat because of my bowel problems”), since these measure aspects of IBS impact not covered by the primary outcomes.
Treatment Adherence
Independent raters listened to audiotaped recordings of therapy sessions for all three treatment conditions and rated treatment adherence on all relevant items for each session. Most ratings were conducted on a 0-6 point scale, with 0 = did not discuss to 6 = extensive discussion. Ratings were conducted on a random sample of two sessions per participant. Treatment adherence ratings were conducted on 76 participants (74 completers; or, 97% of completers and 2 dropouts). Session-by-session items were categorized as: self-monitoring, psychoeducation, cognitive restructuring, relaxation, attentional control, in vivo exposure and interoceptive exposure.
Treatment Credibility Questionnaire (Borkovec & Nau, 1972)
A modified version of Borkovec and Nau's (1972) Reaction to Treatment Questionnaire was completed before the second treatment session. This 7-item questionnaire asks participants to rate the degree to which they perceive their treatment as credible. The scale includes items such as “How confident are you that this treatment will be successful in reducing your bowel symptoms?” and “How competent does this therapist appear to you?” An average treatment credibility score was calculated.
Procedure
Initial eligibility was determined through a standard telephone assessment. Interested individuals who appeared to meet initial eligibility requirements were invited for an initial screening visit. The goals of the screening visit were to provide the participants with information about the study, obtain informed consent, and determine eligibility. At screening, a medical history and physical exam were conducted, including an ADIS diagnostic interview (to assess for anxiety disorders) and diagnostic assessment for IBS conducted by a gastroenterologist or nurse practitioner experienced in the diagnosis of functional bowel disease and the exclusion of organic disease. Eligible participants were then randomized to one of the three treatment conditions. Participants completed a symptom diary for two weeks and then prior to the first treatment session completed their pre-treatment assessment on self-report questionnaires.
Treatment conditions were matched on number and duration of sessions (all sessions were approximately 50 minutes).
Attention control condition (AC)
The AC protocol was based on the control group developed for a prior trial examining CBT for IBS (Toner et al., 1998; Drossman et al., 2003). The components of the AC treatment included: (a) self-monitoring of IBS symptoms that were reviewed thorhoughly; (b) receiving and reading educational material about IBS; and (c) discussing the reading material with the therapist.2
CBT-Stress management (SM)
SM consisted of (a) education about IBS symptoms and their relationship to stress; (b) self-monitoring of IBS symptoms; and (c) skills training in progressive muscle relaxation (PMR); (d) cognitive therapy to identify threat-laden appraisals of life events; and (d) in vivo exposure to items from an individualized hierarchy of external stressful situations (e.g., interpersonal conflict, work deadlines) that were not directly related to the experience of IBS sensations. The goal of SM was to reduce cognitive and physical stressful reactions to daily life events, which was presumed to reduce IBS symptoms as a reaction to stress.
CBT- Interoceptive exposure (IE)
The IE protocol was based on CBT for panic disorder (Barlow & Craske, 2006). The IE protocol adapted for an IBS population (de Cola, 2001) targeted erroneous beliefs about IBS symptoms, hypervigilance to IBS symptoms, fear of IBS symptoms, and maladaptive behavioral responses to IBS symptoms. Treatment consisted of (a) education that IBS symptoms reflect conditional reactions to reminders of gastro-distress (e.g., food intake or leaving the house); (b) self-monitoring of IBS symptoms; (c) attentional control skills to learn to shift attention away from rather than perseverate upon unpleasant visceral sensations (Wells et al., 1997); (d) cognitive therapy to identify and challenge threat-laden appraisals of visceral sensations (e.g., “I have a serious disease”); (e) interoceptive exposure involving repeated exposure to visceral sensations (e.g., tightening stomach to produce gut sensations, wearing tight clothing, delaying entrance to the bathroom, eating feared/avoided foods) to reduce fear of the sensations; and (f) in vivo exposure to feared/avoided situations in which IBS sensations were expected (e.g., long road trips, eating at restaurants, going places in which bathrooms were not accessible) while weaning safety signals or safety behaviors (e.g., additional underclothing).
In summary, whereas SM focused upon reducing stress reactivity to daily life events, IE focused on reducing anxious and avoidant responding to visceral sensations.
Statistical Analysis
One-way ANOVAs (for continuous variables) and chi-square tests (for categorical variables) assessed baseline differences between groups. A one-way ANOVA assessed differential treatment dropout between groups. Logistic regression was used to predict attrition. A 4 × 3 mixed-models approach (SAS/STAT, SAS Institute Inc, Cary, NC) evaluated changes across time (pre, mid, post, and follow-up) and by condition (AC, SM, and IE). Within-group, pre- to follow-up change was assessed by examining slopes across time-points within each treatment. The mixed models approach analyzes repeated measures and accounts for missing cases by estimating the best fitting model with the available data. The comprehensive modeling permitted in SAS mixed models allows for a conservative number of tests, each of which provides within-group and between-group comparisons within a single analysis. A priori comparisons (i.e., IE v. SM, IE v. AC, and SM v. AC) were conducted using the ITT sample, both cross-sectionally (e.g., differences at post-treatment) and over time (i.e., differences between groups on pre to post slopes, pre to follow-up slopes, and post to follow-up slopes). The mixed-models approach estimates data for missing cases in the ITT sample.
