Abstract
Background
Ketamine rapidly improves depressive symptoms in patients with treatment-resistant major depressive disorder (MDD) who do not respond to multiple standard antidepressants. However, it remains unknown whether ketamine is equally effective in patients with MDD who previously also did not respond to electroconvulsive therapy (ECT).
Methods
This study compared 17 patients with treatment-resistant MDD who previously did not respond to ECT and 23 patients with treatment-resistant MDD who had not previously received ECT. All subjects received a single open-label infusion of ketamine (0.5 mg/kg). Patients were evaluated using the Montgomery-Asberg Depression Rating Scale (MADRS) at baseline (60 minutes before the infusion), as well as at 40, 80, 120, and 230 minutes post-infusion.
Results
Depressive symptoms were significantly improved in the ECT-resistant group at 230 minutes with a moderate effect size (p<.001, d=0.50, 95% C.I.: 0.21–0.80). At 230 minutes, the non-ECT exposed group showed significant improvement with a large effect size (p<.001, d=1.00, 95% C.I.: 0.71–1.29).
Conclusion
Ketamine appears to improve depressive symptoms in patients with MDD who had previously not responded to ECT. These preliminary results encourage further investigation with a larger sample size to determine effectiveness compared to other treatment-resistant patients with MDD.
Keywords: depression, electroconvulsive therapy, glutamate, NMDA, treatment-resistant
1. Introduction
Major depressive disorder (MDD) is a common, chronic, severe, and often life threatening illness, with a lifetime prevalence estimated at 16.2% (Kessler et al., 2003). Indeed, the World Health Organization's Global Burden of Disease Study identified MDD as the leading cause of disability in the Western World for those aged 15–44 (The World Health Organization, 2000). Currently available therapeutic approaches for MDD are not effective for a substantial number of patients; furthermore, a considerable therapeutic lag before clinical improvement is seen in those patients who do respond (Rush et al., 2006). Data from inpatient populations with MDD that used algorithm-guided treatments (including electroconvulsive therapy (ECT)) demonstrated remission rates between 50–54%; in addition, these algorithms included the use of medications at higher than FDA-recommended dosages, and had higher dropout rates than treatment-as-usual (Bauer et al., 2009; Birkenhager et al., 2006).
ECT has long been considered the gold standard therapeutic approach for treatment-resistant MDD (Fink, 1990, UK ECT Review Group, 2003), and has also been described as the most effective treatment for MDD due to its having the largest effect size of all available treatments (Fava, 2003). Traditionally, one of the main indications for receiving ECT has been non-response to multiple antidepressant trials (Thase and Rush, 1997). One meta-analysis found that the probability of responding to ECT was almost four times greater than the probability of responding to antidepressants (Pagnin et al., 2004). In addition, ECT has also been associated with more rapid onset of antidepressant effects than standard antidepressants (Fava, 2003). For instance, the Consortium for Research in ECT (CORE) noted that more than half of patients treated with ECT showed improvement during the first week, and 65% achieved remission after 10 sessions (Husain et al., 2004). Another group reported that remission rates with ECT were as high as 70–90% (UK ECT Review Group, 2003). Despite these favorable data, ECT is often reserved as the last choice for treating MDD (Pagnin, de Queiroz, 2004, Thase and Rush, 1997), typically because of its potential for causing adverse cognitive effects and post-ECT relapse (Sackeim et al., 2001).
A related issue is the choice of pharmacological strategies used in patients who do not respond to ECT, a topic on which there is unfortunately little information. Among the few case reports that have addressed this issue, one reported an antidepressant response to venlafaxine in two patients who did not respond to ECT (Iodice and McCall, 2003). In a case series investigating the effects of clozapine in ECT-resistant depressed inpatients, clozapine was found to have no clear antidepressant effects (Quante et al., 2007). With regard to somatic treatments, vagal nerve stimulation (VNS) and deep brain stimulation (DBS) have both shown antidepressant efficacy in patients with MDD who did not respond to ECT (Mayberg et al., 2005, Rush et al., 2005). However, these studies did not systematically explore how effective these interventions were in ECT non-responders. Furthermore, a rapid antidepressant response (within hours or a few days) has not been demonstrated with any of these treatments.
