Abstract
Human immunodeficiency virus (HIV) infection and progression of acquired immunodeficiency syndrome (AIDS) can be modulated by a number of cofactors, including drugs of abuse. Opioids, cocaine, cannabinoids, methamphetamine (METH), alcohol, and other substances of abuse have been implicated as risk factors for HIV infection, as they all have the potential to compromise host immunity and facilitate viral replication. Although epidemiologic evidence regarding the impact of drugs of abuse on HIV disease progression is mixed, in vitro studies as well as studies using in vivo animal models have indicated that drugs of abuse have the ability to enhance HIV infection/replication. Drugs of abuse may also be a risk factor for perinatal transmission of HIV. Because high levels of viral load in maternal blood are associated with increased risk of HIV vertical transmission, it is likely that drugs of abuse play an important role in promoting mother-fetus transmission. Furthermore, because the neonatal immune system differs qualitatively from the adult system, it is possible that maternal exposure to drugs of abuse would exacerbate neonatal immunity defects, facilitating HIV infection of neonate immune cells and promoting HIV vertical transmission. The availability and use of antiretroviral therapy for women infected with HIV increase, there is an increasing interest in determining the impact of drug abuse on efficacy of AIDS Clinical Trials Group (ACTG) -standardized treatment regimens for woman infected with HIV in the context of HIV vertical transmission.
Keywords: Opioids, Methamphetamine, Cocaine, Marjuana/Cannabinoid, Alcohol, HIV
INTRODUCTION
Since the beginning of the global acquired immunodeficiency syndrome (AIDS) epidemic, almost 60 million people have been infected with human immunodeficiency virus (HIV) and 25 million people have died of HIV-related causes. As of 2008, the approximate number of people living with HIV or AIDS was 33.4 million, among them 50% women and 2.1 million children under 15 years of age. In year of 2009, around 2.7 million new HIV infections, including 430,000 newborn children was reported (UNAIDS 2008, 2009). HIV/AIDS is now the third leading cause of death among women aged 25 to 44 years and the leading cause of death among African women in this age group. HIV infection and progression of AIDS are affected by a number of cofactors, including drugs of abuse. HIV epidemic has been driven by drug abusers in the United States and other regions in the world. Injection drug users (IDUs) are at a significant risk for acquiring HIV infection, representing one of the largest reservoirs of HIV infection in the United States and contributes to the fastest spread of the virus (Alcabes and Friedland 1995; Risdahl et al. 1998). One third of HIV-infected individuals in the United States are intravenous drug abusers. In addition to collective use of injecting equipment and drug solution, the negative impact of drug abuse on host immune system is another factor for promoting HIV infection. Thus, drugs of abuse have been suggested to have a cofactor role in the immunopathogenesis of HIV disease. In the following sections, we discuss the effects of some of popularly used drugs including alcohol on HIV infection/replication and their implication in HIV progression and vertical transmission.
OPIOIDS AND HIV
Opioids are agents that bind opioid receptors principally found in the central nervous system (CNS) and gastrointestinal tract, but they are also found on immune cells. Four classes of opioids have been categorized: endogenous opioid peptides; opium alkaloids, such as morphine and codeine; semisynthetic opioids that include heroin and oxycodone; and fully synthetic opioids such as pethidine and methadone that have structures unrelated to the opium alkaloids (Cabral 2006). Because many of opiate abusers (> 90%) use injection as the primary route of administration, opiate abuse contributes significantly to HIV transmission among drug users (Martin et al. 2010). In addition, clinical and epidemiological evidence from early, pre-AIDS studies indicates that opiates have a cofactor role in the pathogenesis of AIDS (Alcabes and Friedland 1995; Donahoe et al. 1993; Ronald et al. 1994; Specter 1994). Furthermore, laboratory studies have demonstrated that morphine enhances susceptibility of the immune cells to HIV infection (Guo et al. 2002; Ho et al. 2003; Li et al. 2003; Nair et al. 1997a; Wang et al. 2005) and increase viral titers in the brain (Stefano et al. 1996). The expression of HIV in co-cultures of chronically infected promonocytes and human brain cells was increased following exposure to morphine (Peterson et al. 1994). Morphine enhances HIV replication in human PBMC co-culture assay (Peterson et al. 1990) and Simian immunodeficiency viruses (SIV) replication in monkey peripheral blood mononuclear cell (PBMC) (Suzuki et al. 2002). Morphine enhances HIV replication in human macrophages (Guo et al. 2002; Ho et al. 2003; Li et al. 2003; Li et al. 2002), T lymphocytes (Chuang et al. 1993; Peterson et al. 2004; Steele et al. 2003), kuffer cells (Schweitzer et al. 1991), human neuroblastoma cells (Squinto et al. 1990) and human brain cells (Chao et al. 1995; Peterson et al. 1999). A study by Nair’s group (Reynolds et al. 2006) found that heroin potentiates HIV replication in normal human astrocytes. With human T lymphoid cell line, Selimova et al demonstrated that heroin increased HIV replication at the early stages of its life cycle (Bobkov et al. 2005; Selimova et al. 2002). Although these in vitro data clearly demonstrate that opioids such as morphine or heroin enhance HIV infection/replication (Table 1), the animal and clinical studies have yielded contradictory data with regard to the role of opioid use in the progression of SIV or HIV disease (Donahoe and Falek 1988; Rothenberg et al. 1987) (Table 2). The studies conducted by Donahoe et al. (Donahoe et al. 1993; Donahoe et al. 2009) showed that long -term opiate dependency seemed to retard the rate of progression to AIDS in the SIVsmm9 monkey model, but Chuang et al. (Chuang et al. 1995) and Kumar et al. (Kumar et al. 2004) observed greater SIV disease progression after opiate injection (Table 2).
Table 1.
In vitro Studies on Impact of Drug Abuse on HIV
| Substance | Effect | Target Cells | Type of Study | Refecrences |
|---|---|---|---|---|
| Heroin | ↑ | Human Astrocytes | in vitro | Reynolds et al. (2006) |
| ↑ | Human MT-4 T cell line | in vitro | Selimova et al. (2002) | |
| Morphine | ↑ | Human Macrophages | in vitro | Guo et al. (2002), Ho et al. (2003) |
| Li et al. (2003) | ||||
| ↑ | Human PBMC | in vitro | Peterson et al. (1990), Li et al. (2003) | |
| ↑ | Monkey PBMC | in vitro | Suzuki et al. (2002)a | |
| ↑ | Human CEMX174 cell line | in vitro | Chuang et al. (1993) | |
| ↑ | Human lymphocytes | in vitro | Steele et al. (2003) | |
| ↑ | Human promonocyes and human brain cells | in vitro | Peterson et al. (1994) | |
| ↑ | Human CD4+ T lymphocytes and microglial cells | in vitro | Peterson et al. (2004) | |
| ↑ | Human Kuffer cells | in vitro | Schweitzer et al. (1991) | |
| ↑ | Human Brain cells | in vitro | Peterson et al. (1999) | |
| ↓ | Human macrophages and T lymphocytes | in vitro | Szabo et al. (2003), Peterson et al. (2004) | |
| Cocaine | ↑ | Human PBMC | in vitro | Peterson et al. (1991), (1992), |
| ↑ | Human microglia cells | in vitro | Gekker et al. (2004), (2006) | |
| ↑ | Human macrophages | in vitro | Dhillon et al. (2007) | |
| ↑ | Human dendritic cells | in vitro | Nair et al (2005) | |
| METH | ↑ | Human macrophages | in vitro | Liang et al. (2008) |
| ↑ | Human dendritic cells | in vitro | Nair et al. (2009) | |
| Marijuana or Cannabinoid | ↑ | Human MT-2 T cell line | in vitro | Noe et al. (1998) |
| ↓ | Human CD4+ T cells and microglia cells | in vitro | Peterson et al. (2004), Rock et al. (2007) | |
| Alcohol | ↑ | Human macrophgaes | in vitro | Wang et al. (2002) |
| ↑ | Human PBMCs | in vitro | Saravolatz et al. (1990), Bagasra et al. (1993) | |
| No effect | Human Lymphocytes | ex vivo | Cook et al. (1997) | |
| ↑ | Human T Lymphocytes | in vitro | Liu et al. (2003), Wang et al. (2006) | |
| ↑ | Human Oral Keratinocytes | in vitro | Chen et al. (2004) | |
| ↑ | Monkey PBMC and alveolar macrophages | in vitro | Bagby et al. (1998) |
Table 2.
