INTRODUCTION
The ability to identify bitter tastants in humans depends on a small gene family that encodes approximately twenty-five bitter taste receptors (1,2). These receptors contribute to the large variations in human bitter taste perception (1,3). One important variation in taste perception is the ability to identify the bitter tasting compounds phenylthiocarbamide (PTC) and 6-n-propyl-thiouracil (PROP). These two chemically related thiourea compounds are perceived as bitter by some individuals, but appear tasteless or nearly tasteless to others (4,5). The frequency of tasters for PROP and PTC may vary by both race and ethnicity (3,6,7). The estimated frequency of PROP/PTC tasters among Caucasians is approximately 70% (3,7), while estimated frequencies of PROP/PTC tasters for Chinese, Japanese, and sub-Saharan African populations are generally higher with estimates that vary between 80 and 90% (3,6,7) However, PROP taster status may be less than 50% in some subgroups that have been studied in India (7). Although both compounds exhibit similar properties, PROP is generally used for taste blindness studies because this compound lacks the sulfurous odor of PTC, and does not exhibit the toxicity that is associated with PTC (8,9).
The genetics of PROP is considered to be intermediate between a Mendelian trait and a complex trait (1). Although other loci have been proposed (10,11), the TAS2R38 (T2R38) gene on the long arm of human chromosome 7 is thought to be primarily responsible for detecting the bitter taste of both PTC and PROP in humans (1,10,12). Genomic PCR analysis has further demonstrated that the human TAS2R38 gene exhibits single nucleotide polymorphisms at five different sites, which result in seven known haplotypes (1). These haplotypes likely explain the wide variability in PTC and PROP taste perception (1). Each single nucleotide polymorphism encodes a different amino acid in the receptor protein (at amino acid positions 49, 262, 296, as well as 80 (rare), and 274 (rare). Of the seven haplotypes, two predominant forms exist at high frequency outside of sub-Saharan Africa (13). These two major haplotypes are generally responsible for the taster and non-taster phenotypes for PTC and PROP. The predominant taster form of the TAS2R38 receptor contains a proline at amino acid position 49, an alanine at position 262, and a valine at position 296 (the PAV haplotype). In contrast, the most common non-taster form of the receptor expresses an alanine, a valine, and an isoleucine at these same three amino acid positions (the AVI haplotype). Individuals who express either one or two PAV-containing alleles are tasters of PROP and PTC, while individuals who express two AVI-containing alleles are non-tasters of both PROP and PTC (1,13,14). In the absence of the PAV allele, AAI, PVI, and AAV haplotypes may confer intermediate taste sensitivities to both PTC and PROP (9,13). Finally, the two rare haplotypes (ARARI and AHACI) have not been phenotypically characterized. Due to their high frequencies, PAV is considered the major taster haplotype while AVI is considered the major non-taster haplotype. These two predominant haplotypes account for the bimodal distribution for PTC and PROP sensitivity in humans (14).
The examination of PROP taster status may be important for human health and nutrition. The ability to taste PROP has been associated with the rejection of other bitter tasting compounds, along with the avoidance of bitter-tasting fruits and vegetables (15). These bitter tasting foods include raw cruciferous vegetables such as cabbage, broccoli and Brussels sprouts (15). Many of these foods are important sources of phytonutrients and antioxidants, both of which may reduce the risk of cancer and other chronic diseases (16). Finally, PTC tasters may have enhanced protection against the overconsumption of bitter tasting dietary goitrogens. These individuals may be less likely to suffer from the formation of goiters in areas where dietary iodine is low, when compared to PTC nontasters (17).
However, current laboratory-based methods for assessing PROP sensitivity are not useful for use in large-scale population studies. One limitation to more fully understanding the role of bitter taste and taste blindness in human health and nutrition is the lack of a simple, rapid, and reliable method for characterizing PROP taster status for epidemiological studies. In general, aqueous solutions or impregnated filter papers are used to present PTC or PROP to the test subject (3,8,18). The use of impregnated filter paper may produce unacceptable proportions of false negative responses (8,19), and may also give limited agreement with the classification of tasters when identified by taste recognition thresholds (20).
The main goal of this study is to determine the sensitivity of PROP taste recognition thresholds when this thioamide is delivered to the oral cavity via edible taste strips. The development of edible taste strips for both threshold and suprathreshold studies will allow the rapid evaluation of PROP taster status in large populations. This approach will in turn be useful for identifying the proposed role of PROP as a broad indicator of taste perception and food selection in humans (3,21). The use of edible taste strips will further allow the rapid examination of PROP phenotypes for comparison to TAS2R38 haplotypes.
