Abstract
The term human epithelial carcinoma antigen (HCA) has been applied collectively to mucin-type high molecular weight (>1000 kDa) glycoproteins that are over-expressed in epithelial cancers. Since the 1990s, over 40 monoclonal antibodies have been raised that recognize HCA. There has been evidence that the antigenic determinants are mostly carbohydrates, but details have been elusive. Here we have carried out carbohydrate microarray in analyses of one of the monoclonal antibodies, AE3, that has been regarded the ‘most carcinoma specific’ in respect to its ability to detect HCA in sera of patients with epithelial cancers. The microarrays encompassed a series of 492 sequence-defined glycan probes in the form of glycolipids and neoglycolipids. We have thus established that the antigen recognized by antibody AE3 is a carbohydrate sequence distinct from the A, B, H, Lewisa/b, Lewisx/y and T antigens, but that it is strongly expressed on the monosulfated tetra-glycosyl ceramide, SM1a, Galβ1-3GalNAcβ1-4(3-O-sulfate)Galβ1-4GlcCer. This is the first report of an anti-HCA to be characterized with respect to its recognition sequence and of the occurrence of the antigen on a glycolipid as well as on glycoproteins. Knowledge of a discrete glycan sequence as target antigen now opens the way to its exploration as a serologic cancer biomarker, namely to determine if the antigen elicits an autoantibody response in early non-metastatic cancer, or if it is shed and immunochemically detectable in more advanced disease.
Keywords: human carcinoma antigen HCA, anti-epiglycanin AE3, epithelial mucin glycoproteins, MUC21, sulfoglycolipids, NGL-based carbohydrate microarrays
Introduction
The term human epithelial carcinoma-associated antigen (HCA) has been applied collectively to mucin-type high molecular weight (>1000 kDa) glycoproteins that are over-expressed in epithelial cancers [1]. Antigenic cross-reactions between HCA and epiglycanin, the major sialomucin glycoprotein (~ 500 kDa) of murine mammary adenocarcinoma TA3 cells [2], have meant that murine monoclonal antibodies raised against epiglycanin could be used by Codington and colleagues as reagents to detect HCA in sera of patients with epithelial carcinomas [3;4].
The molecular identities of the murine epiglycanin and HCA were unknown for a long time, and only recently are being clarified. By differential analyses of murine mammary carcinoma cell variants (TA3-Ha) that express epiglycanin or lack epiglycanin (TA3-St), and by homology searching, Irimura and colleagues have cloned and expressed a human and a murine ortholog [5;6]. These have been shown to be highly glycosylated transmembrane mucins having 28 and 98 tandem repeat domains, respectively, rich in serine and threonine residues, and they have been designated MUC21 [5]. Whether there is a single epiglycanin protein in mouse and human or whether there are different forms of the glycoproteins that are variously upregulated in epithelial cancers remains to be determined.
In excess of 40 monoclonal antibodies have been raised to murine epiglycanin: the majority were of IgM class [3] and two of IgG class [7]. There was evidence that the antigenic determinants recognized by many of the IgM antibodies involve carbohydrate moieties of the glycoprotein. Their binding was reduced after periodate oxidation of epiglycanin, and inhibited in the presence of the plant lectin, peanut agglutinin (PNA), and high concentrations of the blood group T disaccharide, Galβ1-3GalNAc. The binding of one of the antibodies was strongly inhibited in the presence of Ricinus communis agglutinin I (RCA120) [3]. The anti-epiglycanin antibody, designated AE3, was considered the ‘most carcinoma specific’ in respect to its ability to detect HCA in sera of patients with epithelial cancers such as those of breast [8]. This antibody was also reported to strongly immunostain human cancer tissues such as those of the prostate, bladder and esophagus [9–11]. Having found that the binding to epiglycanin was inhibited both by the blood group T disaccharide and synthetic peptides carrying this disaccharide sequence, antibody AE3 was suggested to resemble PNA which recognizes the O-glycan core sequence Galβ1-3GalNAc linked to Ser/Thr. However, the antibody differed in that the concentration of the blood group T disaccharide required for inhibition of binding to epiglycanin was 104 times greater than for PNA. It was thus inferred that the blood group T disaccharide sequence was a part of a larger antigenic determinant recognized by antibody AE3 [4]. This has remained an open question over the ensuing years.