In vivo Studies on Impact of Drug Abuse on HIV
| Effect | Type of Study | Refecrences | |
|---|---|---|---|
| Heroin | ↑ | Human | Martin et al (2010) |
| ↓ | Human | Margolick et al. (1992) | |
| Morphine | ↑ | Monkey | Chuang et al. (1997) |
| ↑ | Monkey | Kumar et al. (2004) | |
| ↓ | Monkey | Donahoe et al (1993) | |
| Cocaine | ↑ | Human | Chaisson et at (1989), Nelson et al (2002) |
| Baum et al. (2009) | |||
| ↑ | Mouse | Roth et al (2002), (2005) | |
| METH | ↑ | Monkey | Burdo et al. (2006), Marcondes et al (2010) |
| ↑ | Human | Kall and Olin (1990), Maragos et al. (2002) | |
| Ellis et al. (2003), Morin et al. (2005), | |||
| Peck et al. (2005), Martin et al (2010) | |||
| Marijuana or Cannabinoid | ↑ | Mouse | Roth et al (2005) |
| ↑ | Human | Tindall et al (1988), Kaslow et al. (1989) | |
| No effect | Human | Abrams et al. (2003), Simbayi et at (2005) | |
| Alcohol | ↑ | Monkey | Bagby et al. (2003), Poonia et al. (2006) |
| Marcondes et al. (2008), Molina et al. (2008) | |||
| ↑ | Human | Szabo et al. (1999), Fisher et al. (2007), | |
| Kalichman et al. (2008), Baliunas et al. (2009) |
Although the role of opioid use in promoting the progress of HIV disease is still debatable, it is well known that opioids exert a profound influence on immunomodulatory activity. Opioid abusers have higher incidence of infectious disease, which may be directly related to impaired immune functions (McCarthy et al. 2001; Nair et al. 1997b; Novick et al. 1989; Ochshorn et al. 1990; Peterson et al. 1993; Risdahl et al. 1998). The administration of morphine to rodents suppresses a variety of immune responses that involve the major cell types in the immune system, including natural killer cells, T cells, B cells, macrophages and polymorphonuclear leukocytes (Bayer et al. 1990). Opioids also inhibits antibody (Lockwood et al. 1994) and cytokine release by the immune cells (Hung et al. 1973; Wang et al. 2003). Morphine suppressed the production of interferon–alpha (IFN-α (Peterson et al. 1989; Wang et al. 2002a), a cytokine that modulates all phases of immune processes and has a central role in host innate immunity against viral infections. We recently showed that morphine, through the suppression of interferon-gamma (IFN-γ), compromised CD8+ T cell-mediated anti-HIV activity in both acute and latently infected cells (Wang et al. 2005). Opioids modulates immune functions via pharmacological activation of endogenous opioid receptors in the immune cells (Bayer et al. 1990; Fecho et al. 1996; Hernandez et al. 1993).
In summary, although the biological impact of opioid use on the progression of HIV disease remains to be determined, opioid use has profound effects on host immune systems, which may form not only HIV transmission, but also viral replication. Certainly, more studies with well-characterized opioid-using population will be needed to definitely establish the role of opioid use in HIV disease progression.
COCAINE AND HIV
Crack cocaine has been identified as an independent risk factor for HIV infection and AIDS epidemic. As a non-injection drug use, cocaine contributes to the spread of HIV through risky sexual behaviors including multiple sex partners, inconsistent condom use, and exchanging sex for drugs or money (Booth et al. 1993; Pechansky et al. 2006). In the earlier cohort studies (Caiaffa et al. 1994; Chaisson et al. 1989; Chiasson et al. 1991; Nelson et al. 2002) different groups found that both intravenous cocaine use and smoking illicit drugs (marijuana, cocaine, crack) were associated with HIV and bacteria infection. Studies that distinguished among the types of drugs used by people positive for HIV found that cocaine, and particularly crack-cocaine, increased the risk of progression to AIDS (Cook et al. 2008; Duncan et al. 2007; Webber et al. 1999). In a more recently cohort study, Baum et al. reported that crack–cocaine use facilitates HIV disease progression by reducing adherence in those on highly active antiretroviral therapy (HAART) and by accelerating disease progression independently of HAART (Baum et al. 2009).
The role of cocaine in promoting HIV disease is also supported by the laboratory investigations (Table 1). In vitro studies indicate that cocaine increases replication of HIV in both stimulated (Bagasra and Pomerantz 1993) and unstimulated human peripheral blood cells (Peterson et al. 1991; Peterson et al. 1992). Cocaine alters both cytokine production and HIV expression in mononuclear phagocytes, including microglia cells (Gekker et al. 2006; Gekker et al. 2004). Cocaine markedly enhanced virus production in simian human immunodeficiency virus (SHIV)-infected macrophages and in a chronically infected promonocytic cell line (Dhillon et al. 2007). Nair et al reported that cocaine exacerbates HIV infection by up-regulating Dendritic Cell-Specific Intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) on dendritic cells and these effects are mediated via dysregulation of mitogen-activated protein kinases (MAPKs) (Nair et al. 2005). In addition to the in vitro findings described above, in vivo animal studies also support the role of cocaine in promoting HIV replication. Using the human peripheral blood mononuclear leukocyte (huPBL) severe combined immunodeficiency (SCID) mouse model, Roth et al demonstrated that systemic exposure to cocaine leads to increase in HIV replication and spread in vivo (Roth et al. 2002; Roth et al. 2005b).
Taken together, findings from laboratory and epidemiological studies indicate that cocaine use has a significant impact on the pathogenesis/progression of HIV disease. This impact maybe mediated by immunological and viralogical factors that influence host susceptibility, use of antiretroviral therapies and underlying corruptibility. This information should be used to guide future research on the mechanism (s) involved in the cocaine action on HIV infection.
METHAMPHETAMINE (METH) AND HIV
Because of the alarming prevalence of HIV infection among METH users (Boddiger 2005), it is becoming increasingly important to investigate the association between psychotropic drug use and HIV infection. METH use is associated with risky sexual behavior, increasing the potential of users to become infected with HIV and hepatitis B virus and hepatitis C virus (Letendre et al. 2005). METH-type substances can be used percutaneously and a high correlation has been reported for injection frequency and HIV transmission (Bruneau et al. 2001). A strong connection between METH dependence and HIV infection has been observed for METH-dependent men who have sex with men (Morin et al. 2005; Peck et al. 2005; Plankey et al. 2007; Shoptaw and Reback 2006, 2007), men who have sex with women (Wohl et al. 2002) and female sex workers (Patterson et al. 2008). More importantly, METH use has been correlated with more rapid progression to AIDS in HIV-infected people (Moore et al. 2004). METH exposure increases virus load in the CNS of HIV-infected patients (Maragos et al. 2002; Marcondes et al. 2010). Active METH users displayed higher levels of HIV loads than non-users (Ellis et al. 2003), which may be attributable to increased viral replication. The effect of METH on HIV may be at the viral entry or integration into host genome levels, but not at the translation level (Gavrilin et al. 2002). We and other have shown METH has the ability to enhance HIV replication in macrophages (Liang et al. 2008) and dendritic cells (Nair et al. 2009).
Similar to other drugs of abuse, METH abuse has immunosuppressive effects and consequently has the potential to increase susceptibility of host immune cells to HIV infection. METH has been shown to exert immunomodulatory effects (Yu et al. 2003). There is the immunosuppressive effect of METH on the T cell-mediated immune response (House et al. 1994; Iwasa et al. 1996). In vivo studies have shown that METH significantly suppressed interlukin-2 (IL-2) and IFN-γ expression (Gavrilin et al. 2002). METH stimulates secretion of Tumor Necrosis Factor-alpha (TNF-α) in splenocytes from retrovirus-infected mice (Yu et al. 2002). METH exposure inhibited macrophage-mediated antiviral and cytotoxic activities and reduced their ability to produce nitric oxide (NO)/TNF-α (In et al. 2004). METH treatment induced an increase in the percentage of CD4+ cells with simultaneous decrease in the percentages of CD8+ and double-positive CD4+ CD8+ in thymus (In et al. 2005). Microarray analysis of human brain tissue from HIV-infected METH users showed significant up-regulation of genes associated with inflammation (Everall et al. 2005), which contributes to enhancement of HIV expression in vivo (Ellis et al. 2003). Taken together, investigations from in vivo and in vitro studies provide evidence to support the possibility that METH may have a cofactor in the immunopathogenesis of HIV infection and progression to AIDS.