Ketamine, a non-competitive high-affinity N-methyl-D-aspartate (NMDA) receptor antagonist used primarily as an anesthetic has been shown in controlled studies to exert rapid and robust antidepressant effects in treatment-resistant patients with either MDD or bipolar depression (Berman et al., 2000, Diazgranados et al., 2010, Zarate et al., 2006). One of the most notable results of these studies is that all enrolled patients had an extensive treatment history, having, on average, failed six or more adequate trials of antidepressants; some had also previously received ECT. Preliminary data from these studies gave the impetus to more systematically examine the antidepressant effects of ketamine in patients who had previously not responded to ECT.
The objective of the present study was to investigate whether a single intravenous infusion of ketamine was associated with a decrease in depressive symptoms in patients with treatment-resistant MDD who had previously not responded to ECT. Results were subsequently compared with a comparison group of concurrently-recruited, ECT-naïve, but otherwise treatment-resistant patients with MDD.
2. Methods
2.1 Patients
Forty-two patients aged 18–65 years participated in this study between October 2006 and December 2009. Participants' diagnoses were ascertained by the Structured Clinical Interview for Diagnosis (SCID) (First et al., 2001). All participants met DSM-IV criteria for MDD, currently in a major depressive episode without psychotic features (American Psychiatric Association, 1994). In addition, all patients had a Montgomery-Asberg Depression Rating Scale (MADRS) total score of ≥22 (Montgomery and Asberg, 1979), a current or past history of lack of response to two adequate antidepressant trials (operationally defined using the Antidepressant Treatment History Form (ATHF; (Sackeim, 2001)), and a current major depressive episode lasting at least four weeks. Patients with a DSM-IV (American Psychiatric Association, 1994) diagnosis of alcohol or substance abuse or dependence within the previous three months, serious, unstable medical illness, or uncorrected hypo- or hyperthyroidism were excluded. All patients were in good physical health with no unstable medical conditions, as determined by medical history, physical examination, routine blood labs, electrocardiogram, urinalysis, and urine toxicology. All subjects had been drug free from any psychotropic medications for at least two weeks (or five weeks for fluoxetine). All patients gave written informed consent. The study was approved by the Combined Neuroscience Institutional Review Board (IRB) of the National Institutes of Health (NIH).
2.2 Ketamine Administration
Ketamine infusion occurred as previously described (Diazgranados, Ibrahim, 2010, Zarate, Singh, 2006). Briefly, patients openly received a single intravenous infusion of ketamine hydrochloride (0.5 mg/kg) over the course of 40 minutes, followed by double-blind randomization to riluzole or placebo six hours post-infusion. In this paper we only report results from the first part of the study—that is, the open-label ketamine intravenous infusion and the first 230 minutes after the infusion. This represented an ideal time point for analyzing early antidepressant effects, given that in our previous placebo-controlled study most patients whose depressive symptoms improved with ketamine (88%) had responded by 230 minutes (Zarate, Singh, 2006). Mood was evaluated using the MADRS at baseline, as well as at 40, 80, 120, and 230 minutes post-infusion. The MADRS was the primary outcome measure. The clinical rater performing the MADRS was blind to the treatment history of the patient. In order to measure dissociative symptoms, we also administered the Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998); CADSS ratings were obtained at the same time points as the MADRS ratings.
2.3 Classification of ECT Treatment History
The ATHF (Sackeim, 2001) was used to assess treatment history. This instrument evaluates the level of treatment failure to various antidepressants, as well as to somatic treatments. ECT treatment non-response was retrospectively defined as non-response to six bilateral or seven unilateral ECT sessions. For this study, patients were divided into two groups: 1) those who had previously not responded to an adequate ECT trial (n=17), and 2) those who had not been exposed to ECT. All patients in the group of ECT non-responders had never met response criteria with ECT treatment; this information was obtained from charts, previous medical records, and by direct questioning of the patients. Patients who had received an ECT trial with fewer sessions (eg, less than six bilateral or seven unilateral sessions) were excluded from the analysis (n=2). Demographic characteristics, clinical characteristics, and response to ketamine were compared between both groups.