In addition, percent achieving responder status on the BSS and VSI was determined, yielding two separate indices of response. Responder status was defined as an improvement of 50% or greater from pre-treatment. While 30% improvement on NRS pain scales has been suggested as a minimally significant change by an international consensus group for low back pain (Lauridsen et al., 2006), we chose a more conservative criteria to indicate a treatment responder, one which has been reported in previous IBS trials (Spiegel et al., 2009). Responder analyses were conducted for the ITT and Completers samples. For the ITT analyses, the last observation carried forward approach was used for missing data to calculate responder status.
Results
Equivalence at baseline
There were no statistically significant differences among the three treatment groups on any demographic variable (all ps > .10). See Table 1 for descriptive information.
Table 1.
Means and SDs of Outcome Measures at all Assessment Periods (Completers)
| Measure | IE | SM | AC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Mid | Post | FU | Pre | Mid | Post | FU | Pre | Mid | Post | FU | |
| VSI | 40.63 (18.56) | 35.15 (16.11) | 29.37 (18.51) | 23.96a (16.60) | 43.61 (17.27) | 39.57 (17.15) | 34.66 (17.28) | 30.13 (16.26) | 45.18 (12.29) | 36.32 (17.19) | 35.95 (16.55) | 39.69 (13.74) |
| PVAQ | 45.87 (16.24) | 43.68c (14.24) | 37.42a (17.12) | 33.52ab (13.48) | 49.12 (12.90) | 47.76 (13.00) | 41.06 (14.28) | 41.35 (17.11) | 51.32 (9.10) | 53.25 (12.85) | 44.23 (10.40) | 46.98 (12.61) |
| BSS | .40 (.64) | - | -.54 (.74) | -.78 (.60) | .25 (.65) | - | -.25 (.83) | -.53 (.74) | .52 (.47) | - | -.25d (.98) | -.25d(.97) |
| IBS-QOL Food Avoidance | 47.96 (27.87) | - | 65.74 (28.44) | 73.00 (24.33) | 47.50 (33.24) | - | 57.76 (30.40) | 64.49 (33.26) | 46.21 (24.22) | - | 50.42 (29.80) | 57.74 (22.75) |
| IBS-QOL Interference | 69.91 (22.45) | - | 79.07 (20.81) | 80.71 (20.77) | 60.89 (27.81) | - | 74.26 (25.15) | 75.00 (25.02) | 57.31 (22.55) | - | 68.39 (23.21) | 66.33 (24.77) |
outperformed AC, p < .05
=outperformed SM, p = .06
= outperformed AC, p = .07
= outperformed AC, p ≤ .08
Note: BSS scores represent standardized (i.e., between -1 and 1) scores because the composite BSS severity index required individual items to be standardized due to different measurement scales (see Measures).
Note: Higher scores on IBS-QOL measures indicate greater improvement in quality of life
Attrition Analyses
The treatment groups did not differ in terms of dropout/completer status, χ2 (df=1, N=111) = 3.98, p = .14. None of the demographic variables [i.e., sex, age, race (Caucasian v. non-Caucasian), bowel habit, income, marital status] nor pre-treatment severity variables (BSS, VSI and PVAQ) significantly predicted attrition (all p-values for the ORs >.10). Further, there was no difference among groups in number of sessions completed (p = .21).
Treatment adherence
No difference emerged across the three groups in amount of time spent on psychoeducational material during treatment, F (2, 59) = 0.13, p = .88. There was a main effect of Group for self-monitoring [F (2, 44) =38.69, p < .001]; post hoc tests revealed that significantly more self-monitoring was discussed/implemented in IE compared to SM (p < .001) and AC (p < .001), with no differences between SM and AC (p = .42). A main effect of Group was found for cognitive restructuring [F (2, 59) = 119.27, p < .001]; post hoc tests indicated that cognitive restructuring was covered more in IE than AC (p < .001) and more in SM than AC (p < .001), with no differences between IE and SM (p = .57). A main effect of Group was found for relaxation [F (2, 43) = 126.63, p < .001]; relaxation was conducted more in SM than IE (p < .001) and AC (p < .001), with no differences between IE and AC (p = .85). Group effects were significant for attentional control techniques [F (2, 43) = 51.54, p < .001], with more attentional control addressed in IE compared to both SM and AC (both ps < .001) and no differences between SM and AC (p = .90). Group effects were significant for in vivo exposure [F (2, 56) = 43.00, p < .001]; more exposure was conducted/discussed in IE vs SM and AC (ps < .001) and SM vs AC (p < .001). Also, a main effect of Group for interoceptive exposure [F (2, 48) = 143.98, p < .001] was due to higher levels in IE vs SM and AC (ps < .001) with no differences between SM and AC (p = 1.0).