MARIJUANA, CANABINOID AND HIV
There have been a limited number of studies that have addressed the impact of marijuana or cannabinoids on HIV infection and AIDS. It remains to be determined whether the use of marijuana or administration of cannabinoids in a therapeutic mode has potential risks and/or hazards associated with HIV infection. Earlier epidemiologic studies suggested marijuana as a potential cofactor in the development and progression of HIV infection. Using univariant and multivariant analyses, Tindall et al reported there was an association between marijuana use and progression of HIV seropositivity to development of symptomatic AIDS (Tindall et al. 1988). In contrast, a number of studies have documented that marijuana or cannabinoid pro ducts have little impact on the immune system or on HIV infection (Bredt et al. 2002; Coates et al. 1990; Di Franco et al. 1996; Kaslow et al. 1989; Miller and Goodridge 2000; Persaud et al. 1999; Struwe et al. 1993; Wallace et al. 1998). Marijuana use was found to have little effect on non-AIDS mortality in men and on total mortality in women (Cabral 2006). It was reported that antenatal marijuana use was unrelated to sexually transmitted infections during pregnancy (Miller and Goodridge 2000). Smoked and oral cannabinoids did not appear to be a risk in individuals with HIV infection with respect to HIV RNA levels, CD4+and CD8 +cell counts, or protease inhibitor levels (Abrams et al. 2003). In vitro studies also showed contradictory effect of cannabinoid receptor agonist on HIV infection. Klein’s team found that cannabimimetic drugs may enhance HIV infection of human T cell line (Noe et al. 1998). However, Peterson’s group showed that the synthetic cannabinoid WIN 55,212-2 was found to potently inhibit HIV expression in a concentration-and time-dependent manner in CD4+lymphocyte and microglial cell cultures (Peterson et al. 2004; Rock et al. 2007). Using the huPBL SCID mouse model, Roth et al demonstrated that exposure to Tetrahydrocannabinol (THC) in vivo could suppress immune function, increase HIV coreceptor expression, and act as a cofactor to significantly enhance HIV replication (Roth et al. 2005a). Clearly, more in vitro and in vivo studies are needed in order to establish the association between marijuana or cannabinoids and HIV infection/replication. Particularly, it is necessary to develop in vitro model and systems that resemble to human and real-world HIV infection. At the same time, there is a need for longitudinal epidemiological studies on human populations.
ALCOHOL AND HIV
Alcohol is the most commonly used and abused drug in the United States. Approximately 14 million American meet criteria for alcohol abuse or dependence (Bryant 2001). The adverse effects of alcohol abuse are directly associated with induction of immune deficiencies and with increased incidence and prevalence of infectious diseases, including HIV. Since alcohol abuse is widespread and often heavy among HIV-infected individuals and among the most sexually-active age groups at risk of HIV infection, it has been a great interest to investigate the role of alcohol abuse in promoting HIV transmission and infection. It was suggested that alcohol use, both acute and chronic, may increase host susceptibility to HIV infection (Szabo 1997, 1999). Alcohol consumption, especially heavy consumption and abuse, was strongly related to incidence of HIV infection (O’Leary and Hatzenbuehler 2009). Alcohol consumption and alcohol abuse have been identified as potential behavioral risk factors for the transmission of HIV/AIDS, in the form of drinking before risky sexual events or frequent binge drinking as associated with HIV incidence (Erbelding et al. 2004; Fisher et al. 2007; Kalichman et al. 2007). Alcohol consumption, particularly high consumption, has been shown to have a direct influence on adherence to medication in general (Weiss 2004) and specifically to HIV medication (Cook et al. 2001; Hendershot et al. 2009; Meyerhoff 2001; Petry 1999). Chander et al found that hazardous levels of alcohol use were associated with decreased antiretroviral utilization, adherence, and viral suppression independent of active drug use. Combined alcohol and drug use was associated with lower odds of adherence and viral suppression than either drugs or alcohol alone (Chander et al. 2006).
Several lines of evidence has demonstrated there is a strong associations between alcohol consumption and worsening HIV disease. In a cross-sectional study of HIV disease in intravenous drug users, the relative risk of AIDS was 3.8 times higher in heavier drinkers than moderate drinkers (Lake-Bakaar and Grimson 1996). Pol et al. showed that HIV-infected alcohol abusers had a 41% increase in the number of CD4+ T cells after cessation of alcohol use, whereas only a 15% increase was seen in uninfected control subjects who stopped drinking (Pol et al. 1998). Among patients who have a history of alcohol problems and are receiving antiretroviral treatment, alcohol consumption was associated with higher HIV RNA levels and lower CD4+T cell counts (Samet et al. 2003). In a prospective study of HIV-infected drug abusers, alcohol abuse did not correlate with changes in the percentage of CD4+ T cells, however, the percentage of CD8+T cells significantly increased among the heaviest drinkers (Crum et al. 1996).
The findings from the epidemiological studies are supported by several in vitro studies indicating that alcohol augmented HIV replication in PBMC (Table 1) (Bagasra et al. 1996; Bagasra et al. 1989; Bagasra et al. 1993; Saravolatz et al. 1990). Our group demonstrated that alcohol potentiates HIV infection of human blood mononuclear phagocytes (Wang et al. 2002b), T lymphocytes (Wang et al. 2006). Liu et al. showed that alcohol enhanced the entry of CXCR4-tropic HIV into peripheral blood lymphocytes (PBLs) ten-fold compared to untreated cells (Liu et al. 2003). Alcohol enhances HIV infection of normal human oral keratinocytes by up-regulating CXCR4 expression (Chen et al. 2004). These in vitro findings are validated in the animal studies. Bagby et al. found that alcohol could promote SIV infection and replication in both PBMC and alveolar macrophages from rhesus monkeys (Bagby et al. 1998). Several recent reports also showed that under physiologic conditions, chronic alcohol consumption accelerates progression of SIV disease (Bagby et al. 2003; Marcondes et al. 2008; Molina et al. 2008; Poonia et al. 2006).
It is apparent that alcohol consumption has an association with HIV infection/replication. This impact of alcohol on HIV may be a consequence of immunesuppression on immune cells such as T cells and macrophages, the tragets for HIV. However, the mechanism(s) by which alcohol increases suscesitibility of the immune cells to HIV infection are not been fully deliberated. It is difficult to extrapolate results from in vitro and in vivo animal studies to the human conditions. Nevertheless, the studies that have been reviewed suggest that alcohol act, at least as a cofactor that can increase the severity of HIV infection.
IMPACT OF DRUG ABUSE ON MOTHER-TO-FETUS TRANSMISSION OF HIV
Although rates of vertical transmission of HIV in the U.S. and other resource rich areas of the world have declined dramatically since the introduction of antiretroviral therapy, vertical transmission remains a very serious problem in resource poor nations and among poor populations in resource rich countries. Perinatal HIV transmission rates range from 5 ~ 8% to as high as 25 ~ 40%, particularly in settings where little to no prenatal care is available, and in resource-poor countries where there is no access to or no resources for ACTG-standardized treatment regimens (Abrams et al. 1995; Fowler et al. 2000). In order to develop effective strategies to prevent mother to infant transmission of HIV and to prevent disease progression in HIV-infected children, it is essential to understand the host and environment factors that influence perinatal transmission. Although drugs of abuse have been identified as a risk factor for HIV transmission, we know little about the role of drug abuse in vertical transmission of HIV.
Earlier studies demonstrated that HIV-infected women who used illicit drugs during pregnancy had a higher risk of transmitting HIV to their infants than did HIV-infected pregnant women who did not use drugs (Keegan et al. 2010; Shankaran et al. 2007). There is a strong association between the abuse of alcohol, other substances and acquisition, progression of HIV/AIDS among women. Increased levels of alcohol consumption are associated with diminished immune function, as evidenced by reduced levels of CD4+and CD8+ T cell activity. In addition to the pathological relationship between alcohol use and increased susceptibility to HIV infection, the effects of alcohol on accelerated progression to AIDS among infected women are unknown. Although the mechanisms for the role of substance abuse in perinatal transmission of HIV are yet to be determined, it has been demonstrated that such drugs of abuse as opioid, cocaine, and METH have the ability to enhance HIV replication in the target cells (Table 1). In addition, systemic exposure of several drugs of abuse (morphine, cocaine, METH, and alcohol) enhances HIV replication and spread in vivo (Table 2).