Here we have investigated the determinant recognized by AE3 antibody by carbohydrate microarray analyses using sequence-defined glycan probes, and, unexpectedly, we have identified an antigen-positive sequence on a glycolipid.
Materials and Methods
The murine hybridoma IgM antibody, AE3, was produced by mouse immunization (CBL57/J) with asialo-epiglycanin [4;9]. The antibody, enriched by size exclusion chromatography at Maine Biotechnology Services (Portland, Maine), was from Egenix (Rochester, NY). The biotinylated plant lectins PNA and RCA120 were from Vector Laboratories (Peterborough, UK).
Carbohydrate microarray analyses of antibody AE3 were performed using the neoglycolipid (NGL)-based microarray system that contains sequence-defined-lipid linked glycan probes: glycolipids and NGLs [12;13]. The repertoire of 492 probes (Supplementary Table 1), encompassed a variety of mammalian type sequences, representative of N-glycans (high-mannose-type and neutral and sialylated complex-type), peripheral regions of O-glycans; blood group antigen-related sequences (A, B, H, Lewisa, Lewisb, Lewisx, and Lewisy) on linear or branched backbones and their sialylated and/or sulfated analogs; linear and branched poly-N-acetyllactosamine sequences; gangliosides, oligosaccharide fragments of glycosaminoglycans and polysialic acid. The arrays also included microbial and plant-derived homo-oligomers of glucose and of other monosaccharides.
The lipid-linked glycan probes were printed non-covalently on 16-pad nitrocellulose-coated glass slides, in duplicate at two levels, 2 and 5 fmol/spot, as described [13;14]. Microarray screening analyses of antibody AE3 and biotinylated PNA and RCA120, and the imaging of binding signals were performed essentially as described [13]. In brief, after blocking with 3% w/v bovine serum albumin in Hepes buffered saline (5 mM Hepes, pH 7.4, 150 mM NaCl, 5 mM CaCl2), the arrayed slides were probed with AE3 antibody (10 μg/ml), followed by biotinylated anti-mouse IgM, 1:200 (Sigma, Dorset, UK); or with biotinylated PNA and RCA120 at 5 μg/ml. Binding was detected using Alexa Fluor-647-labeled streptavidin from Molecular Probes (1 μg/ml). After quantitation, data analysis and presentation were carried out using dedicated software developed by Mark S. Stoll of the Glycosciences Laboratory [15]. Binding signals were related to dose of probe spotted; results at 5 fmol per spot are presented.
In order to closely compare the binding preferences of AE3 and PNA, a focused microarray was generated in a ‘dose-response’ format, which included the glycolipids asialo-GM1, GM1, SM1a and SB1a. For this, the probes were quantified at the same time and printed in duplicate at four levels: 0.3, 1, 2 and 5 fmol/spot.