MATERIALS AND METHODS
Preparation of Edible Taste Strips
Edible taste strips were prepared as previously described (22). Pullulan (α-1,4-; α-1,6-glucan) was obtained from NutriScience Innovations, LLC (Trumball, CT). Pullulan was combined with the polymer hydroxypropyl methylcellulose (HPMC) (a gift from Dow Chemical Co., Midland, MI) at a weight ratio of 11.5:1. For preparation of edible films, HPMC powder was first added to a rapidly stirred solution of distilled water at 75 to 80° C, followed by addition of pullulan powder to yield a final concentration of 3.25% wt/vol of polymers. The mixture was cooled to room temperature, food coloring was added to aid in visualization of strips, and then tastant was added. Films were poured onto non-stick surfaces, dried, and cut into one inch squares. Strips were stored in the dark at 4 °C. Control strips were prepared as described above except that no tastant was included. PROP was obtained from Sigma Chemical Co. (St. Louis, MO), and food coloring (McCormick & Co., Hunt Valley, MD) was obtained from a local supermarket.
Test Subjects
Individuals were recruited through posted flyers and by word of mouth from Temple University and the surrounding community. A total of 81 healthy volunteers (39 men and 42 women) ranging in age from 18 to 64 years (average age of 25.2, SD = +/− 10.1 years) participated in this study. Approximately 55% of subjects were of Asian or South Asian descent, 35% of subjects were Caucasian, and 10% were of Black/African descent. Seventy-nine participants were nonsmokers, and each subject was asked to refrain from eating or drinking (except water) for a minimum of 30 minutes before their scheduled session. All subjects were healthy by self-report, and no subjects had been previously diagnosed with diabetes or any neurological disorder that would compromise their taste function. In addition, no test subjects had visited a dentist in the previous 48 hours. The Temple University Human Subjects Review committee fully approved the experimental protocol. All subjects provided informed written consent, and were reimbursed for their participation.
Threshold measurements
Subjects were instructed to place an edible taste strip on their tongue, touch the roof of their mouth, and wait five seconds before giving a response (sweet, sour, salty, bitter, or no taste). Subjects practiced with a control strip before the start of the experiment.
Taste recognition thresholds were determined by two separate protocols. One threshold procedure utilized a single series ascending method of limits, with PROP increasing in fifth log steps. In this protocol, taste stimuli were presented in triads (1 stimulus and 2 blanks). The stimulus series was begun with a low amount of tastant in the strip, and the task of the subject was to report which one of the three stimuli differed from the other two, and to correctly identify its taste quality as sweet, sour, salty, or bitter. When a miss occurred within a triad, a subsequent triad was presented at the next higher stimulus amount. When the first triad was performed correctly, an additional triad was presented where the amount of taste stimulus remained the same. If correct performance again occurred, a third triad was presented at the same stimulus concentration. The stimulus amount where three successful triads occurred (where all 3 successful triads contained the same stimulus amount) was taken as the threshold value if one additional triad was successfully performed that contained the next higher amount of tastant. The probability of correctly guessing the strip with tastant in four successive triads (12 presentations) was 0.01.
Taste recognition thresholds were also measured by the method of reversals according to Pribitkin et al. (23), except that each trial of two strips was presented in duplicate. A two-alternative staircase procedure was used to determine PROP thresholds. A reversal was defined as either an increase in PROP (fifth log step) if one or both choices in a trial was incorrect, or a decrease in the amount of PROP (fifth log step) if two sequential trials at the same amount of PROP were correct (four up, one down). The threshold for PROP was calculated as the geometric mean of the last four of five reversals. Subjects were tested on different days for the two PROP threshold procedures. For statistical analysis, the Mann-Whitney U test was used as the non-parametric significance test for both the ascending limits method and the method of reversals.
In order to examine taste function in the absence of olfactory cues, subjects were also tested for PROP taste thresholds where airflow through the nose was fully blocked by plastic clamps (Phipps and Bird, Richmond, VA). The method of ascending limits was used for these threshold measurements.
Genotyping
Genotyping of TAS2R38 at three polymorphic sites was carried out at the Monell Chemical Senses Center (Philadelphia, PA). Genomic DNA was obtained from cheek cells with a sterile swab (Epicentre Biotechnologies, Madison, WI). Genomic DNA was amplified using TaqMan primers (Applied Biosystems, Foster City, CA). Alleles of TAS2R38 were genotyped at each of the three variant single nucleotide sites using allele-specific fluorescent probes from Applied Biosystems (reference SNP numbers included A49P = rs713598, V262A = rs1726866, and I296V = rs10246939). The ability of these nucleotide probes to fully hybridize to genomic DNA resulted in the loss of quenching of the terminal fluorophore, and was the criterion for identifying the specific nucleotide at each polymorphic site.