2.4 Statistics
A linear mixed model with restricted maximum likelihood estimates was used to examine the time course of response to ketamine in patients with prior non-response to ECT and those without prior exposure to an adequate trial of ECT. Baseline ratings were used as covariates to minimize potential group differences. A first order autoregressive covariance structure was the best fitting model following the lowest Schwarz's Bayesian criteria score. Bonferroni post hoc tests were used to evaluate differences at each time point between patient groups. A separate model included baseline as a separate time point instead of as a covariate in order to examine changes from baseline in each group; this allowed a priori comparisons of baseline to 230 minutes post-infusion to be made for each group. Fisher's exact test was used to compare the percentage of patients whose depressive symptoms improved (50% decrease from baseline) between the groups. Significance was evaluated at p<.05, two-tailed. Demographic characteristics were examined with Student's t-tests for continuous measures and chi-square tests for categorical ones.
3. Results
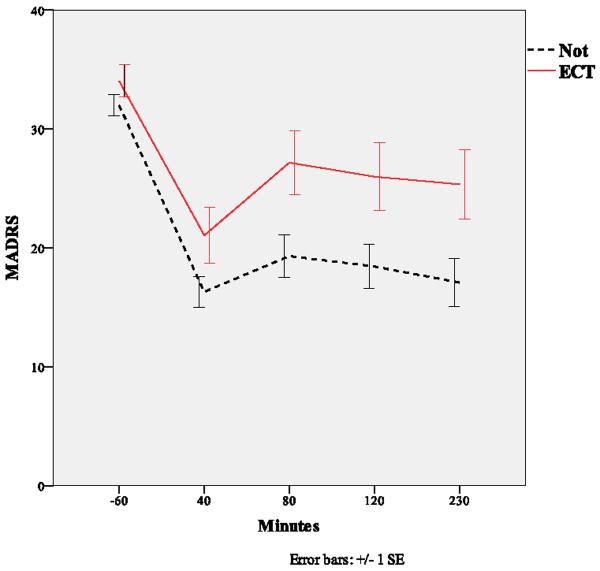
Table 1 shows the clinical and demographic characteristics of patients who had previously not responded to ECT and those who were previously not exposed to ECT. The only significant group difference was a greater prevalence of lifetime hospitalization in the ECT-exposed group. A linear mixed model using baseline as a covariate indicated significant differences in MADRS scores between ECT-resistant and non-ECT exposed patients (F=4.97, df=1, 39, p=.03), and no interaction between group and time (F=0.90, df=3,106, p=.44). The effect size for the group comparison at 230 minutes post-infusion was d=0.60 (95% C.I.: 0.10–1.10), reflecting a moderately sized difference. When baseline was included as a time point in the mixed model, the ECT-resistant group in general had significantly higher scores than the non-ECT exposed group (F=7.46, df=1, 46, p=.009). The interaction between group and time was not significant (F=1.16, df=4,140, p=.33). The ECT-resistant group showed significant improvement at 230 minutes with a moderate effect size (p<.001, d=0.50, 95% C.I.: 0.21–0.80), while the non-ECT exposed group showed significant improvement with a large effect size (p<.001, d=1.00, 95% C.I.: 0.71–1.29). The effect size for the group comparison at 230 minutes post-infusion was moderate (d=0.62, 95% C.I.: 0.21–1.004) (Figure 1). The proportion of patients exhibiting a substantial improvement in depressive symptoms (50%) did not significantly differ between the two groups (p=.33).
Table 1.
Demographics.