Results and Discussion
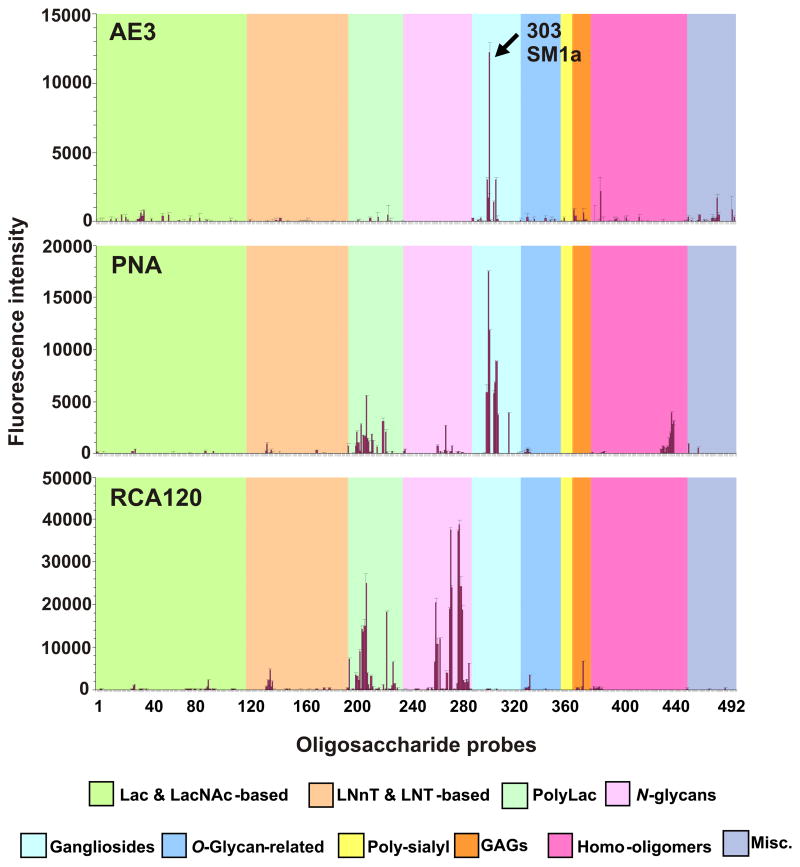
Microarray screening analyses of AE3 antibody showed that the specificity of the antibody is unrelated to the major blood group antigens, A, B, H, Lewisa and Lewisb. Instead, a binding profile for antibody AE3 was observed that partially overlapped with that of PNA (Fig. 1 and Supplementary Table 1) and was clearly distinct from that of the plant lectin RCA120. A potent AE3 antigen-positive probe was detected at position 303 of the array, which was also strongly bound by PNA. This is SM1a, which is the sulfoglycolipid analog of the sialoglycolipid GM1 (position 306).
Fig. 1.
Microarray screening analyses of monoclonal antibody AE3 and the lectins peanut agglutinin (PNA) and Ricinus communis agglutinin I (RCA120). The results are the means of fluorescence intensities of duplicate spots, printed at 5 fmol. The error bars represent half of the difference between the two values. In the glycan array the 492 lipid-linked probes (> 370 mammalian type) are grouped according to their backbone sequences as annotated by the colored panels: disaccharide based: lactose (Lac) and N-acetyllactosamine (LacNAc); tetrasaccharide based: lacto-N-neo-tetraose (LNnT) and lacto-N-tetraose (LNT); poly-N-acetyllactosamine (PolyLac); N-glycans; gangliosides; O-glycan-related; polysialyl; glycosaminoglycans (GAGs); homo-oligomers of glucose and of other monosaccharides, and other non-classified sequences (miscellaneous, Misc).