The enhancing effect of these abused substances on HIV infectivity/replication could diminish the effectiveness of the anti-HIV drugs (Arnsten et al. 2002; Chander et al. 2006; Golin et al. 2002; Kapadia et al. 2005; Lucas et al. 2002; Lucas et al. 2006). As the availability and use of antiretroviral therapy increase, it is becoming extremely important to determine the impact of drug abuse on efficacy of ACTG-standardized treatment regimens used for treating HIV-infected pregnant women. It is known that high levels of viral load in maternal blood are associated with increased risk of HIV vertical transmission. Increased risk of perinatal HIV transmission may also result from neonatal factors. The neonatal immune system differs qualitatively from the adult system (Merkerova et al. 2009; Millet et al. 1999). It is highly possible that exposure to drugs of abuse would exacerbate neonatal immunity defects, facilitating HIV infection of neonate immune cells and promoting HIV vertical transmission. We and others have shown that neonatal macrophages are more susceptible to HIV infection than paired maternal macrophages (Ho et al. 1992; Reinhardt et al. 1995; Sundaravaradan et al. 2006; Wellensiek et al. 2009). Morphine treatment of placental cord blood monocyte-derived macrophages enhanced HIV infection (Li et al, 2003). In animal models, alcohol administration to a mother during pregnancy affects the fetal immune system (Jacobson et al. 1993; Seelig et al. 1996). It was demonstrated that alcohol also modulates cytokine secretion and synthesis in the human fetus (Ahluwalia et al. 2000).
The mechanism(s) of HIV transmission in utero is poorly understood. In addition to the fact that the placental transmission of HIV is influenced by maternal viral load and maternal/neonatal immunity, placenta itself also has a fundamental role in the vertical HIV transmission from the mothers to the fetus. It is likely that environment factors such as drugs of abuse affect HIV transmission through their detrimental effects on placenta. Abused drugs taken by a pregnant woman reach the fetus primarily by crossing the placenta, the same route taken by oxygen and nutrients, which are needed for fetus’s growth and development. It is known that alcohol exposure is associated with placental dysfunction, decreased placental size, impaired blood flow and nutrient transport, endocrine changes, increased rates of stillbirth and abruption, umbilical cord vasoconstriction, and low birth weight (Burd et al. 2007). There is strong supporting evidence to indicate that the placental transport systems are either direct or indirect targets for the drugs of abuse, including cocaine, amphetamines, nicotine, and cannabinoids (Ganapathy et al. 1999; Malek et al. 2009; Paakki et al. 2000). A recent study using placenta perfusion system demonstrated that maternal use of cocaine and heroin increased the permeability of the placenta (Malek et al. 2009). In spite of advance of our knowledge about the interaction of drugs of abuse with placenta, there are still many unaddressed questions about the impact of drug abuse on placenta structure and function changes. Particularly, we need to know whether drugs of abuse impair innate immunity of placenta such as function of macrophages (Hofbauer cells) and T cells in the placenta. Placental cells produce large quantities of cytokines and chemokines that participate in the anti-HIV activity. Therefore, it is of importance to determine whether drugs of abuse modulate the expression of these cytokines and chemokines.
CONCLUSIONS
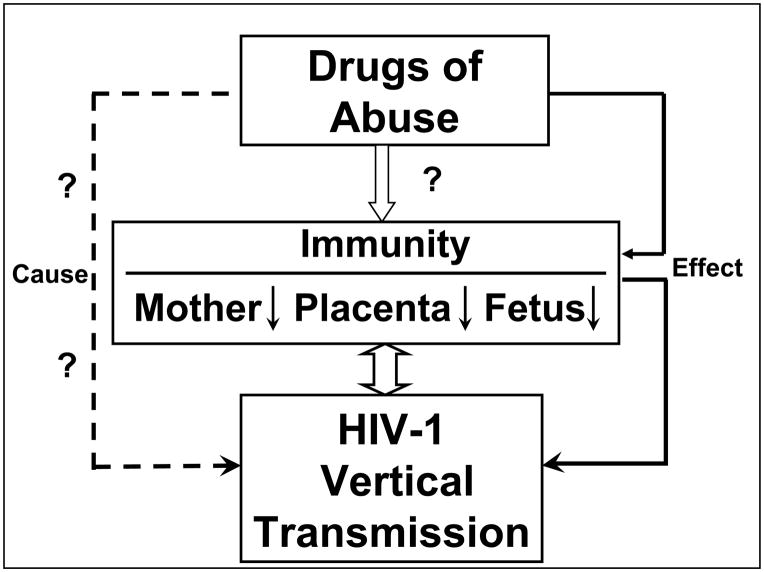
Overall, this review has provided evidence that there is a strong association between drugs of abuse and HIV infection/replication. Although the role of drugs of abuse in promoting HIV disease progression is still debatable, it is known that many of abused drugs exert a profound and detrimental effort on host immunity that has a critical role in restricting HIV replication. It is likely that exposure to drugs of abuse directly or indirectly impairs maternal/neonatal/placental immunity, that facilitating perinatal HIV transmission (Figure 1). However, much remains to be learned about the role of drugs of abuse in vertical HIV transmission. Apparently, more extensive studies are needed in order to determine the specific impact of drugs of abuse on maternal/neonatal/placental immunity and on HIV infection of neonatal/placental immune cells. In addition, it is necessary to determine the effects of drugs of abuse on efficacy of HAART in the context of perinatal transmission.
Figure 1.
The impact of drug abuse on maternal/neonatal/placental immunity and HIV vertical transmission.
Acknowledgments
Supported by the National Institutes of Health grants DA12815, DA27550, DA-25477, DA-022177 and the grant A0901 from W.W. Smith Charitable Trust to Dr. Wen-Zhe Ho.
Footnotes
CONFLICT Of INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. 2003;139 (4):258–66. doi: 10.7326/0003-4819-139-4-200308190-00008. [DOI] [PubMed] [Google Scholar]
- Abrams EJ, Matheson PB, Thomas PA, Thea DM, Krasinski K, Lambert G, Shaffer N, Bamji M, Hutson D, Grimm K, et al. Neonatal predictors of infection status and early death among 332 infants at risk of HIV-1 infection monitored prospectively from birth. New York City Perinatal HIV Transmission Collaborative Study Group. Pediatrics. 1995;96 (3 Pt 1):451–8. [PubMed] [Google Scholar]
- Ahluwalia B, Wesley B, Adeyiga O, Smith DM, Da-Silva A, Rajguru S. Alcohol modulates cytokine secretion and synthesis in human fetus: an in vivo and in vitro study. Alcohol. 2000;21 (3):207–13. doi: 10.1016/s0741-8329(00)00076-8. [DOI] [PubMed] [Google Scholar]
- Alcabes P, Friedland G. Injection drug use and human immunodeficiency virus infection. Clinical Infectious Diseases. 1995;20 (6):1467–79. doi: 10.1093/clinids/20.6.1467. [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17 (5):377–81. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Bachman SE, Jew L, Tawadros R, Cater J, Boden G, Ryan I, Pomerantz RJ. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathogenic mechanisms. J Infect Dis. 1996;173 (3):550–8. doi: 10.1093/infdis/173.3.550. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Kajdacsy-Balla A, Lischner HW. Effects of alcohol ingestion on in vitro susceptibility of peripheral blood mononuclear cells to infection with HIV and of selected T-cell functions. Alcohol Clin Exp Res. 1989;13 (5):636–43. doi: 10.1111/j.1530-0277.1989.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Kajdacsy-Balla A, Lischner HW, Pomerantz RJ. Alcohol intake increases human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells. Journal of Infectious Diseases. 1993;167 (4):789–97. doi: 10.1093/infdis/167.4.789. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Pomerantz RJ. Human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells in the presence of cocaine. J Infect Dis. 1993;168 (5):1157–64. doi: 10.1093/infdis/168.5.1157. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Stoltz DA, Zhang P, Bohm RP, Jr, Nelson S. Simian immunodeficiency virus, infection, alcohol, and host defense. Alcohol Clin Exp Res. 1998;22 (5 Suppl):193S–5S. doi: 10.1111/j.1530-0277.1998.tb03999.x. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Stoltz DA, Zhang P, Kolls JK, Brown J, Bohm RP, Jr, Rockar R, Purcell J, Murphey-Corb M, Nelson S. The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2003;27 (3):495–502. doi: 10.1097/01.ALC.0000057947.57330.BE. [DOI] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50 (1):93–9. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- Bayer BM, Daussin S, Hernandez M, Irvin L. Morphine inhibition of lymphocyte activity is mediated by an opioid dependent mechanism. Neuropharmacology. 1990;29 (4):369–74. doi: 10.1016/0028-3908(90)90096-a. [DOI] [PubMed] [Google Scholar]