Treatment credibility
On average, participants rated the treatments to be more than moderately credible (M = 7.28, SD = 1.26) with no differences across groups (p =.13).
Outcome Measures: Continuous Analyses in Mixed Models
Table 1 reports descriptive information for the outcome measures. Table 2 reports within-group effect sizes from pre to post, pre to follow-up, and post to follow-up. Table 3 reports between-group effect sizes for each of the three a priori comparisons at post and follow-up.
Table 2.
Within-group Effect Sizes (Cohen's d) for each treatment group
| IE | SM | AC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Po | Pre-FU | Po-FU | Pre-Po | Pre-FU | Po-FU | Pre-Po | Pre-FU | Po-FU | |
| BSS | 1.32 | 1.71 | 0.37 | 0.68 | 1.17 | 0.50 | 1.04 | 1.23 | 0.25 |
| VSI | 0.58 | 0.94 | 0.40 | 0.45 | 0.78 | 0.37 | 0.65 | 0.52 | -0.15 |
| PVAQ | 0.46 | 0.86 | 0.46 | 0.40 | 0.55 | 0.19 | 0.23 | 0.47 | 0.27 |
| IBS-FA | 0.60 | 0.91 | 0.31 | 0.36 | 0.64 | 0.30 | 0.15 | 0.34 | 0.19 |
| IBS-IA | 0.73 | 0.78 | 0.09 | 0.34 | 0.41 | 0.07 | 0.54 | 0.67 | 0.11 |
Cohen's d interpretation: 0.3=small, 0.5=medium, 0-8=large effect size. BSS=Bowel Symptom Questionnaire; VSI=Visceral Sensitivity Index; PVAQ=Pain Vigilance and Awareness Questionnaire; IBS-FA= IBS Quality of Life-Food Avoidance subscale; IBS-IA = IBS Quality of Life- Interference with Acitivity subscale; IE=interoceptive exposure; SM=stress management; AC=attentional control; Co=Completers sample; Note: effect sizes are for Intent-to-treat sample
Table 3.
Between-group effect sizes (Cohen's d) at post-treatment and follow-up on outcome measures.
| Post | Follow-up | |||||
|---|---|---|---|---|---|---|
| IE v AC | IE v SM | SM v AC | IE v AC | IE v SM | SM v AC | |
| BSQ | 0.43 | 0.44 | 0.00 | 0.70 | 033 | 0.30 |
| VSI | 0.24 | 0.32 | -0.08 | 0.76 | 0.42 | 0.35 |
| PVAQ | 0.64 | 0.29 | 0.35 | 0.82 | 0.56 | 0.25 |
| IBS-FA | 0.50 | 0.25 | 0.25 | 0.67 | 0.28 | 0.38 |
| IBS-IA | 0.42 | 0.46 | 003 | 0.45 | 0.54 | -0.09 |
Cohen's d interpretation: 0.3=small, 0.5=medium, 0-8=large effect size. BSQ=Bowel Symptom Questionnaire; VSI=Visceral Sensitivity Index; PVAQ=Pain Vigilance and Awareness Questionnaire; IBS-FA= IBS Quality of Life-Food Avoidance subscale; IBS-IA = IBS Quality of Life- Interference with Acitivity subscale; IE=interoceptive exposure; SM=stress management; AC=attentional control; Co=Completers sample; Note: effect sizes are for Intent-to-treat sample
Intent-to-Treat Analyses
Bowel Symptom Severity Composite: Between-group differences on change slopes
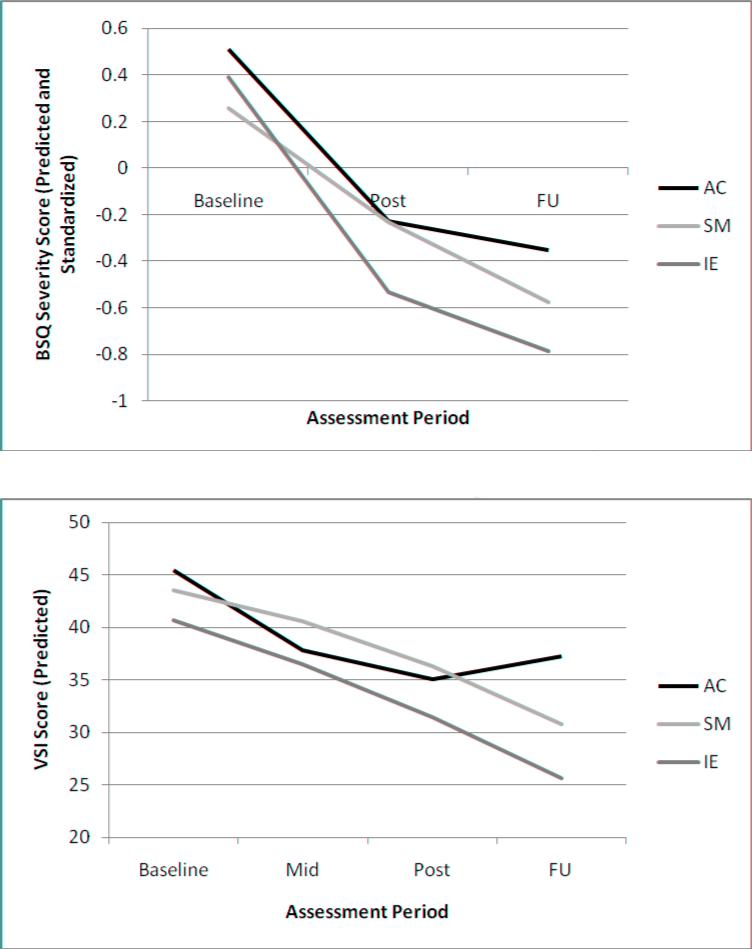
A significant effect of Time was observed for all three treatment groups; IE [t (189) = 6.54, p < .001]; SM [t (195) = 4.29, p < .001]; and AC [t (184) = 3.31, p < .001]. IE showed a steeper decline in BSS (see Figure 2) from pre- to post-treatment than SM [t (165) = -2.03, p < .05]. No other group differences in slopes were observed from pre- to post-treatment, pre- to follow-up, or post to follow-up (all ps > .19).
2.