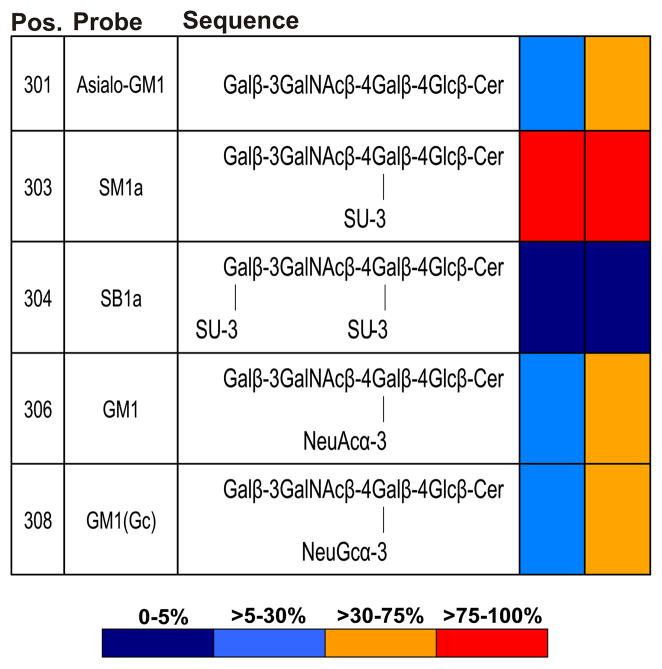
In a matrix presentation in Fig. 2, the relative binding intensities of antibody AE3 and of PNA are shown toward SM1a and four of the other GM1-related glycolipids included in the screening arrays (Fig. 1). Neither AE3 nor PNA gave binding signals with the disulfated analog, SB1a (position 304). AE3 gave binding signals of lower relative intensity than PNA with the three other structurally-related nonsulfated glycolipids. These were the asialo-GM1 (position 301) and the N-acetyl and N-glycolyl (NeuGc) forms of GM1 (positions 306 and 308, respectively).
Fig. 2.
Relative intensities of binding of antibody AE3 and PNA to five glycolipids sharing the asialo-GM1 backbone sequence, Galβ-3GalNAcβ-4Galβ-4Glc in the microarray screening analyses. Relative binding intensities are shown as the percentage of the fluorescence signal intensity relative to that given by SM1a, the glycolipid most strongly bound by each protein at 5 fmol per spot. Pos., positions in the microarray in Fig. 1 and Supplementary Table 1.
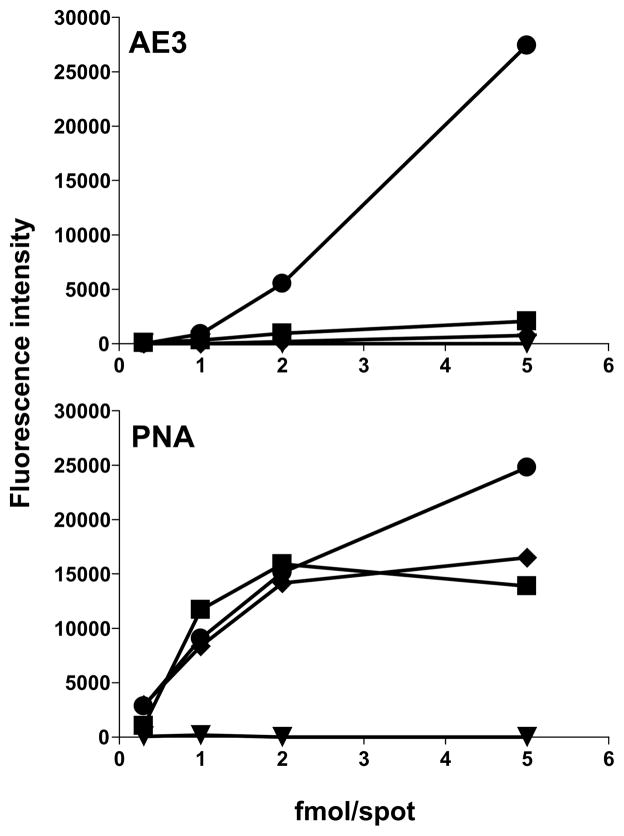
For a closer comparison of the specificities of antibody AE3 and PNA we carried out microarray analyses in dose-response format using asialo-GM1 (301), SM1a (303), SB1a (304), and GM1 (306) glycolipids (Fig. 3). Here also, neither AE3 nor PNA elicited binding signals with SB1a. In its strong preference for SM1a, antibody AE3 clearly differed from PNA, which gave equivalent binding to SM1a, GM1 and asialo-GM1 except at the highest dose tested (5 fmol) where the binding to SM1a was stronger.
Fig. 3.
Microarray dose-response analyses of the binding of antibody AE3 and PNA to asialo-GM1 (■), SM1a (●), SB1a (◆) and GM1 (▼), printed at 0.3, 1, 2 and 5 fmol/spot.