| ECT (n=17) | No ECT (n=23) | ||
|---|---|---|---|
| N (%) |
N (%) |
p |
|
| Gender (Male) | 10 (59) | 14 (61) | 0.99 |
| Education (College Graduate) | 13 (77) | 10 (50) | 0.17 |
| Abuse | |||
| • Physical | 6 (35) | 4 (20) | 0.46 |
| • Sexual | 1 (6) | 4 (21) | 0.19 |
| Substance Abuse | |||
| • Self | 7 (41) | 6 (29) | 0.50 |
| • ETOHAbuse | |||
| • Self | 7 (41) | 5 (24) | 0.31 |
| • Family (1st Degree) | 5 (31) | 7 (37) | 0.99 |
| • Family (2nd Degree) | 2 (13) | 4 (21) | 0.67 |
| Family History | |||
| • Depression | 15 (88) | 19 (91) | 0.99 |
| • Suicide | 6 (38) | 8 (35) | 0.88 |
| MDD Subtype | 0.41 | ||
| • Atypical | 3 (19) | 7 (37) | |
| • Melancholic | 7 (44) | 5 (26) | |
| • Neither | 6 (38) | 7 (37) | |
| Illness History | |||
| • Hospitalization | 14 (82) | 9 (39) | 0.01 |
| • Suicide | |||
| • Attempt | 7 (41) | 8 (35) | 0.75 |
| • Ideation | 13 (77) | 12 (52) | 0.19 |
| • Ideation at Admission | 5 (29) | 9 (39) | 0.74 |
| Mean (SD) |
Mean (SD) |
p |
|
|---|---|---|---|
| Age | 46.5 (10.8) | 46.6 (14.8) | 0.99 |
| BMI | 28.9 (5.7) | 30.9 (7.7) | 0.38 |
| Illness History | |||
| • Age of Onset | 21.5 (12.3) | 21.5 (11.6) | 0.99 |
| • Length of Current Episode (Months) | 99.1 (108.8) | 103.0 (163.4) | 0.93 |
| • Length of Illness (Years) | 25.4 (10.2) | 25.3 (15.3) | 0.99 |
| • Previous Episodes | 32.3 (46.5) | 17.3 (35.3) | 0.28 |
| Clinical Scales | |||
| • MADRS | 34.1 (5.6) | 32.0 (4.3) | 0.20 |
| • BPRS | 35.8 (6.1) | 36.0 (5.6) | 0.93 |
| • CADSS | 4.7 (10.1) | 4.0 (5.6) | 0.81 |
| • Suicide Ideation Scale | 4.8 (6.7) | 2.1 (4.7) | 0.15 |
Figure 1.
Course of depressive symptoms (MADRS) following ketamine infusion in ECT naïve and exposed groups.
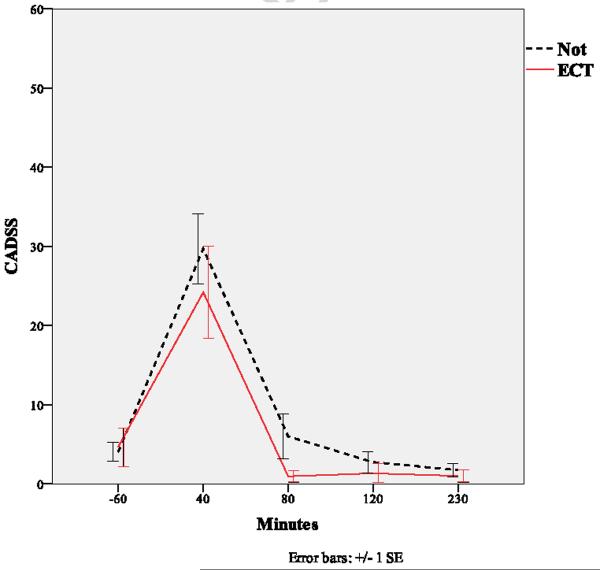
Because ketamine is well known to cause dissociative effects, we also administered the CADSS (Figure 2), a widely-used measure of dissociation (Bremner et al., 1998). Dissociation scores increased significantly at 40 minutes, and returned to normal by 80 minutes; no difference was noted between patient groups (p=.19).
Figure 2.
Course of dissociative symptoms (CADSS) following ketamine infusion in ECT naïve and exposed groups.
4. Discussion
The present study investigated whether a single intravenous infusion of an NMDA antagonist would have antidepressant effects in patients with treatment-resistant MDD who had previously not responded to an adequate trial of ECT. The results suggested that such patients experienced a decrease in depressive symptoms comparable to that of ECT-naive patients. A significant improvement in depressive symptoms was seen within 230 minutes post-infusion. The effect size within the ECT-resistant group between baseline and endpoint was moderate. Because of the limited sample size, the estimate of the effect size may be less reliable than that provided by a larger study.