BSI Decline Slopes and VSI Decline Slopes for all Three Treatment Groups Across all Assessment Periods (Baseline through Follow-up)
Bowel Symptom Severity Composite: Cross-sectional between-group differences
A priori contrasts revealed no significant cross-sectional differences at post-treatment or follow-up across the three groups.
VSI: Between-group differences on change slopes
A significant effect of Time was observed for IE [t (310) = 4.65, p < .0001] and SM [t (314) = 3.61, p < .001], whereas the effect did not attain statistical significance in AC [t (306) = 1.81, p = .07]. In terms of slopes, IE showed greater post-treatment to follow-up symptom decline compared to AC [t (244) = -2.19, p < .05] as did SM compared to AC [t (244) = -2.06, p < .05], with no other significant inter-group differences on any of the slope comparisons (all ps > .22). Figure 3 shows the slopes for all three groups across all assessment periods.
VSI: Cross-sectional between-group differences
A priori contrasts of cross-sectional differences among groups revealed lower VSI scores at follow-up in IE compared to AC [t (244) = 2.27, p < .05], with no other significant inter-group differences (all ps > .19).
PVAQ: Between-group differences in change slopes
There were significant declines in PVAQ scores across Time for IE [t (325) = 3.89, p < .0001] and SM [t (325) = 2.28, p < .05], with no significant change for AC (p = .14). There were no significant differences between groups in slopes of symptom decline over time (all ps > .28).
PVAQ: Cross-sectional between-group differences
A priori comparisons revealed significantly lower scores for IE than AC at post-treatment [t (175) = 2.25, p < .05] and follow-up assessment [t (200) = 2.39, p < .05]. No other cross-sectional differences were observed (all ps > .24). In addition, PVAQ was lower in IE than SM at follow-up, but this difference did not attain statistical significance [t (272) = 1.89, p = .06].
IBS-QOL Interference Subscale: Between-group differences in change slopes
All groups showed significant improvement over Time on the interference subscale; IE [t (161) = -3.55, p < .001]; SM [t (167) = -2.66, p < .05]; and AC [t (158) = -3.46, p < .001]. No slope differences were observed between groups (ps > .19).
IBS-QOL Interference Subscale: Cross-sectional between-group differences
IE showed greater improvement than SM at post-treatment [t (151) = -1.83, p < .07] and at follow-up [t (173) = -1.82, p = .07], but these findings did not attain statistical significance. No other cross-sectional differences were significant (ps > .12)
IBS-QOL Food Avoidance Subscale: Between-group differences in change slopes
IE [t (185) = -4.85, p < .001] and SM [t (195) = -3.18, p < .01] showed significant improvement over Time on the food avoidance subscale, whereas AC did not (p = .18). Pre to post [t (182) = -1.77, p < .08] and pre to follow-up [t (158) = -1.76, p < .08] slopes were somewhat steeper for IE compared to AC, although statistical significance was not attained. No other differences in slopes emerged between groups (all ps > .27).