RESULTS
Taste Recognition Thresholds
All subject data was included in analyses except as described below. During the early stages of testing, one subject detected PROP at the lowest amount (5.2 nanomoles). Two lower PROP amounts (1.6 and 4.1 nanomoles) were then added to the threshold study, and this subject (along with all subsequently tested subjects) was tested with strips that included lower amounts of PROP. Additionally, one subject misunderstood the directions for the study, and was retested at a later date. The second set of threshold results for this subject was used for data analysis.
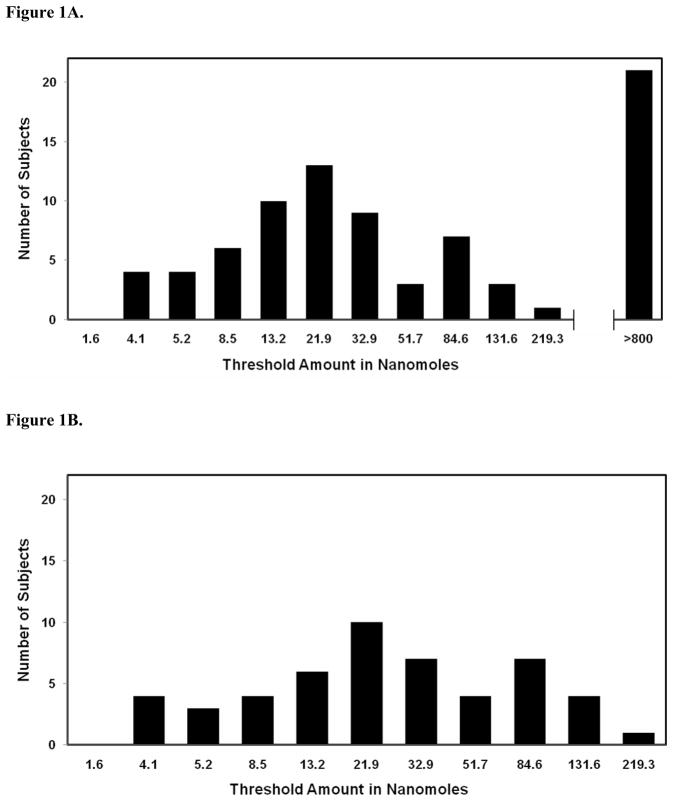
Based on threshold distributions obtained from the ascending method of limits, sixty subjects were determined to be PROP tasters (74%) and twenty-one subjects (26%) were identified as non-tasters. Figure 1 shows the threshold distribution of PROP tasters by the method of ascending limits. The average taste recognition threshold amount for PROP was 36.1 nanomoles, and this distribution of PROP tasters formed a bell-shaped curve (see Fig 1A). In our population of PROP tasters, the threshold range varied from 4.1 to 219.3 nanomoles of PROP, with the majority of thresholds well below 100 nanomoles. No statistical differences in mean values were observed between males and females in this group (41.6 nanomoles and 29.6 nanomoles respectively, P >= 0.05, two-tailed test). The cumulative frequency distribution of thresholds for PROP tasters obtained by the method of ascending limits produced a hyperbolic plot that spanned less than two orders of magnitude, and displayed a R2 value of 0.93 (see Figure 1C).
Figure 1.
Human taste recognition thresholds for PROP that were obtained with edible taste strips. Various amounts of PROP were incorporated into one-inch square edible taste strips, and taste recognition thresholds were determined by the ascending limits method. (A). Taste recognition thresholds with unobstructed airflow through the nasal cavity. Note that the horizontal axis of the graph is non-linear. (B). Taste recognition thresholds for PROP tasters when airflow through the nose is completely blocked. (C). Cumulative frequency distribution of taste recognition thresholds for PROP with unobstructed air flow through the nasal cavity. R2 = 0.93, where R2 is the coefficient of determination. (D). Cumulative frequency distribution of taste recognition thresholds for PROP when airflow through the nose is blocked. R2 = 0.97.
PROP taste recognition thresholds when nasal air flow is blocked
If volatilization of a tastant occurs in the oral cavity, then blocked airflow through the nasopharynx would minimize odor release, and would likely raise thresholds in subjects with normal chemosensory function. A subgroup of PROP tasters was retested for taste recognition thresholds when airflow through the nose was completely blocked (n = 50). The method of ascending limits was used to measure taste recognition thresholds in the absence of nasal airflow. Figure 1B shows a graph of PROP taste recognition thresholds for individuals whose airflow through the nose was completely blocked. The average threshold value was 42.6 nanomoles. Although not statistically significant, this value was slightly above the average taste recognition threshold obtained with normal airflow through the nasopharynx. In our study, 26 of 50 subjects had identical taste recognition thresholds in both the presence and absence of nasal airflow.