Taken together the results show, first, that antibody AE3 recognizes an antigen that is expressed on a glycolipid as well as on mucin-type glycoproteins; second, the specificity of the AE3 is distinct from those of the plant lectins PNA and RCA120; and third, AE3 has strong selectivity for the carbohydrate moiety of the glycolipid SM1a, a member of the ganglio series glycosphingolipids that lacks sialic acid but instead has a sulfate at position C3 of the inner galactose residue.
This is to our knowledge the first report of a glycolipid carrier of HCA, and it illustrates the power of modern carbohydrate microarray technology with an increasing repertoire of sequence-defined oligosaccharide probes to discover hitherto unsuspected ligands and antigenic determinants. In early studies, several of the other IgM antibodies that recognize HCA had been examined for binding to the glycolipids GM3, GD3, GM2, GM1, Fuc-GM1, GT1b, GD1a or GD1, with negative results [3].
Our results now provide an explanation for the observation [4] that the disaccharide Galβ1-3GalNAc, corresponding to the outer disaccharide sequence of SM1a, can inhibit, at high concentrations, the binding of antibody AE3 to epiglycanin. Our results moreover highlight the importance of the non-substituted outer galactose residue for the recognition, as its substitution with a sulfate at position C3, as in the di-sulfated analog SB1a, masks the AE3 antigen.
Sulfated sequences have been reported on glycolipids as well as on mucin type glycoproteins of normal and neoplastic epithelia and on endothelia in humans [16–19]. It is interesting that the carbohydrate sequence of SM1a has not been reported among these to our knowledge. We review briefly below the salient information.
Kidney epithelia contain a variety of sulfated glycolipid analogs of gangliosides and their levels increase markedly in renal cell carcinoma concomitantly with enhanced activity of glycolipid sulfotransferase in the cancer tissues [20;21]. This enhancement was reported to involve protein kinase C and tyrosine kinase signalling pathways [22]. There are also reports of the occurrence of increased levels of sulfated glycolipids in human colon cancer cells [23;24]. These are largely of the lactosamine series with sulfate at position C3 of terminal galactose or at position C6 of subterminal N-acetylglucosamine. The accumulation of sulfated glycolipids has also been reported in human hepatocellular carcinomas [25;26]. These are of the ganglio series, and among them, the di-sulfated glycolipid SB1a has been shown to behave as a tumour associated antigen [25]. Thus far, SM1a has been described only as minor component in rat kidney [27] and in a monkey kidney cell line (Verots S3) and postulated to be a precursor of the di-sulfated glycolipid SB1a [20]. Thus our assignment of SM1a as the cancer-associated antigen recognized by antibody AE3 is a novel finding.
As for sulfate modifications of O-glycans of mucin type glycoproteins, these have been identified at position C3 of galactose or at C6 of N-acetylglucosamine on human fetal mucins (meconium), also at position C3 of galactose on mucins of normal adult small intestine [28] and descending colon [18], and on an ovarian cystadenoma glycoprotein [29]. Sulfotransferase activities toward peripheral galactose residues on O-glycans were described to be upregulated in human colon and breast cancer cell lines [30]. These were 3-O sulfotransferase activities toward the core 3 sequence, Galβ1-3GalNAc, and the lactosamine and globo sequences, Galβ1-4GlcNAc, and Galβ1-3GalNAcβ1-3Gal, respectively.
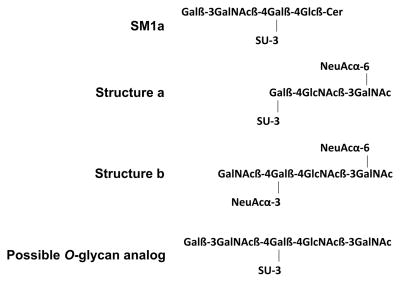
Oligosaccharide sequences related to that of SM1a and based on the core 3 have been described among O-glycans of glycoproteins in normal descending colon: structures a and b (Scheme 1) [18]. This leads us to predict that an O-glycan analog of SM1a with a sulfate on an inner galactose and with an outer Galβ1-3GalNAc sequence (Scheme 1) would be among antigen-positive glycans of HCA and the other glycoproteins bound by antibody AE3.