IBS-QOL Food Avoidance Subscale: Cross-sectional between-group differences
IE showed some non-statistically significant improvement over AC at post-treatment [t (173) = -1.73, p < .09] and follow-up [t (204) = -1.94, p = .05], with no other between-group differences (all ps > .27).
Treatment Response
BSS-Composite
At follow-up, 62% of IE, 54% of SM, and 59% of AC achieved responder status, with no differences among groups (p = .74). In the Completers sample, 89% of IE, 82% of SM, and 56% of AC achieved responder status. In planned comparisons of the Completers sample, IE outperformed AC, χ2 (df =1, N=28) = 4.17, p < .05, with no other between-group differences (all ps ≥ .14).
VSI
At follow-up, 30% of IE, 15% of SM, and 5% of AC achieved responder status. IE outperformed AC, χ2 (df=1, N=69) = 5.61, p < .05, with differences between IE and SM not attaining statistical significance, χ2 (df=1, N=88) = 2.86, p = .09, and no differences between AC and SM. Percentages of responder status were higher in the Completers sample, with 44% of IE, 23% of SM, and 0% of AC. Pairwise comparisons revealed that the percentage of responders in IE was significantly higher than AC (p < .01), with no other between-group differences.
Discussion
The present study had two general aims. The first was to design and test a new theory-driven version of CBT that focused on changing fear and avoidance of IBS symptoms using cognitive restructuring, attentional control, and behavioral exposures to IBS-related sensations and situations. The second aim was to compare this new treatment procedure against both a stress management oriented CBT and an active educational control condition. All three treatment conditions led to significant reductions in IBS symptoms, with the majority reporting a greater than 50% symptom reduction in both the intent-to-treat and the completer samples, although responder status was less for the measure of anxiety about IBS symptoms (i.e., the VSI). Most significantly, there was some evidence for the superiority of IE treatment. Specifically, the IE group had a significantly greater decline in symptoms from pre to post compared to SM, and, in the completer sample, showed a significantly greater number of symptom responders than the AC group. Similarly, on the VSI measure, the IE group showed a greater response rate and lower follow-up scores than AC, and a marginally greater response rate than SM. For the secondary measure of pain vigilance, IE also showed significantly lower scores than AC at both post and follow-up, and marginally lower scores than SM at follow-up. On the two measures of life interference (activity interference and food avoidance) there were no significant group differences but several trends, all in the direction of greater change for IE than either the SM or AC groups. Importantly, only one variable (VSI slope from post-treatment to follow-up) showed greater responses in the SM group compared to either the IE or AC.
The superiority of the IE group is not explained by process variables, since attrition rates (23%) and treatment credibility did not differ across groups. Furthermore, adherence analysis indicated that the three groups were delivered with integrity and in ways that were distinctly different from each other as intended. Thus, this well-controlled comparative trial shows some potential added benefits for version of CBT for IBS that directly targets fear and avoidance of visceral sensations.
Previous trials of CBT for IBS have utilized a variety of measures to determine symptom responder status, but most report responder rates above 60% (e.g., Drossman et al., 2003; Lackner et al., 2010). Responder rates for IE are comparable (62% for IBS symptoms). However, the size of our between-group effects was likely mitigated by our very stringent attention control condition. That is, many of the positive trials of psychological therapies for IBS (e.g., Lackner et al., 2004; Blanchard & Scharff, 2002) utilized treatment-as-usual or wait list control comparisons, which tend to yield substantially larger between-group effects than do active control comparisons. One large IBS trial by the Drossman group (2003) that included an educational control group also found only modest between-group effects, similar to those observed herein. The improvement observed in our attention control condition may have derived from positive expectancy, therapist support and provision of a treatment rationale (i.e., placebo), factors that have been judged to be sufficient for an effective treatment (Zeiss, 1979). The effectiveness of the attention control condition may have additionally derived from active ingredients such as education and structured review of symptoms, especially since other research has shown the effectiveness of psychoeducation group treatments relative to usual care in IBS (Ringstrom et al., 2010; Colwell et al. 1998; Saito et al., 2004).
To the extent that IE exerted specific effects on IBS symptoms, anxiety about IBS symptoms, and vigilance, the feasibility and potential utility of incorporating exposure elements into IBS treatment is validated. The IE treatment was based on a fear of visceral sensations conceptualization of IBS symptoms, and use of graduated exposures to decrease fear and avoidance and to correct misappraisals of both interoceptive and exteroceptive cues associated with IBS symptoms. The interoceptive and in vivo exposures were well-tolerated and patients were able to rapidly generate hierarchies and understand the concepts of exposure and non-avoidance of anxiety. Similar fear and avoidance treatments have recently been proposed and in part supported for other pain-related problems, including low back pain (Vlaeyen & Linton, 2000). A unique feature of the fear and avoidance model is that it narrows the focus of treatment to cues associated with the target symptoms, unlike more general stress management which attempts to alter coping responses to a broad range of life stressors.