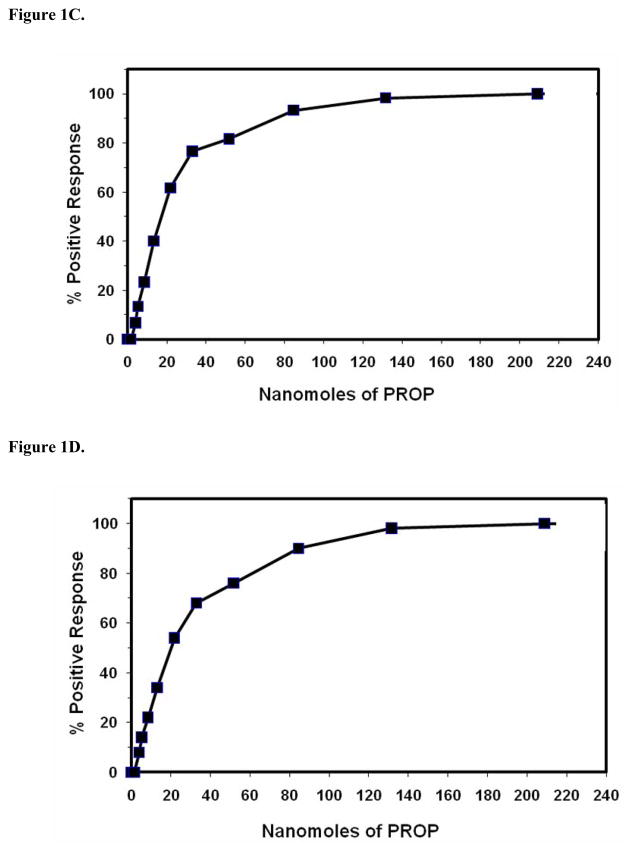
The threshold for females was 36.7 nanomoles, and was 48.4 nanomoles for males. Again, no statistical differences in thresholds were observed by sex (P >= 0.05, two-tailed test). The cumulative frequency distribution of PROP taste recognition thresholds measured in the presence of nose clamps was similar to the frequency distribution in the absence of nose clamps, and no statistical differences in thresholds were observed in the absence or presence of nose clamps (P >= 0.05, two-tailed test. See Fig 1D). However, the amount of PROP that represented the value where 50% of test subjects could detect the bitter taste of PROP was lower in the absence of nose clamps (half maximal value of ~17 nanomoles) than in the presence of nose clamps (half maximal value of ~20 nanomoles). These results suggest that some minimal volatilization of PROP occurred in the oral cavity when this compound was presented by means of edible taste strips. Thus, PROP taste strips primarily measure gustatory cues, with some minimal contribution of olfactory stimulation when the strips dissolve in the oral cavity. See Figure 2 for a direct comparison of PROP taste recognition thresholds for individuals who were tested in either the absence or presence of normal airflow through the nasal passages.
Figure 2.
Comparison of PROP taste recognition thresholds in the presence and absence of airflow through the nasal cavity (n =50 subjects). Taste recognition thresholds were detected by the method of ascending limits (R2 = 0.82).