Scheme 1.
A SM1a analog as depicted in Scheme 1 would represent a novel sequence on O-glycans, and is under current investigation by the ‘designer’ microarray approach, the term we have coined for arrays from targeted ligand- or antigen-positive glycoconjugates [13], this time from HCA-positive glycoproteins. If this sequence is corroborated on tumor-associated mucins, questions will arise as to the enzymes involved in the sulfation and chain extension in neoplastic epithelia.
Studies of the involvement of the SM1a-related sequence(s) in the behavior of the epithelial cancer cells, and the possible exploitation of the antigen as a serologic cancer biomarker are among topics for future studies. Now that a specific antigenic structure has been identified, the way is open to serologic studies, to determine if the antigen elicits an autoantibody response in early (non-metastatic) cancer or if it is shed and immunochemically detectable in advanced disease.
Supplementary Material
Acknowledgments
The authors thank Donna Peehl for valuable discussions, Zeqi Zhou for providing the antibody AE3. The late Ineo Ishizuka is acknowledged for the SM1a and related glycolipids. This work has been supported by NCI Alliance of Glycobiologists for Detection of Cancer and Cancer Risk (U01 CA128416); UK Research Councils’ Basic Technology Initiative ‘Glycoarrays’ (GRS/79268) and EPSRC Translational Grant (EP/G037604/1). ASP is a fellow of the Fundação para a Ciência e Tecnologia (SFRH/BPD/26515/2006, Portugal).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thingstad T, Haavik S, Hansen K, Sletten K, Codington JF, Barsett H. Human carcinoma-associated antigen (HCA), isolated from the endometrial carcinoma cell line KLE-1 and ascitic fluid of a patient with ovarian carcinoma; comparison with epiglycanin. Eur J Pharm Sci. 1998;6:121–129. doi: 10.1016/s0928-0987(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 2.Codington JF, Sanford BH, Jeanloz RW. Glycoprotein coat of the TA3 cell. Isolation and partial characterization of a sialic acid containing glycoprotein fraction. Biochemistry. 1972;11:2559–2564. doi: 10.1021/bi00764a001. [DOI] [PubMed] [Google Scholar]
- 3.Haavik S, Codington JF, Davison PF. Development and characterization of monoclonal antibodies against a mucin-type glycoprotein. Glycobiology. 1992;2:217–224. doi: 10.1093/glycob/2.3.217. [DOI] [PubMed] [Google Scholar]
- 4.Haavik S, Nilsen M, Thingstad T, Barsett H, Renouf DV, Hounsell EF, Codington JF. Specificity studies of an antibody developed against a mucin-type glycoprotein. Glycoconj J. 1999;16:229–236. doi: 10.1023/a:1007080405162. [DOI] [PubMed] [Google Scholar]
- 5.Itoh Y, Kamata-Sakurai M, da-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, Okada K, Goto A, Fukayama M, Irimura T. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74–83. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]