In the current study, IE not only outperformed AC, and to a lesser extent SM, but in no case was SM intervention superior to IE, and in all but one comparison, the SM group was not significantly different than the AC. Thus, the overall results suggest that IE was more effective than SM, which mostly was no different than an attention control. The current study was the first in this field to directly test a general stress management approach against a fear of visceral sensations approach for IBS.
There were several limitations to the study, including sample size, especially when considering the drop-out rate of 23%. The substantial pair-wise IE vs. AC effect sizes (ds of 0.71 and 0.84 for BSS and VSI, respectively, at 6 months) suggest that a larger sample, especially for the control participants, may have resulted in a more consistent pattern of statistical IE superiority. The current study was cognizant of the ROME foundation working groups’ reports on IBS trial design (Levine et al, 2006, and Spiegel, et al., 2008) and included recommended design features of an active control condition, use of a broad sample of patients all fitting the ROME IBS criteria, examination of intent-to-treat and per-protocol analysis, use of both a pre-specified responder criteria and assessment of absolute change, and an extended follow-up period. These aspects of a rigorous and conservative study design clearly impact the outcome and interpretation of the results in that they heighten generalizability and confidence in the conclusions that outcome differences resulted from differences in intervention content. However, the use of an active control and a 50% responder criterion does lead to smaller effect sizes than studies with treatment as usual or wait-list designs. Since there are no objective markers for IBS or universally agreed upon symptom scales, the choice of self-report measures also may have significantly impacted on the outcomes. Future studies may consider independent clinician ratings and/or behavioral measures of adaptive functioning and fear and avoidance of IBS symptoms and activities.
In sum, a treatment approach that focused on fear and avoidance of visceral sensations associated with IBS was found to be superior to an active attention control condition on several measures, including a measure of symptom severity as well as anxiety about symptoms and overall body vigilance. Furthermore, the IE approach outperformed a standard stress management approach to treatment, which in turn showed very minimal benefits over the active control condition. Thus, these results highlight the positive effects of a focused and theoretically cohesive cognitive behavioral approach to IBS, something that has been advocated for cognitive behavioral approaches to pain management in general (Eccleston et al., 2009).
Acknowledgements
The authors wish to acknowledge Drs. Joseph DeCola, Jayson Mystkowski, and Janice Jones for their role as therapist on this study, as well as Dr. Brenda Toner for her help with the Attention Control Protocol and Suzanne Smith NP RN for participant screening.
Supported in part by NIH Grants NR007768 (BDN), P50 DK64539 (EAM) and VA Medical Research (BDN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although bloating is not a specific Rome criterion, it is one of the most common and bothersome of the IBS symptoms (Ringel et al., 2009) that make up “abdominal discomfort,” which is a criteria symptom for IBS.
Protocols for all three conditions are available from the corresponding author.
References
- Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomised controlled trials. Gut. 2001;48:272–282. doi: 10.1136/gut.48.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH, Craske MG. Mastery of your anxiety and panic: Client workbook. 4th Edition Oxford University Press; New York: 2006. [Google Scholar]
- Beck AT, Emery G, Greenberg RL. Anxiety disorders and phobias: A cognitive perspective. Basic Books; New York: 1985. [Google Scholar]
- Blanchard EB, Scharff L. Psychosocial aspects of assessment and treatment of irritable bowel syndrome in adults and recurrent abdominal pain in children. Journal of Consulting and Clinical Psychology. 2002;70:725–738. doi: 10.1037//0022-006x.70.3.725. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Scharff L, Payne A, Schwarz SP, Suls JM, Malamood H. Prediction of outcome from cognitive-behavioral treatment of irritable bowel syndrome. Behaviour Research and Therapy. 1992;30:D647–650. doi: 10.1016/0005-7967(92)90011-5. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Nau SD. Credibility of analogue therapy rationales. Journal of Behavior Therapy and Experimental Psychiatry. 1972;3:257–260. [Google Scholar]