Method of Reversals
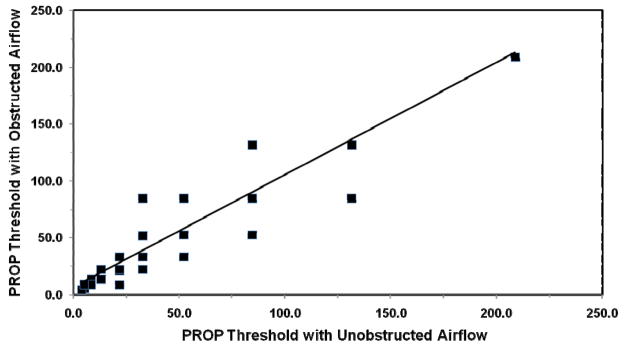
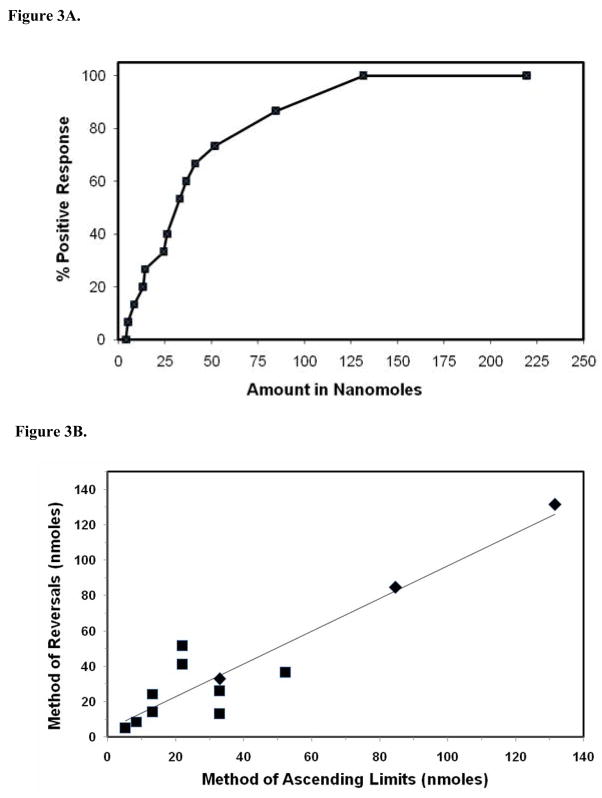
Next, taste recognition thresholds for PROP were determined by the method of reversals in 15 individuals who had previously been tested by the method of ascending limits. As shown in Figure 3, thresholds varied between 5.2 and 131.6 nanomoles of PROP, and taste recognition thresholds again formed a bell-shaped curve. These taste recognition thresholds were then compared to threshold results obtained by the method of ascending limits for these same 15 individuals. Figure 3 compares threshold data for these subjects whose thresholds were identified by either protocol (R2 = 0.92).
Figure 3.
PROP taste recognition thresholds obtained by the method of reversals during normal airflow through the nasal cavity. (A). Cumulative frequency of PROP taste recognition threshold by the method of ascending limits (R2 for a logarithmic curve = 0.96). (B). Comparison of PROP taste recognition thresholds by the method of ascending limits, and the method of reversals (n = 15 subjects). Diamonds represent two identical data points in the graph.
TAS2R38 Genotypes
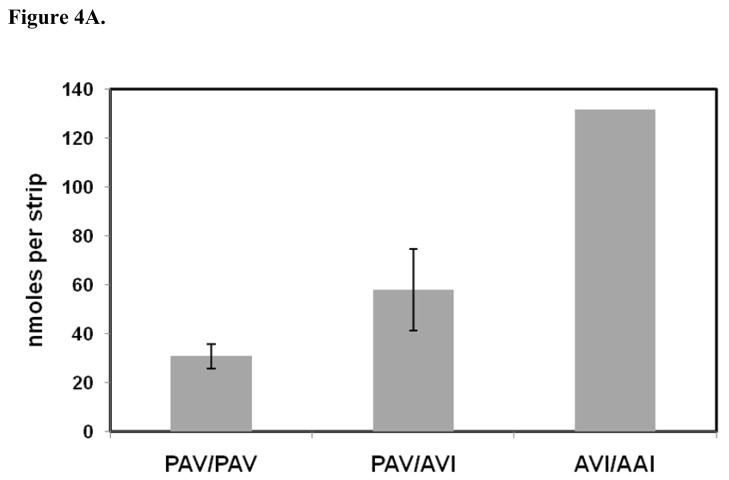
Since a close association between absolute detection thresholds and TAS2R38 haplotypes has been reported (9), genotype data for the TAS2R38 gene was obtained for 44 subjects who had been previously tested for PROP taste recognition thresholds. The genetic data were used to determine whether or not taste recognition thresholds obtained with PROP taste strips could discriminate between PAV homozygotes and PAV/AVI heterozygotes. Secondly, genotype data could determine whether or not AVI homozygotes were PROP non-tasters when this taste stimulus was presented by means of edible strips. In this study, all PAV/PAV and PAV/AVI test subjects could detect PROP in strips at thresholds at or below 131.6 nanomoles (see Figure 4). PAV/PAV individuals on average tended to have lower thresholds for PROP when compared to PAV/AVI test subjects (see Fig 4A). Also, PAV/PAV test subjects reported taste recognition thresholds that occurred over a narrower range when compared to PAV/AVI test subjects. However, taste recognition thresholds for the two genotypes were not statistically significant (P >= 0.05, two-tailed test).