These findings are particularly noteworthy in that the patient population studied was severely ill both in terms of the length of their depressive episode and the overall length of their illness. Notably, studies have shown that in patients with MDD, the severity of treatment resistance predicts poor response to acute treatment (Zarate et al., 2000). These patients had, on average, experienced their first episode of illness 26 years earlier, and had previously not responded to trials of diverse antidepressants. The severity of illness of the ECT-resistant group compared with the non-ECT exposed group was underscored by this group's higher MADRS scores at baseline (though this difference was not statistically significant).
It is interesting to note that ketamine, when used as an anesthetic before ECT, has been associated with positive cognitive and antidepressant effects, particularly in its ability to spare memory function after ECT (McDaniel et al., 2006). Ketamine has been found to dissociate the mossy-cell layer thickening in the hippocampus after seizure induction in rats, and this thickening may be the mechanism underlying its cognitive side effects after ECT (Lamont et al., 2005). Ketamine has also been found to block widespread and nonspecific hippocampal long term potentiation (LTP) following ECT, thus sparing LTP for further memory acquisition and guarding against memory loss after ECT (Brun et al., 2001).
It has also been argued that using ketamine instead of methohexital anesthesia could improve the cognitive side effects associated with ECT, because ketamine has fewer anticonvulsant properties (Krystal et al., 2003). More recently, Okamoto and colleagues reported that ketamine anesthesia during ECT had rapid antidepressant effects in patients with treatment-resistant MDD (Okamoto et al., 2010). In that study, 31 inpatients with treatment-resistant MDD who continued to take their existing medications were assigned (according to patient preference) to propofol (n=20) or ketamine (n=11) anesthesia prior to ECT. The investigators found that 17-item Hamilton Depression Rating Scale (HAM-D) scores improved earlier in the ketamine group than in the propofol group (differences were seen at the second and fourth ECT sessions, but not at the sixth or eighth sessions) and that, furthermore, these improvements were more pronounced in the ketamine group (Okamoto, Nakai, 2010). Only two out of 11 patients in the ketamine group had a history of ECT, but it is unknown whether these subjects had undergone an adequate ECT trial. Thus, to our knowledge, the present study is the first to offer data regarding ketamine's efficacy in treatment-resistant patients with MDD who had previously not responded to an adequate trial of ECT.
A limitation of this study is that ketamine was administered in an open-label fashion, which could have biased the reported effects; however, the severity of depressive symptoms, the extent of improvement in mood, the rapidity of antidepressant effects, and the resistance to previous standard treatment make a placebo effect unlikely. In fact, patients in this study who received ketamine showed a similar pattern of improvements in depressive symptoms at 230 minutes post-infusion as MDD and bipolar depression patients in our previous placebo-controlled studies (Diazgranados, Ibrahim, 2010, Zarate, Singh, 2006). Placebo-response rates in those studies were low. In addition, the CADSS—which measures dissociation—showed that while dissociation scores increased significantly at 40 minutes, they had returned to normal by 80 minutes post-infusion, and that there was no difference between the two patient groups throughout; furthermore, ketamine's antidepressant effects lasted for at least 230 minutes, suggesting that the improvement in depressive symptoms was not due to dissociation. The question remains whether the continued but smaller reduction in MADRS scores after the CADSS had returned to baseline is a true antidepressant improvement. Future studies that incorporate an active placebo group will be needed to fully elucidate the issue of placebo improvement.
5. Conclusion
Ketamine appears to be an effective treatment in ECT-resistant patients with MDD. Future studies would need to examine whether patients receiving ECT could benefit from the concomitant anesthetic use of ketamine, regardless of whether or not they previously responded to ECT. Ketamine is widely used to induce anesthesia and is sometimes administered to patients receiving ECT. Thus, it is possible that the combination of both agents could enhance time to improvement. However, this conclusion is speculative and requires further study. The design of future studies should also make it possible to investigate remission rates and biological markers for treatment response in this group of treatment-resistant patients.