- Bobkov AF, Selimova LM, Khanina TA, Zverev SY, Pokrovsky VV, Weber JN, Bobkov EN, Rylkov AV. Human immunodeficiency virus type 1 in illicit-drug solutions used intravenously retains infectivity. J Clin Microbiol. 2005;43 (4):1937–9. doi: 10.1128/JCM.43.4.1937-1939.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddiger D. Metamphetamine use linked to rising HIV transmission. Lancet. 2005;365 (9466):1217–8. doi: 10.1016/S0140-6736(05)74794-2. [DOI] [PubMed] [Google Scholar]
- Booth RE, Watters JK, Chitwood DD. HIV risk-related sex behaviors among injection drug users, crack smokers, and injection drug users who smoke crack. Am J Public Health. 1993;83 (8):1144–8. doi: 10.2105/ajph.83.8.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt BM, Higuera-Alhino D, Shade SB, Hebert SJ, McCune JM, Abrams DI. Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J Clin Pharmacol. 2002;42 (11 Suppl):82S–9S. doi: 10.1002/j.1552-4604.2002.tb06007.x. [DOI] [PubMed] [Google Scholar]
- Bruneau J, Lamothe F, Soto J, Lachance N, Vincelette J, Vassal A, Franco EL. Sex-specific determinants of HIV infection among injection drug users in Montreal. CMAJ. 2001;164 (6):767–73. [PMC free article] [PubMed] [Google Scholar]
- Bryant K. Alcohol and AIDS: A Guide to Research Issues and Opportunities. NIAAA. 2001 [Google Scholar]
- Burd L, Roberts D, Olson M, Odendaal H. Ethanol and the placenta: A review. J Matern Fetal Neonatal Med. 2007;20 (5):361–75. doi: 10.1080/14767050701298365. [DOI] [PubMed] [Google Scholar]
- Cabral GA. Drugs of abuse, immune modulation, and AIDS. J Neuroimmune Pharmacol. 2006;1 (3):280–95. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- Caiaffa WT, Vlahov D, Graham NM, Astemborski J, Solomon L, Nelson KE, Munoz A. Drug smoking, Pneumocystis carinii pneumonia, and immunosuppression increase risk of bacterial pneumonia in human immunodeficiency virus-seropositive injection drug users. Am J Respir Crit Care Med. 1994;150 (6 Pt 1):1493–8. doi: 10.1164/ajrccm.150.6.7952605. [DOI] [PubMed] [Google Scholar]
- Chaisson RE, Bacchetti P, Osmond D, Brodie B, Sande MA, Moss AR. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA. 1989;261 (4):561–5. [PubMed] [Google Scholar]
- Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43 (4):411–7. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Hu S, Sheng WS, Portoghese PS, Peterson PK. Upregulation of HIV-1 expression in cocultures of chronically infected promonocytes and human brain cells by dynorphin. Biochemical Pharmacology. 1995;50 (5):715–22. doi: 10.1016/0006-2952(95)00176-z. [DOI] [PubMed] [Google Scholar]
- Chen H, Zha J, Gowans RE, Camargo P, Nishitani J, McQuirter JL, Cole SW, Zack JA, Liu X. Alcohol enhances HIV type 1 infection in normal human oral keratinocytes by up-regulating cell-surface CXCR4 coreceptor. AIDS Res Hum Retroviruses. 2004;20 (5):513–9. doi: 10.1089/088922204323087769. [DOI] [PubMed] [Google Scholar]
- Chiasson MA, Stoneburner RL, Hildebrandt DS, Ewing WE, Telzak EE, Jaffe HW. Heterosexual transmission of HIV-1 associated with the use of smokable freebase cocaine (crack) AIDS. 1991;5 (9):1121–6. doi: 10.1097/00002030-199109000-00011. [DOI] [PubMed] [Google Scholar]
- Chuang LF, Killam KF, Jr, Chuang RY. Increased replication of simian immunodeficiency virus in CEM x174 cells by morphine sulfate. Biochem Biophys Res Commun. 1993;195 (3):1165–73. doi: 10.1006/bbrc.1993.2167. [DOI] [PubMed] [Google Scholar]
- Chuang RY, Chuang LF, Li Y, Kung HF, Killam KF., Jr SIV mutations detected in morphine-treated Macaca mulatta following SIVmac239 infection. Adv Exp Med Biol. 1995;373:175–81. doi: 10.1007/978-1-4615-1951-5_24. [DOI] [PubMed] [Google Scholar]
- Coates RA, Farewell VT, Raboud J, Read SE, MacFadden DK, Calzavara LM, Johnson JK, Shepherd FA, Fanning MM. Cofactors of progression to acquired immunodeficiency syndrome in a cohort of male sexual contacts of men with human immunodeficiency virus disease. Am J Epidemiol. 1990;132 (4):717–22. doi: 10.1093/oxfordjournals.aje.a115713. [DOI] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, Golub ET, Schwartz RM, Howard AA, Ponath C, Plankey MW, Levine AM, Grey DD. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22 (11):1355–63. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16 (2):83–8. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum RM, Galai N, Cohn S, Celentano DD, Vlahov D. Alcohol use and T-lymphocyte subsets among injection drug users with HIV-1 infection: a prospective analysis. Alcohol Clin Exp Res. 1996;20 (2):364–71. doi: 10.1111/j.1530-0277.1996.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Dhillon NK, Williams R, Peng F, Tsai YJ, Dhillon S, Nicolay B, Gadgil M, Kumar A, Buch SJ. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007;13 (6):483–95. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- Di Franco MJ, Sheppard HW, Hunter DJ, Tosteson TD, Ascher MS. The lack of association of marijuana and other recreational drugs with progression to AIDS in the San Francisco Men’s Health Study. Ann Epidemiol. 1996;6 (4):283–9. doi: 10.1016/s1047-2797(96)00022-1. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Byrd LD, McClure HM, Fultz P, Brantley M, Marsteller F, Ansari AA, Wenzel D, Aceto M. Consequences of opiate-dependency in a monkey model of AIDS. Adv Exp Med Biol. 1993;335:21–8. doi: 10.1007/978-1-4615-2980-4_4. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Falek A. Neuroimmunomodulation by opiates and other drugs of abuse: relationship to HIV infection and AIDS. Advances in Biochemical Psychopharmacology. 1988;44:145–58. [PubMed] [Google Scholar]
- Donahoe RM, O’Neil SP, Marsteller FA, Novembre FJ, Anderson DC, Lankford-Turner P, McClure HH. Probable deceleration of progression of Simian AIDS affected by opiate dependency: studies with a rhesus macaque/SIVsmm9 model. J Acquir Immune Defic Syndr. 2009;50 (3):241–9. doi: 10.1097/QAI.0b013e3181967354. [DOI] [PubMed] [Google Scholar]
- Duncan R, Shapshak P, Page JB, Chiappelli F, McCoy CB, Messiah SE. Crack cocaine: effect modifier of RNA viral load and CD4 count in HIV infected African American women. Front Biosci. 2007;12:1488–95. doi: 10.2741/2162. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188 (12):1820–6. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- Erbelding EJ, Hutton HE, Zenilman JM, Hunt WP, Lyketsos CG. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted disease risk. Sex Transm Dis. 2004;31 (1):8–12. doi: 10.1097/01.OLQ.0000105326.57324.6F. [DOI] [PubMed] [Google Scholar]
- Everall I, Salaria S, Roberts E, Corbeil J, Sasik R, Fox H, Grant I, Masliah E. Methamphetamine stimulates interferon inducible genes in HIV infected brain. J Neuroimmunol. 2005;170 (1–2):158–71. doi: 10.1016/j.jneuroim.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Dykstra LA, Lysle DT. Assessment of the involvement of central nervous system and peripheral opioid receptors in the immunomodulatory effects of acute morphine treatment in rats. J Pharmacol Exp Ther. 1996;276 (2):626–36. [PubMed] [Google Scholar]
- Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sex Transm Dis. 2007;34 (11):856–63. doi: 10.1097/OLQ.0b013e318067b4fd. [DOI] [PubMed] [Google Scholar]
- Fowler MG, Simonds RJ, Roongpisuthipong A. Update on perinatal HIV transmission. Pediatr Clin North Am. 2000;47 (1):21–38. doi: 10.1016/s0031-3955(05)70193-0. [DOI] [PubMed] [Google Scholar]
- Ganapathy VV, Prasad PD, Ganapathy ME, Leibach FH. Drugs of abuse and placental transport. Adv Drug Deliv Rev. 1999;38 (1):99–110. doi: 10.1016/s0169-409x(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Gavrilin MA, Mathes LE, Podell M. Methamphetamine enhances cell-associated feline immunodeficiency virus replication in astrocytes. J Neurovirol. 2002;8 (3):240–9. doi: 10.1080/13550280290049660. [DOI] [PubMed] [Google Scholar]
- Gekker G, Hu S, Sheng WS, Rock RB, Lokensgard JR, Peterson PK. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. Int Immunopharmacol. 2006;6 (6):1029–33. doi: 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gekker G, Hu S, Wentland MP, Bidlack JM, Lokensgard JR, Peterson PK. Kappa -opioid receptor ligands inhibit cocaine-induced HIV-1 expression in microglial cells. J Pharmacol Exp Ther. 2004;309 (2):600–6. doi: 10.1124/jpet.103.060160. [DOI] [PubMed] [Google Scholar]