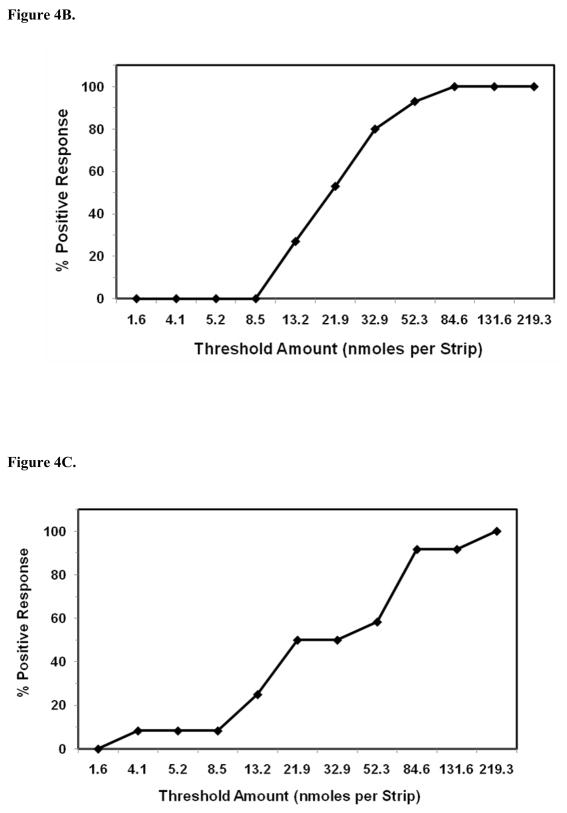
Figure 4.
Comparison of PROP taste recognition thresholds to TAS2R38 genotypes. (A). Genotypes for PAV/PAV (n = 12), PAV/AVI (n = 15), and AVI/AAI (n = 1) subjects. PROP thresholds for AVI/AVI non-tasters (n = 12) is not shown. (B). Cumulative frequency distribution of taste recognition thresholds for PROP as a function of PAV/PAV genotype. (C). Cumulative frequency distribution of taste recognition thresholds for PROP as a function of PAV/AVI genotype.
Almost all AVI/AVI subjects were unable to detect the bitter taste of PROP. A clear separation of AVI/AVI subjects from PAV/AVI and PAV/PAV subjects was observed by our method of threshold analysis. However, at least one of the 15 AVI/AVI subjects in this study was able to detect the bitter taste of PROP. This subject could identify both 800 and 1000 nanomole PROP strips as bitter in a forced-choice test. In addition, a second subject [PROP taster] who was initially identified as AVI/AVI was genotyped a second time. Probe rs713598 (A49P) yielded a weak signal, which suggested an unusual amino acid at position 49 for both TAS2R38 alleles from this individual.
Finally, two subjects expressed rare TAS2R38 genotypes in this study. One subject expressed an AVI/AAI genotype, and this participant had a higher taste recognition threshold for PROP than either the PAV/PAV or PAV/AVI subjects (Fig 4A). A second subject expressed an AVI/AAV genotype. This subject was a non-taster, and could not detect the bitter taste of PROP at amounts as high as 1000 nanomoles per taste strip.
DISCUSSION
Taste recognition thresholds represent the lowest amount for which a taste quality can be correctly identified. Measurements of PROP thresholds are one method to separate taster and nontaster phenotypes. This separation can be based on a positive taste response at the antimodal concentration (8), or can be based on comparison of PROP thresholds to an antimodal cutoff concentration (24).
Our studies demonstrate that edible taste strips are readily adapted to studies on taste recognition thresholds, and for studies on taste blindness. Edible strips should also have application to other methods of threshold analysis of taste, such as the modified Harris-Kalmus procedure (25). Our studies further show that the method of ascending limits and the method of reversals yield almost identical taste recognition thresholds for PROP. If this observation is true for other taste stimuli, then taste recognition thresholds can be readily obtained by either the method of ascending limits or by the method of reversals. In our hands, the method of ascending limits requires fewer trials, and results in a shorter testing time. This shorter testing time may be an important concern when testing younger children, older subjects, subjects with neurological disorders, or subjects with a shortened attention span.
These findings also indicate that edible taste strips can be used to detect taste recognition thresholds for PROP at amounts lower than published threshold values from impregnated filter papers (23), and equal to or lower than published values obtained with aqueous solutions (9). Thus, edible taste strips permit high sensitivity for detecting the bitterness of PROP, with taste recognition thresholds generally occurring over a narrower range when compared with other methods.