- Brandt LJ, Locke GR, Olden K, Quigley E, Schoenfeld P, Schuster M, et al. An evidence based approach to the management of irritable bowel syndrome in North America. American Journal of Gastroenterology. 2002;97:S1–S26. doi: 10.1016/s0002-9270(02)05657-5. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Choi MG. Review article: irritable bowel syndrome. Alimentary Pharmacology & Therapeutics. 1997;11:3–15. doi: 10.1046/j.1365-2036.1997.84256000.x. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Caputo GC, Bright P, Gallagher R. Assessment of fear of fear in agoraphobics: the body sensations questionnaire and the agoraphobic cognitions questionnaire. Journal of Consulting and Clinical Psychology. 1984;52:1090–1097. doi: 10.1037//0022-006x.52.6.1090. [DOI] [PubMed] [Google Scholar]
- Colwell LJ, Prather CM, Phillips SF, Zinsmeister AR. Effects of an irritable bowel syndrome educational class on health-promoting behaviors and symptoms. American Journal of Gastroenterology. 1998;93:901–905. doi: 10.1111/j.1572-0241.1998.00273.x. [DOI] [PubMed] [Google Scholar]
- Craske MG, Barlow DH. Panic disorder and agoraphobia. In: Barlow DH, editor. Clinical handbook of psychological disorders. 2nd Edition Guilford Press; New York: 1993. pp. 1–47. [Google Scholar]
- Creed F, Fernandes L, Guthrie E, Palmer S, Ratcliffe J, Read N, et al. The cost effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology. 2003;124:303–317. doi: 10.1053/gast.2003.50055. [DOI] [PubMed] [Google Scholar]
- DeCola JP. Deliberate exposure to interoceptive sensations: a cognitive-behavioral treatment for irritable bowel syndrome. Dissertation Abstracts International: Section B: The Sciences and Engineering. 2001;62:1073. [Google Scholar]
- DiNardo PA, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV) Graywind Publications; Albany, NY: 1994. [Google Scholar]
- Drossman DA, Leserman J, Li Z, Keefe F, Hu YJ, Toomey TC. Effects of coping on health outcome among women with gastrointestinal disorders. Psychosomatic Medicine. 2000;62:309–317. doi: 10.1097/00006842-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Drossman D, Li Z, Andruzzi E, Temple R, Talley N, Thompson G, et al. U.S. householder survey of functional gastrointestinal disorders. Digestive Diseases and Sciences. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Evidence Based Mental Health. 2009;12:118–210. doi: 10.1002/14651858.CD007407.pub2. [DOI] [PubMed] [Google Scholar]
- Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. Journal of Gastroenterology and Hepatology. 2010;25:691–699. doi: 10.1111/j.1440-1746.2009.06120.x. [DOI] [PubMed] [Google Scholar]
- Hazlett-Stevens H, Craske MG, Mayer EA, Chang L, Naliboff BD. Prevalence of irritable bowel syndrome among university students: the roles of worry, neuroticism, anxiety sensitivity and visceral anxiety. Journal of Psychosomatic Research. 2003;55:501–505. doi: 10.1016/s0022-3999(03)00019-9. [DOI] [PubMed] [Google Scholar]
- Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Alimentary Pharmacology & Therapeutics. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- Labus J, Mayer EA, Bolus R, et al. Gastrointestinal-specific anxiety: further validation of the visceral sensitivity index. Gastroenterology. 2005;128:A–67. [Google Scholar]
- Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosomatic medicine. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- Lackner JM, Gudleski GD, Keefer L, Kraspner SS, Powell C, Katz LA. Rapid response to cognitive behavior therapy predicts treatment outcome in patients with irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2010;8:426–432. doi: 10.1016/j.cgh.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Holroyd K. Self administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clinical Gastroenterology and Hepatology. 2008;6:899–906. doi: 10.1016/j.cgh.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. Journal of Consulting and Clinical Psychology. 2004;72:1100–1113. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]
- Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet-Nilsson N. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskeletal Disorders. 2006;7:82–98. doi: 10.1186/1471-2474-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesbros-Pantoflickova D, Michetti P, Fried M, Beglinger C, Blum AL. Meta analysis: the treatment of irritable bowel syndrome. Alimentary Pharmacology & Therapeutics. 2004;20:1253–1269. doi: 10.1111/j.1365-2036.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- Longstreth GF. Definition and classification of irritable bowel syndrome: current consensus and controversies. Gastroenterology Clinics of North America. 2005;32:173–187. doi: 10.1016/j.gtc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Wilson A, Knight K, Wong J, Chiou CF, Barghout V, et al. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. American Journal of Gastroenterology. 2003;98:600–607. doi: 10.1111/j.1572-0241.2003.07296.x. [DOI] [PubMed] [Google Scholar]
- Lydiard RB. Anxiety and irritable bowel syndrome. Psychiatric Annals. 1992;22:612–618. [PubMed] [Google Scholar]
- Lynch PM, Zamble E. A controlled behavioral treatment study of irritable bowel syndrome. Behavior Therapy. 1989;20:509–523. [Google Scholar]