6. Acknowledgements
The authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, and Department of Health & Human Services (IRP-NIMH-NIH-DHHS). Dr. Machado-Vieira would also like to thank the Stanley Medical Research Institute (SMRI). Ioline Henter provided outstanding editorial assistance.
Abbreviations
- CADSS
Clinician-Administered Dissociative States Scale
- ECT
electroconvulsive therapy
- MADRS
Montgomery-Asberg Depression Rating Scale
- MDD
major depressive disorder
- NMDA
N-methyl-D-aspartate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bauer M, Pfennig A, Linden M, Smolka MN, Neu P, Adli M, et al. Efficacy of an algorithm-guided treatment compared with treatment as usual. J Clin Psychopharmacology. 2009;29:327–33. doi: 10.1097/JCP.0b013e3181ac4839. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Birkenhager TK, Broek WW, Moleman P, Bruijn JA, et al. Outcome of a 4-step treatment algorithm for depressed inpatients. J Clin Psychaitry. 2006;67:1266–71. doi: 10.4088/jcp.v67n0814. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–36. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Brun VH, Ytterbo K, Morris RG, Moser MB, Moser EI. Retrograde amnesia for spatial memory induced by NMDA receptor-mediated long-term potentiation. J Neurosci. 2001;21:356–62. doi: 10.1523/JNEUROSCI.21-01-00356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consensus conference Electroconvulsive therapy. JAMA. 1985;254:2103–8. [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–59. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- Fink M. The 1990 APA Task Force Report: a quiet revolution. Convuls Ther. 1990;6:75–8. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams AR. Structured Clinical Interview for DSM-IV TR Axis I Disorders. Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research; New York: 2001. [Google Scholar]
- Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004;65:485–91. doi: 10.4088/jcp.v65n0406. [DOI] [PubMed] [Google Scholar]
- Iodice AJ, McCall WV. ECT resistance and early relapse: two cases of subsequent response to venlafaxine. J ECT. 2003;19:238–41. doi: 10.1097/00124509-200312000-00012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Weiner RD, Dean MD, Lindahl VH, Tramontozzi LA, 3rd, Falcone G, et al. Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J Neuropsychiatry Clin Neurosci. 2003;15:27–34. doi: 10.1176/jnp.15.1.27. [DOI] [PubMed] [Google Scholar]
- Lamont SR, Stanwell BJ, Hill R, Reid IC, Stewart CA. Ketamine pre-treatment dissociates the effects of electroconvulsive stimulation on mossy fibre sprouting and cellular proliferation in the dentate gyrus. Brain Res. 2005;1053:27–32. doi: 10.1016/j.brainres.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McDaniel WW, Sahota AK, Vyas BV, Laguerta N, Hategan L, Oswald J. Ketamine appears associated with better word recall than etomidate after a course of 6 electroconvulsive therapies. J ECT. 2006;22:103–6. doi: 10.1097/00124509-200606000-00005. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT. 2010;26:223–7. doi: 10.1097/YCT.0b013e3181c3b0aa. [DOI] [PubMed] [Google Scholar]
- Pagnin D, de Queiroz V, Pini S, Cassano GB. Efficacy of ECT in depression: a meta-analytic review. J ECT. 2004;20:13–20. doi: 10.1097/00124509-200403000-00004. [DOI] [PubMed] [Google Scholar]
- Quante A, Zeugmann S, Bajbouj M, Anghelescu I. Clozapine in medication- and electroconvulsive therapy-resistant, depressed inpatients: a case series. J Clin Psychopharmacol. 2007;27:715–7. doi: 10.1097/JCP.0b013e31815a57ef. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58:347–54. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA. 2001;285:1299–307. doi: 10.1001/jama.285.10.1299. [DOI] [PubMed] [Google Scholar]
- Thase ME, Rush AJ. When at first you don't succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(Suppl 13):23–9. [PubMed] [Google Scholar]
- The World Health Organization . World Health Report 2001. WHO; Geneva: 2000. [Google Scholar]
- UK ECT Review Group Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361:799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Rothschild A, Fletcher KE, Madrid A, Zapatel J. Clinical predictors of acute response with quetiapine in psychotic mood disorders. J Clin Psychiatry. 2000;61:185–9. doi: 10.4088/jcp.v61n0307. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]