Based on TAS2R38 genotype data, taste recognition thresholds that were obtained with edible taste strips could predict taster-nontaster status with excellent accuracy. The insensitivity of the majority of homozygous AVI subjects to 800 nanomoles of PROP is best explained by the observed lack of a response of the AVI haplotype to PROP in the majority of our subjects. This lack of response might be due to diminished binding of PROP to the TAS2R38 receptor, modified conformational changes of the receptor upon ligand binding, and/or a diminished interaction of the receptor with the stimulatory GTP-binding protein(s) that is involved in bitter taste transduction. However, at least one AVI/AVI individual could detect PROP in strips as mildly bitter at high amounts (800 nanomoles or greater). Recent in vitro experiments have demonstrated that a small number of AVI/AVI individuals may depend on an additional TAS2R gene or genes to perceive the bitter taste of PTC or PROP (9). Finally, papillae density may further modify bitter taste perception in some AVI/AVI individuals (26).
Only one subject expressed the rare AAI allele, and this individual showed one of the highest threshold values observed in this study. This observation further suggests that the rare AAI haplotype in AVI/AAI subjects is less sensitive to PROP than PAV homozygotes or PAV/AVI heterozygotes (9). Along with data obtained from the confirmed AVI/AVI individual in this study, these results further indicate that a single TAS2R38 PAV allele may not be necessary for PROP taster status.
Some phenotypic variation of subjects with either a PAV/PAV or a PAV/AVI genotype was observed for taste recognition thresholds. The greater variation in thresholds and slightly higher average threshold value observed in subjects expressing PAV/AVI when compared to PAV/PAV genotypes may be due to the expression of only one taster allele, which may decrease expression levels of functional receptor. One possibility is that the TAS2R38 gene may show incomplete penetrance where the expression of two taster alleles, rather than one allele, could more narrowly define taste threshold values. Variations in plasma membrane TAS2R38 receptor protein levels could also affect the number of functional TAS2R38 receptors per taste receptor cell, or possibly the number of taste receptor cells that express the TAS2R38 receptor in a taste bud. These variations could in turn modulate taste recognition thresholds.
The results from this subject population indicate that taste recognition thresholds that are obtained with PROP strips cannot conclusively separate individuals with PAV/PAV from PAV/AVI genotypes. However, a somewhat greater variability in thresholds, and a greater range in thresholds, was observed for our population of PAV/AVI heterozygotes when compared to PAV homozygotes. Overall, these results indicate that threshold studies with edible taste strips are a valid means to identify PROP taster status in the human population. PROP strips should be useful for examining how PROP taster status may affect the ability to perceive other taste stimuli.
Edible taste strips do not require the preparation and storage of solutions or the impregnation of insoluble filter paper strips for testing. As opposed to the filter paper method, no biological hazardous waste is generated by testing with this method. Due to the ease of use of edible strips, these strips have potential for testing young children, as well as older adults. Finally, these edible taste strips may be useful for examining relevant clinical populations for the detection of subtle disturbances in bitter taste function.
CONCLUSION
Edible taste strips may be used to readily detect PROP thresholds at values that are equal to or lower than PROP solutions or PROP-impregnated filter papers. In addition, the forced choice method almost exclusively measured taste stimuli, with little or no contribution from olfactory stimuli. The comparison of threshold data with TAS2R38 genotype analysis validates the use of edible taste strips for identifying taste insensitivity to PROP in the human population. These edible taste strips should be useful for examining taste blindness, and subtle taste disturbances in the human population.
Acknowledgments
This work was supported by NIDCD 2R44 DC007291, and by a contract awarded to Richard C. Gershon from the institutes and centers that form the NIH Blueprint for Neuroscience Research (The NIH Toolbox for Neurological and Behavioral Function, contract# HHS-N-260-2006-00007-C). The authors thank Sahbina Ebba, M. Hakan Ozdener, Ray Abarintos, and Janis Zambrano for their valuable assistance. The authors also thank Danielle Reed and Fujiko Duke for assistance with genotyping, and Dow Chemical Company for the hydroxypropyl methylcellulose.