- Mayer EA, Bradesi S, Chang L, Spiegel BMR, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. Journal of Clinical Psychiatry. 2001;62:28–36. [PubMed] [Google Scholar]
- McCracken LM. Attention to pain in persons with chronic pain: a behavioural approach. Behaviour Therapy. 1997;28:271–284. [Google Scholar]
- McNally RJ, Lorenz M. Anxiety sensitivity in agoraphobics. Journal of Behavior Therapy and Experimental Psychiatry. 1987;18:3–11. doi: 10.1016/0005-7916(87)90065-6. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, et al. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson OS, Turner MJ, Johnson DA, Burnett CK, Whitehead WE. Hypnosis treatment for severe irritable bowel syndrome: investigation of mechanism and effects on symptoms. Digestive Diseases and Sciences. 2002;47:2605–2614. doi: 10.1023/a:1020545017390. [DOI] [PubMed] [Google Scholar]
- Pare P, Gray J, Lam S, Balshaw R, Khorasheh S, Barbeau M, et al. Health related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clinical Therapeutics. 2006;28:1726–1735. doi: 10.1016/j.clinthera.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Digestive Diseases and Sciences. 1998;43:400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- Ringel Y, Williams RE, Kalilani L, Cook SF. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2009;7:68–72. doi: 10.1016/j.cgh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Ringstrom G, Storsrud S, Posserud I, Lundgvist S, Westman B, Simren M. Structured patient education is superior to written information in the management of patients with irritable bowel syndrome: a randomized controlled study. European Journal of Gastroenterology & Hepatology. 2010;22:420–428. doi: 10.1097/MEG.0b013e3283333b61. [DOI] [PubMed] [Google Scholar]
- Saito YA, Prather CM, Van Dyke CT, Fett S, Lock GR., III Effects of multidisciplinary education on outcomes in patients with irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2004;2:576–584. doi: 10.1016/s1542-3565(04)00241-1. [DOI] [PubMed] [Google Scholar]
- Saito YA, Schoenfeld P, Locke R. The epidemiology of irritable bowel syndrome in North America: a systematic review. American Journal of Gastroenterology. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Lerew DR, Trakowski JH. Body vigilance in panic disorder: evaluating attention to bodily perturbations. Journal of Consulting and Clinical Psychology. 1997;65:214–220. doi: 10.1037//0022-006x.65.2.214. [DOI] [PubMed] [Google Scholar]
- Spiegel B, Strickland A, Naliboff BD, Mayer EA, Chang L. Predictors of patient–assessed illness severity in irritable bowel syndrome. American Journal of Gastroenterology. 2008;103:2536–2543. doi: 10.1111/j.1572-0241.2008.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel B, Camilleri M, Bolus R, Andresen V, Chey WD, Fehnel S, Mangel A, Talley NJ, Whitehead WE. Psychometric evaluation of patient-reported outcomes in irritable bowel syndrome randomized controlled trials: a Rome Foundation report. Gastroenterology. 2009;137:1944–53. doi: 10.1053/j.gastro.2009.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley NJ, Owen BK, Boyce P, Paterson K. Psychological treatments for irritable bowel syndrome: a critique of controlled treatment trials. American Journal of Gastroenterology. 1996;91:277–283. [PubMed] [Google Scholar]
- Thompson WG. Irritable bowel syndrome. Strategy for the family physician. Canadian Family Physician. 1994;40:307–310. 313–316. [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus JS, Naliboff BD, Bolus R, Shetzline M, Mayer EA, et al. Characterization of the alternating bowel habit subtype in patients with irritable bowel syndrome. American Journal of Gastroenterology. 2005;100:896–904. doi: 10.1111/j.1572-0241.2005.41211.x. [DOI] [PubMed] [Google Scholar]
- Toner BB, Segal ZV, Emmott S, Myran D, Ali A, DiGasbarro I, et al. Cognitive behavioral group therapy for patients with irritable bowel syndrome. International Journal of Group Psychotherapy. 1998;48:215–243. doi: 10.1080/00207284.1998.11491537. [DOI] [PubMed] [Google Scholar]
- van Dulmen AM, Fennis JF, Bleijenberg G. Cognitive-behavioral group therapy for irritable bowel syndrome: effects and long-term follow-up. Psychosomatic Medicine. 1996;58:508–514. doi: 10.1097/00006842-199609000-00013. [DOI] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- Wells A, White J, Carter K. Attention training: effects on anxiety and beliefs in panic and social phobia. Clinical Psychology & Psychotherapy. 1997;4:226–232. [Google Scholar]
- Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- Whorwell PJ, Prior A, Colgan SM. Hypnotherapy in severe irritable bowel syndrome: further experience. Gut. 1987;28:423–425. doi: 10.1136/gut.28.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiss AM, Lewinsohn PM, Munoz R. Non-specific improvement effects in depression using interpersonal skills training, pleasant activity schedules, or cognitive training. Journal of Consulting and Clinical Psychology. 1979;48:730–735. doi: 10.1037//0022-006x.47.3.427. [DOI] [PubMed] [Google Scholar]