- 6.Yi Y, Kamata-Sakurai M, da-Nagai K, Itoh T, Okada K, Ishii-Schrade K, Iguchi A, Sugiura D, Irimura T. Mucin 21/epiglycanin modulates cell adhesion. J Biol Chem. 2010;285:21233–21240. doi: 10.1074/jbc.M109.082875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou DX, Wang H, Zhou H, Li S, Qu HX, Kou X, Li J, Wang H, Hu HP. Over-expression of human carcinoma-associated antigen in intrahepatic cholangiocellular carcinoma. Biochem Biophys Res Commun. 2011 doi: 10.1016/j.bbrc.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Codington JF, Haavik S, Nikrui N, Kuter I, Vassileva C, Zhang H, Matson S, Chen X, Wu Z. Immunologic quantitation of the carcinoma specific human carcinoma antigen in clinical samples. Cancer. 2002;94:803–813. doi: 10.1002/cncr.10124. [DOI] [PubMed] [Google Scholar]
- 9.Li RS, Yao JL, Bourne PA, di Sant’Agnese PA, Huang JT. Frequent expression of human carcinoma-associated antigen, a mucin-type glycoprotein, in cells of prostatic carcinoma. Archives of Pathology & Laboratory Medicine. 2004;128:1412–1417. doi: 10.5858/2004-128-1412-FEOHCA. [DOI] [PubMed] [Google Scholar]
- 10.Yao JL, Bourne PA, Yang Q, Lei J, di Sant’Agnese PA, Huang J. Overexpression of human carcinoma-associated antigen in urothelial carcinoma of the bladder. Arch Pathol Lab Med. 2004;128:785–787. doi: 10.5858/2004-128-785-OOHCAI. [DOI] [PubMed] [Google Scholar]
- 11.Liang S, Yao J, Bourne PA, di Sant’Agnese PA, Huang J, Lei JY. Overexpression of human carcinoma-associated antigen in esophageal adenocarcinoma and its precursor lesions. Am J Clin Pathol. 2004;122:747–751. doi: 10.1309/02AP-A6AP-GL43-GCTR. [DOI] [PubMed] [Google Scholar]
- 12.Feizi T. Neoglycolipid Technology - An approach to deciphering the information content of the glycome. In: Teeri TT, Svensson B, Gilbert HJ, Feizi T, editors. Carbohydrate Bioengineering: Interdisciplinary Approaches. The Royal Society of Chemistry; 2002. pp. 186–193. [Google Scholar]
- 13.Palma AS, Feizi T, Zhang Y, Stoll MS, Lawson AM, Diaz-Rodríguez E, Campanero-Rhodes AS, Costa J, Brown GD, Chai W. Ligands for the beta-glucan receptor, Dectin-1, assigned using ‘designer’ microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J Biol Chem. 2006;281:5771–5779. doi: 10.1074/jbc.M511461200. [DOI] [PubMed] [Google Scholar]
- 14.Palma AS, Liu Y, Muhle-Goll C, Butters TD, Zhang Y, Childs R, Chai W, Feizi T. Multifaceted approaches including neoglycolipid oligosaccharide microarrays to ligand discovery for malectin. Methods Enzymol. 2010;478:265–286. doi: 10.1016/S0076-6879(10)78013-7. [DOI] [PubMed] [Google Scholar]
- 15.Stoll MS, Feizi T. In: Kettner C, editor. Software tools for storing, processing and displaying carbohydrate microarray data; Proceeding of the Beilstein Symposium on Glyco-Bioinformatics; 4–8 October, 2009; Potsdam, Germany. Frankfurt, Germany: Beilstein Institute for the Advancement of Chemical Sciences; 2009. pp. 123–140. [Google Scholar]
- 16.Ishizuka I. Chemistry and functional distribution of sulfoglycolipids. Prog Lipid Res. 1997;36:245–319. doi: 10.1016/s0163-7827(97)00011-8. [DOI] [PubMed] [Google Scholar]
- 17.Capon C, Leroy Y, Wheruszeski JM, Ricardi G, Strecker G, Montreuil J, Fournet B. Structures of O-glycosidically linked oligosaccharides isolated from human meconium glycoproteins. Eur J Biochem. 1989;182:139–152. doi: 10.1111/j.1432-1033.1989.tb14810.x. [DOI] [PubMed] [Google Scholar]
- 18.Capon C, Maes E, Michalski JC, Leffler H, Kim YS. Sd(a)-antigen-like structures carried on core 3 are prominent features of glycans from the mucin of normal human descending colon. Biochem J. 2001;358:657–664. doi: 10.1042/bj3580657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen SD. L-Selectin and its biological ligands. Histochemistry. 1993;100:185–191. doi: 10.1007/BF00269091. [DOI] [PubMed] [Google Scholar]