Footnotes
Conflict of Interest: None
BIBLIOGRAPHY
- 1.Kim U-K, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 2.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 3.Tepper BJ. Nutritional implications of genetic taste variation: The Role of PROP sensitivity and other taste phenotypes. Ann Rev Nutr. 2008;28:367–388. doi: 10.1146/annurev.nutr.28.061807.155458. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. Science news: Some advances in the sciences during 1931. Science (Suppl) 1931;74:14a. [Google Scholar]
- 5.Griffin F, Fischer R. Differential reactivity of saliva from ‘tasters’ and ‘non-tasters’ of 6-n-propylthiouracil. Nature. 1960;187:417–419. doi: 10.1038/187417b0. [DOI] [PubMed] [Google Scholar]
- 6.Guo SW, Shen FM, Wang YD, Zheng CJ. Threshold distributions of phenylthiocarbamide (PTC) in the Chinese population. Ann N Y Acad Sci. 1998;855:810–812. doi: 10.1111/j.1749-6632.1998.tb10664.x. [DOI] [PubMed] [Google Scholar]
- 7.Guo SW, Reed DR. The genetics of phenylthiocarbamide perception. Ann Hum Biol. 2001;28:111–142. doi: 10.1080/03014460151056310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawless H. A comparison of different methods used to assess sensitivity to the taste of phenylthiocarbamide (PTC) Chem Sens. 1980;5:247–256. [Google Scholar]
- 9.Bufe B, Breslin PAS, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim U-K, Drayna D, Meyerhof W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drayna D, Coon H, Kim U-K, Elsner T, Cromer K, Otterud B, Baird L, Peiffer AP, Leppert M. Genetic analysis of a complex trait in the Utah Genetic Reference Project: a major locus for PTC taste ability on chromosome 7q and a secondary locus on chromosome 16p. Hum Genet. 2003;12:567–572. doi: 10.1007/s00439-003-0911-y. [DOI] [PubMed] [Google Scholar]
- 11.Reed DR, Nanthakumar E, North M, Bell C, Bartoshuk LM, Price RA. Localization of a gene for bitter-taste perception to human chromosome 5p15. Am J Hum Genet. 1999;64:1478–1480. doi: 10.1086/302367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy VB, Davidson AC, Kidd JR, Kidd KK, Speed WC, Pakstis AJ, Reed DR, Snyder DJ, Bartoshuk LM. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res. 2004;28:1629–1637. doi: 10.1097/01.ALC.0000145789.55183.D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim U-K, Drayna D. Genetics of individual differences in bitter taste perception: lessons from the PTC gene. Clin Genet. 2005;67:275–280. doi: 10.1111/j.1399-0004.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- 14.Reed DR, Bartoshuk LM, Duffy V, Marino S, Price RA. Propylthiouracil tasting: determination of underlying threshold distributions using maximum likelihood. Chem Sens. 1995;20:529–533. doi: 10.1093/chemse/20.5.529. [DOI] [PubMed] [Google Scholar]
- 15.Kaminski LC, Henderson SA, Drewnowski A. Young women’s food preferences and taste responsiveness to 6-n-propylthiouracil (PROP) Physiol Behav. 2000;68:691–697. doi: 10.1016/s0031-9384(99)00240-1. [DOI] [PubMed] [Google Scholar]
- 16.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 17.Sandell MA, Breslin PAS. Variability in a taste-receptor gene determines whether we taste toxins in food. Curr Biol. 2006;16:R792–R794. doi: 10.1016/j.cub.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Tepper BJ, Christensen CM, Cao J. Development of brief methods to classify individuals by PROP taster status. Physio Behav. 2001;73:571–577. doi: 10.1016/s0031-9384(01)00500-5. [DOI] [PubMed] [Google Scholar]
- 19.Azevedo E, Krieger H, Mi MP, Morton NE. PTC taste sensitivity and endemic goiter in Brazil. Amer J Hum Genet. 1965;17:87–90. [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann G. Application of individual taste difference toward phenylthiocarbamide in genetic investigations. Ann Eugen. 1939;9:123–135. [Google Scholar]
- 21.Chang WI, Chung JW, Kim YK, Chung SC, Kho HS. The relationship between phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP) taster status and taste thresholds for sucrose and quinine. Arch Oral Biol. 2006;51:427–432. doi: 10.1016/j.archoralbio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Smutzer G, Lam S, Hastings L, Desai H, Abarintos RA, Sobel M, Sayed N. A test for measuring gustatory function. Laryngoscope. 2008;118:1411–1416. doi: 10.1097/MLG.0b013e31817709a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pribitkin E, Rosenthal MD, Cowart BJ. Prevalence and causes of severe taste loss in a chemosensory clinic population. Ann Otol Rhinol Laryngol. 2003;112:971–978. doi: 10.1177/000348940311201110. [DOI] [PubMed] [Google Scholar]
- 24.Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav. 1994;56:1165–1171. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 25.Harris H, Kalmus H. Chemical specificity in genetical differences of taste sensitivity. Ann Eugen. 1949;15:32–45. doi: 10.1111/j.1469-1809.1949.tb02420.x. [DOI] [PubMed] [Google Scholar]
- 26.Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Sens. 2008;33:255–265. doi: 10.1093/chemse/bjm084. [DOI] [PubMed] [Google Scholar]