- Golin CE, Liu H, Hays RD, Miller LG, Beck CK, Ickovics J, Kaplan AH, Wenger NS. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17 (10):756–65. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. Journal of Investigative Medicine. 2002;50 (6):435–42. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52 (2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MC, Flores LR, Bayer BM. Immunosuppression by morphine is mediated by central pathways. Journal Pharmacology and Experimental Therapeutics. 1993;267 (3):1336–41. [PubMed] [Google Scholar]
- Ho WZ, Guo CJ, Yuan CS, Douglas SD, Moss J. Methylnaltrexone antagonizes opioid-mediated enhancement of HIV infection of human blood mononuclear phagocytes. J Pharmacol Exp Ther. 2003;307(3):1158–62. doi: 10.1124/jpet.103.056697. Epub 2003 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WZ, Lioy J, Song L, Cutilli JR, Polin RA, Douglas SD. Infection of cord blood monocyte-derived macrophages with human immunodeficiency virus type 1. J Virol. 1992;66 (1):573–9. doi: 10.1128/jvi.66.1.573-579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House RV, Thomas PT, Bhargava HN. Comparison of immune functional parameters following in vitro exposure to natural and synthetic amphetamines. Immunopharmacol Immunotoxicol. 1994;16 (1):1–21. doi: 10.3109/08923979409029897. [DOI] [PubMed] [Google Scholar]
- Hung CY, Lefkowitz SS, Geber WF. Interferon inhibition by narcotic analgesics. Proceedings of the Society for Experimental Biology and Medicine. 1973;142 (1):106–11. doi: 10.3181/00379727-142-36968. [DOI] [PubMed] [Google Scholar]
- In SW, Son EW, Rhee DK, Pyo S. Modulation of murine macrophage function by methamphetamine. J Toxicol Environ Health A. 2004;67 (23–24):1923–37. doi: 10.1080/15287390490514589. [DOI] [PubMed] [Google Scholar]
- In SW, Son EW, Rhee DK, Pyo S. Methamphetamine administration produces immunomodulation in mice. J Toxicol Environ Health A. 2005;68 (23–24):2133–45. doi: 10.1080/15287390500177156. [DOI] [PubMed] [Google Scholar]
- Iwasa M, Maeno Y, Inoue H, Koyama H, Matoba R. Induction of apoptotic cell death in rat thymus and spleen after a bolus injection of methamphetamine. Int J Legal Med. 1996;109 (1):23–8. doi: 10.1007/BF01369597. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Kaplan-Estrin MG. Teratogenic effects of alcohol on infant development. Alcohol Clin Exp Res. 1993;17 (1):174–83. doi: 10.1111/j.1530-0277.1993.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prev Sci. 2007;8 (2):141–51. doi: 10.1007/s11121-006-0061-2. [DOI] [PubMed] [Google Scholar]
- Kapadia F, Cook JA, Cohen MH, Sohler N, Kovacs A, Greenblatt RM, Choudhary I, Vlahov D. The relationship between non-injection drug use behaviors on progression to AIDS and death in a cohort of HIV seropositive women in the era of highly active antiretroviral therapy use. Addiction. 2005;100 (7):990–1002. doi: 10.1111/j.1360-0443.2005.01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow RA, Blackwelder WC, Ostrow DG, Yerg D, Palenicek J, Coulson AH, Valdiserri RO. No evidence for a role of alcohol or other psychoactive drugs in accelerating immunodeficiency in HIV-1-positive individuals. A report from the Multicenter AIDS Cohort Study. JAMA. 1989;261 (23):3424–9. [PubMed] [Google Scholar]
- Keegan J, Parva M, Finnegan M, Gerson A, Belden M. Addiction in pregnancy. J Addict Dis. 2010;29 (2):175–91. doi: 10.1080/10550881003684723. [DOI] [PubMed] [Google Scholar]
- Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, Donahoe RM, Kraiselburd E, Stephens EB, Kumar A. Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol. 2004;78 (20):11425–8. doi: 10.1128/JVI.78.20.11425-11428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake-Bakaar G, Grimson R. Alcohol abuse and stage of HIV disease in intravenous drug abusers. J R Soc Med. 1996;89 (7):389–92. doi: 10.1177/014107689608900709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, Heaton RK, McCutchan JA, Grant I. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS. 2005;19 (Suppl 3):S72–8. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- Li Y, Merrill JD, Mooney K, Song L, Wang X, Guo CJ, Savani RC, Metzger DS, Douglas SD, Ho WZ. Morphine enhances HIV infection of neonatal macrophages. Pediatric Research. 2003;54 (2):282–8. doi: 10.1203/01.PDR.0000074973.83826.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang X, Tian S, Guo CJ, Douglas SD, Ho WZ. Methadone enhances human immunodeficiency virus infection of human immune cells. Journal of Infectious Diseases. 2002;185 (1):118–22. doi: 10.1086/338011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, Wang YJ, Zhou L, Ho WZ. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172 (6):1617–24. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zha J, Nishitani J, Chen H, Zack JA. HIV-1 infection in peripheral blood lymphocytes (PBLs) exposed to alcohol. Virology. 2003;307 (1):37–44. doi: 10.1016/s0042-6822(02)00031-4. [DOI] [PubMed] [Google Scholar]
- Lockwood LL, Silbert LH, Fleshner M, Laudenslager ML, Watkins LR, Maier SF. Morphine-induced decreases in in vivo antibody responses. Brain Behavior and Immunity. 1994;8 (1):24–36. doi: 10.1006/brbi.1994.1003. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16 (5):767–74. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163 (5):412–20. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- Malek A, Obrist C, Wenzinger S, von Mandach U. The impact of cocaine and heroin on the placental transfer of methadone. Reprod Biol Endocrinol. 2009;7:61. doi: 10.1186/1477-7827-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83 (4):955–63. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Flynn C, Watry DD, Zandonatti M, Fox HS. Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. Am J Pathol. 2010;177 (1):355–61. doi: 10.2353/ajpath.2010.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes MC, Watry D, Zandonatti M, Flynn C, Taffe MA, Fox H. Chronic alcohol consumption generates a vulnerable immune environment during early SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2008;32 (9):1583–92. doi: 10.1111/j.1530-0277.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Vanichseni S, Suntharasamai P, Mock PA, Griensven FV, Pitisuttithum P, Tappero JW, Chiamwongpaet S, Sangkum U, Kitayaporn D, Gurwith M, Choopanya K. Group ftBVE. Drug use and the risk of HIV infection amongst injection drug users participating in an HIV vaccine trial in Bangkok, 1999–2003. International Journal of Drug Policy. doi: 10.1016/j.drugpo.2009.12.0022010. [DOI] [PubMed] [Google Scholar]
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug and Alcohol Dependence. 2001;62 (2):111–23. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- Merkerova M, Vasikova A, Bruchova H, Libalova H, Topinka J, Balascak I, Sram RJ, Brdicka R. Differential gene expression in umbilical cord blood and maternal peripheral blood. Eur J Haematol. 2009;83 (3):183–90. doi: 10.1111/j.1600-0609.2009.01281.x. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ. Effects of alcohol and HIV infection on the central nervous system. Alcohol Res Health. 2001;25 (4):288–98. [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Jr, Goodridge C. Antenatal marijuana use is unrelated to sexually transmitted infections during pregnancy. Infect Dis Obstet Gynecol. 2000;8 (3–4):155–7. doi: 10.1155/S106474490000020X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet V, Lacroze V, Bodiou AC, Dubus JC, D’Ercole C, Unal D. Ontogeny of the immune system. Arch Pediatr. 1999;6 (Suppl 1):14S–9S. doi: 10.1016/s0929-693x(99)80241-3. [DOI] [PubMed] [Google Scholar]