- 20.Niimura Y, Ishizuka I. Isolation and identification of nine sulfated glycosphingolipids containing two unique sulfated gangliosides from the African green monkey kidney cells, Verots S3, and their possible metabolic pathways. Glycobiology. 2006;16:729–735. doi: 10.1093/glycob/cwj114. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Honke K, Kamio K, Sakakibara N, Gasa S, Miyao N, Tsukamoto T, Ishizuka I, Miyazaki T, Makita A. Sulfolipids and glycolipid sulfotransferase activities in human renal cell carcinoma cells. Br J Cancer. 1993;67:76–80. doi: 10.1038/bjc.1993.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honke K, Tsuda M, Hirahara Y, Miyao N, Tsukamoto T, Satoh M, Wada Y. Cancer-associated expression of glycolipid sulfotransferase gene in human renal cell carcinoma cells. Cancer Res. 1998;58:3800–3805. [PubMed] [Google Scholar]
- 23.Misonou Y, Shida K, Korekane H, Seki Y, Noura S, Ohue M, Miyamoto Y. Comprehensive clinico-glycomic study of 16 colorectal cancer specimens: elucidation of aberrant glycosylation and its mechanistic causes in colorectal cancer cells. J Proteome Res. 2009;8:2990–3005. doi: 10.1021/pr900092r. [DOI] [PubMed] [Google Scholar]
- 24.Shida K, Misonou Y, Korekane H, Seki Y, Noura S, Ohue M, Honke K, Miyamoto Y. Unusual accumulation of sulfated glycosphingolipids in colon cancer cells. Glycobiology. 2009;19:1018–1033. doi: 10.1093/glycob/cwp083. [DOI] [PubMed] [Google Scholar]
- 25.Hiraiwa N, Iida N, Ishizuka I, Itai S, Shigeta K, Kannagi R, Fukuda Y, Imura H. Monoclonal antibodies directed to a disulfated glycosphingolipid, SB1a (GgOse4Cer-II3IV3-bis-sulfate), associated with human hepatocellular carcinoma. Cancer Res. 1988;48:6769–6774. [PubMed] [Google Scholar]
- 26.Hiraiwa N, Fukuda Y, Imura H, Tadano-Aritomi K, Nagai K, Ishizuka I, Kannagi R. Accumulation of highly acidic sulfated glycosphingolipids in human hepatocellular carcinoma defined by a series of monoclonal antibodies. Cancer Res. 1990;50:2917–2928. [PubMed] [Google Scholar]
- 27.Tadano K, Ishizuka I. Isolation and characterization of sulfated gangliotriaosylceramide from rat kidney. J Biol Chem. 1982;257:1482–1490. [PubMed] [Google Scholar]
- 28.Robbe-Masselot C, Maes E, Rousset M, Michalski JC, Capon C. Glycosylation of human fetal mucins: a similar repertoire of O-glycans along the intestinal tract. Glycoconj J. 2009;26:397–413. doi: 10.1007/s10719-008-9186-9. [DOI] [PubMed] [Google Scholar]
- 29.Yuen CT, Lawson AM, Chai W, Larkin M, Stoll MS, Stuart AC, Sullivan FX, Ahern TJ, Feizi T. Novel sulfated ligands for the cell adhesion molecule E-selectin revealed by the neoglycolipid technology among O-linked oligosaccharides on an ovarian cystadenoma glycoprotein. Biochemistry. 1992;31:9126–9131. doi: 10.1021/bi00153a003. [DOI] [PubMed] [Google Scholar]
- 30.Chandrasekaran EV, Xue J, Neelamegham S, Matta KL. The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: a predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydr Res. 2006;341:983–994. doi: 10.1016/j.carres.2006.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.