- Molina PE, Lang CH, McNurlan M, Bagby GJ, Nelson S. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res. 2008;32 (1):138–47. doi: 10.1111/j.1530-0277.2007.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr. 2004;35 (1):46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- Morin SF, Steward WT, Charlebois ED, Remien RH, Pinkerton SD, Johnson MO, Rotheram-Borus MJ, Lightfoot M, Goldstein RB, Kittel L, Samimy-Muzaffar F, Weinhardt L, Kelly JA, Chesney MA. Predicting HIV transmission risk among HIV-infected men who have sex with men: findings from the healthy living project. J Acquir Immune Defic Syndr. 2005;40 (2):226–35. doi: 10.1097/01.qai.0000166375.16222.eb. [DOI] [PubMed] [Google Scholar]
- Nair MP, Mahajan SD, Schwartz SA, Reynolds J, Whitney R, Bernstein Z, Chawda RP, Sykes D, Hewitt R, Hsiao CB. Cocaine modulates dendritic cell-specific C type intercellular adhesion molecule-3-grabbing nonintegrin expression by dendritic cells in HIV-1 patients. J Immunol. 2005;174 (11):6617–26. doi: 10.4049/jimmunol.174.11.6617. [DOI] [PubMed] [Google Scholar]
- Nair MP, Saiyed ZM, Nair N, Gandhi NH, Rodriguez JW, Boukli N, Provencio-Vasquez E, Malow RM, Miguez-Burbano MJ. Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. J Neuroimmune Pharmacol. 2009;4 (1):129–39. doi: 10.1007/s11481-008-9128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MP, Schwartz SA, Polasani R, Hou J, Sweet A, Chadha KC. Immunoregulatory effects of morphine on human lymphocytes. Clinical and Diagnostic Laboratory Immunology. 1997a;4 (2):127–32. doi: 10.1128/cdli.4.2.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MPN, Schwartz SA, Polasani R, Hou J, Sweet A, Chadha KC. Immunoregulatory effects of morphine on human lymphocytes. Clinical and Diagnostic Laboratory Immunology. 1997b;4 (2):127–32. doi: 10.1128/cdli.4.2.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KE, Galai N, Safaeian M, Strathdee SA, Celentano DD, Vlahov D. Temporal trends in the incidence of human immunodeficiency virus infection and risk behavior among injection drug users in Baltimore, Maryland, 1988–1998. Am J Epidemiol. 2002;156 (7):641–53. doi: 10.1093/aje/kwf086. [DOI] [PubMed] [Google Scholar]
- Noe SN, Nyland SB, Ugen K, Friedman H, Klein TW. Cannabinoid receptor agonists enhance syncytia formation in MT-2 cells infected with cell free HIV-1MN. Adv Exp Med Biol. 1998;437:223–9. doi: 10.1007/978-1-4615-5347-2_25. [DOI] [PubMed] [Google Scholar]
- Novick DM, Ochshorn M, Ghali V, Croxson TS, Mercer WD, Chiorazzi N, Kreek MJ. Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term methadone maintenance patients. Journal Pharmacology and Experimental Therapeutics. 1989;250 (2):606–10. [PubMed] [Google Scholar]
- O’Leary A, Hatzenbuehler M. Alcohol and AIDS. In: Korsmeyer P, Kranzler HR, editors. Addictive Behaviors. 3. Vol. 1. Detroit, MI: Macmillian Reference USA; 2009. [Google Scholar]
- Ochshorn M, Novick DM, Kreek MJ. In vitro studies of the effect of methadone on natural killer cell activity. Israel Journal of Medical Sciences. 1990;26 (8):421–5. [PubMed] [Google Scholar]
- Paakki P, Stockmann H, Kantola M, Wagner P, Lauper U, Huch R, Elovaara E, Kirkinen P, Pasanen M. Maternal drug abuse and human term placental xenobiotic and steroid metabolizing enzymes in vitro. Environ Health Perspect. 2000;108 (2):141–5. doi: 10.1289/ehp.00108141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Semple SJ, Staines H, Lozada R, Orozovich P, Bucardo J, Philbin MM, Pu M, Fraga M, Amaro H, Torre Ade L, Martinez G, Magis–Rodriguez C, Strathdee SA. Prevalence and correlates of HIV infection among female sex workers in 2 Mexico-US border cities. J Infect Dis. 2008;197 (5):728–32. doi: 10.1086/527379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechansky F, Woody G, Inciardi J, Surratt H, Kessler F, Von Diemen L, Bumaguin DB. HIV seroprevalence among drug users: an analysis of selected variables based on 10 years of data collection in Porto Alegre, Brazil. Drug Alcohol Depend. 2006;82 (Suppl 1):S109–13. doi: 10.1016/s0376-8716(06)80017-7. [DOI] [PubMed] [Google Scholar]
- Peck JA, Shoptaw S, Rotheram-Fuller E, Reback CJ, Bierman B. HIV-associated medical, behavioral, and psychiatric characteristics of treatment-seeking, methamphetamine-dependent men who have sex with men. J Addict Dis. 2005;24 (3):115–32. doi: 10.1300/J069v24n03_10. [DOI] [PubMed] [Google Scholar]
- Persaud NE, Klaskala W, Tewari T, Shultz J, Baum M. Drug use and syphilis. Co-factors for HIV transmission among commercial sex workers in Guyana. West Indian Med J. 1999;48 (2):52–6. [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Brummitt C, Pentel P, Bullock M, Simpson M, Hitt J, Sharp B. Suppression of human peripheral blood mononuclear cell function by methadone and morphine. Journal of Infectious Diseases. 1989;159 (3):480–7. doi: 10.1093/infdis/159.3.480. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Chao CC, Schut R, Molitor TW, Balfour HH., Jr Cocaine potentiates HIV-1 replication in human peripheral blood mononuclear cell cocultures. Involvement of transforming growth factor-beta. J Immunol. 1991;146 (1):81–4. [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Chao CC, Schut R, Verhoef J, Edelman CK, Erice A, Balfour HH., Jr Cocaine amplifies HIV-1 replication in cytomegalovirus-stimulated peripheral blood mononuclear cell cocultures. J Immunol. 1992;149 (2):676–80. [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Anderson WR, Kravitz F, Portoghese PS, Balfour HH, Jr, Chao CC. Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J Neuroimmunol. 1994;50 (2):167–75. doi: 10.1016/0165-5728(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Cabral G, Lokensgard JR. Cannabinoids and morphine differentially affect HIV-1 expression in CD4(+) lymphocyte and microglial cell cultures. J Neuroimmunol. 2004;147 (1–2):123–6. doi: 10.1016/j.jneuroim.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC. Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implication of an atypical mu-opioid receptor. Neuropharmacology. 1999;38 (2):273–8. doi: 10.1016/s0028-3908(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC. Mechanisms of morphine-induced immunomodulation. Biochem Pharmacol. 1993;46 (3):343–8. doi: 10.1016/0006-2952(93)90508-t. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4 (9):869–73. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Petry NM. Alcohol use in HIV patients: what we don’t know may hurt us. Int J STD AIDS. 1999;10 (9):561–70. doi: 10.1258/0956462991914654. [DOI] [PubMed] [Google Scholar]
- Plankey MW, Ostrow DG, Stall R, Cox C, Li X, Peck JA, Jacobson LP. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45 (1):85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol S, Lamorthe B, Thi NT, Thiers V, Carnot F, Zylberberg H, Berthelot P, Brechot C, Nalpas B. Retrospective analysis of the impact of HIV infection and alcohol use on chronic hepatitis C in a large cohort of drug users. Journal of Hepatology. 1998;28 (6):945–50. doi: 10.1016/s0168-8278(98)80341-3. [DOI] [PubMed] [Google Scholar]
- Poonia B, Nelson S, Bagby GJ, Zhang P, Quniton L, Veazey RS. Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Res Hum Retroviruses. 2006;22 (6):589–94. doi: 10.1089/aid.2006.22.589. [DOI] [PubMed] [Google Scholar]
- Reinhardt PP, Reinhardt B, Lathey JL, Spector SA. Human cord blood mononuclear cells are preferentially infected by non-syncytium-inducing, macrophage-tropic human immunodeficiency virus type 1 isolates. J Clin Microbiol. 1995;33 (2):292–7. doi: 10.1128/jcm.33.2.292-297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JL, Mahajan SD, Sykes D, Nair MP. Heroin-Induces Differential Protein Expression by Normal Human Astrocytes (NHA) Am J Infect Dis. 2006;2 (2):49–57. doi: 10.3844/ajidsp.2006.49.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risdahl JM, Khanna KV, Peterson PK, Molitor TW. Opiates and infection. Journal of Neuroimmunology. 1998;83 (1–2):4–18. doi: 10.1016/s0165-5728(97)00216-6. [DOI] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cabral GA, Martin BR, Peterson PK. WIN 55,212-2-mediated inhibition of HIV-1 expression in microglial cells: involvement of cannabinoid receptors. J Neuroimmune Pharmacol. 2007;2 (2):178–83. doi: 10.1007/s11481-006-9040-4. [DOI] [PubMed] [Google Scholar]
- Ronald PJ, Robertson JR, Elton RA. Continued drug use and other cofactors for progression to AIDS among injecting drug users. Aids. 1994;8 (3):339–43. doi: 10.1097/00002030-199403000-00007. [DOI] [PubMed] [Google Scholar]
- Roth MD, Tashkin DP, Choi R, Jamieson BD, Zack JA, Baldwin GC. Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J Infect Dis. 2002;185 (5):701–5. doi: 10.1086/339012. [DOI] [PubMed] [Google Scholar]
- Roth MD, Tashkin DP, Whittaker KM, Choi R, Baldwin GC. Tetrahydrocannabinol suppresses immune function and enhances HIV replication in the huPBL-SCID mouse. Life Sci. 2005a;77 (14):1711–22. doi: 10.1016/j.lfs.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol. 2005b;78 (6):1198–203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- Rothenberg R, Woelfel M, Stoneburner R, Milberg J, Parker R, Truman B. Survival with the acquired immunodeficiency syndrome. Experience with 5833 cases in New York City. New England Journal of Medicine. 1987;317 (21):1297–302. doi: 10.1056/NEJM198711193172101. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Traphagen ET, Lyon SM, Freedberg KA. Alcohol consumption and HIV disease progression: are they related? Alcohol Clin Exp Res. 2003;27 (5):862–7. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- Saravolatz LD, Cerra RF, Pohlod DJ, Smereck S. The effect of alcohol on HIV infection in vitro. Prog Clin Biol Res. 1990;325:267–71. [PubMed] [Google Scholar]
- Schweitzer C, Keller F, Schmitt MP, Jaeck D, Adloff M, Schmitt C, Royer C, Kirn A, Aubertin AM. Morphine stimulates HIV replication in primary cultures of human Kupffer cells. Res Virol. 1991;142 (2–3):189–95. doi: 10.1016/0923-2516(91)90056-9. [DOI] [PubMed] [Google Scholar]
- Seelig LL, Jr, Steven WM, Stewart GL. Effects of maternal ethanol consumption on the subsequent development of immunity to Trichinella spiralis in rat neonates. Alcohol Clin Exp Res. 1996;20 (3):514–22. doi: 10.1111/j.1530-0277.1996.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Selimova LM, Khanina TA, Kazennova EV, Zverev S, Pokrovskii VV, Bobkov AF. Effect of heroin-containing substances on the infectivity of the human immunodeficiency virus type 1 in vitro. Vopr Virusol. 2002;47 (5):16–20. [PubMed] [Google Scholar]
- Shankaran S, Lester BM, Das A, Bauer CR, Bada HS, Lagasse L, Higgins R. Impact of maternal substance use during pregnancy on childhood outcome. Semin Fetal Neonatal Med. 2007;12 (2):143–50. doi: 10.1016/j.siny.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ. Associations between methamphetamine use and HIV among men who have sex with men: a model for guiding public policy. J Urban Health. 2006;83 (6):1151–7. doi: 10.1007/s11524-006-9119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ. Methamphetamine use and infectious disease-related behaviors in men who have sex with men: implications for interventions. Addiction. 2007;102 (Suppl 1):130–5. doi: 10.1111/j.1360-0443.2006.01775.x. [DOI] [PubMed] [Google Scholar]
- Specter S. Drugs of abuse and infectious diseases. Journal of the Florida Medical Association. 1994;81 (7):485–7. [PubMed] [Google Scholar]
- Squinto SP, Mondal D, Block AL, Prakash O. Morphine-induced transactivation of HIV-1 LTR in human neuroblastoma cells. AIDS Research and Human Retroviruses. 1990;6 (10):1163–8. doi: 10.1089/aid.1990.6.1163. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309 (1):99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Scharrer B, Smith EM, Hughes TK, Jr, Magazine HI, Bilfinger TV, Hartman AR, Fricchione GL, Liu Y, Makman MH. Opioid and opiate immunoregulatory processes. Crit Rev Immunol. 1996;16 (2):109–44. doi: 10.1615/critrevimmunol.v16.i2.10. [DOI] [PubMed] [Google Scholar]
- Struwe M, Kaempfer SH, Geiger CJ, Pavia AT, Plasse TF, Shepard KV, Ries K, Evans TG. Effect of dronabinol on nutritional status in HIV infection. Ann Pharmacother. 1993;27 (7–8):827–31. doi: 10.1177/106002809302700701. [DOI] [PubMed] [Google Scholar]
- Sundaravaradan V, Saxena SK, Ramakrishnan R, Yedavalli VR, Harris DT, Ahmad N. Differential HIV-1 replication in neonatal and adult blood mononuclear cells is influenced at the level of HIV-1 gene expression. Proc Natl Acad Sci U S A. 2006;103 (31):11701–6. doi: 10.1073/pnas.0602185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Carlos MP, Chuang LF, Torres JV, Doi RH, Chuang RY. Methadone induces CCR5 and promotes AIDS virus infection. FEBS Lett. 2002;519 (1–3):173–7. doi: 10.1016/s0014-5793(02)02746-1. [DOI] [PubMed] [Google Scholar]
- Szabo G. Alcohol’s contribution to compromised immunity. Alcohol Health Res World. 1997;21 (1):30–41. [PMC free article] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34 (6):830–41. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Tindall B, Cooper DA, Donovan B, Barnes T, Philpot CR, Gold J, Penny R. The Sydney AIDS Project: development of acquired immunodeficiency syndrome in a group of HIV seropositive homosexual men. Aust N Z J Med. 1988;18 (1):8–15. doi: 10.1111/j.1445-5994.1988.tb02232.x. [DOI] [PubMed] [Google Scholar]
- UNAIDS. Report on the Global HIV/AIDS Epidemic: Executive Summary. Geneva, Switzerland: UNAIDS; 2008. [Google Scholar]
- UNAIDS. AIDS epidemic update. Geneva, Switzerland: UNAIDS; 2009. [Google Scholar]
- Wallace JM, Lim R, Browdy BL, Hopewell PC, Glassroth J, Rosen MJ, Reichman LB, Kvale PA. Risk factors and outcomes associated with identification of Aspergillus in respiratory specimens from persons with HIV disease. Pulmonary Complications of HIV Infection Study Group. Chest. 1998;114 (1):131–7. doi: 10.1378/chest.114.1.131. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Loh HH, Roy S. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem. 2003;278 (39):37622–31. doi: 10.1074/jbc.M301224200. [DOI] [PubMed] [Google Scholar]
- Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. The immunosuppressive effects of chronic morphine treatment are partially dependent on corticosterone and mediated by the mu-opioid receptor. J Leukoc Biol. 2002a;71 (5):782–90. [PubMed] [Google Scholar]
- Wang X, Douglas SD, Metzger DS, Guo CJ, Li Y, O’Brien CP, Song L, Davis-Vogal A, Ho WZ. Alcohol potentiates HIV-1 infection of human blood mononuclear phagocytes. Alcohol Clin Exp Res. 2002b;26 (12):1880–6. doi: 10.1097/01.ALC.0000042148.50808.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Peng JS, Metzger DS, O’Brien CP, Zhang T, Ho WZ. Naltrexone inhibits alcohol-mediated enhancement of HIV infection of T lymphocytes. J Leukoc Biol. 2006;30:30. doi: 10.1189/jlb.1105642. [DOI] [PubMed] [Google Scholar]
- Wang X, Tan N, Douglas SD, Zhang T, Wang YJ, Ho WZ. Morphine inhibits CD8+ T cell-mediated, noncytolytic, anti-HIV activity in latently infected immune cells. Journal of Leukocyte Biology. 2005;78 (3):772–6. doi: 10.1189/jlb.0305167. [DOI] [PubMed] [Google Scholar]
- Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13 (2):257–62. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- Weiss RD. Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction. 2004;99 (11):1382–92. doi: 10.1111/j.1360-0443.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- Wellensiek BP, Ramakrishnan R, Sundaravaradan V, Mehta R, Harris DT, Ahmad N. Differential HIV-1 integration targets more actively transcribed host genes in neonatal than adult blood mononuclear cells. Virology. 2009;385 (1):28–38. doi: 10.1016/j.virol.2008.10.052. [DOI] [PubMed] [Google Scholar]
- Wohl AR, Johnson DF, Lu S, Jordan W, Beall G, Currier J, Simon PA. HIV risk behaviors among African American men in Los Angeles County who self-identify as heterosexual. J Acquir Immune Defic Syndr. 2002;31 (3):354–60. doi: 10.1097/00126334-200211010-00013. [DOI] [PubMed] [Google Scholar]
- Yu Q, Larson DF, Watson RR. Heart disease, methamphetamine and AIDS. Life Sci. 2003;73 (2):129–40. doi: 10.1016/s0024-3205(03)00260-1. [DOI] [PubMed] [Google Scholar]
- Yu Q, Zhang D, Walston M, Zhang J, Liu Y, Watson RR. Chronic methamphetamine exposure alters immune function in normal and retrovirus-infected mice. Int Immunopharmacol. 2002;2 (7):951–62. doi: 10.1016/s1567-5769(02)00047-4. [DOI] [PubMed] [Google Scholar]