Abstract
Objectives/Hypothesis
Prostaglandin (PG)E2 has been implicated in a variety of disease processes. It has been described as antifibrotic in the lower airway, yet scar-inducing in the skin. We seek to describe the effects of PGE2 on vocal fold fibroblasts and its interactions with TGF-β1. In addition, we describe a novel organotypic model, a critical step in the development of therapeutic trials.
Study Design
In vitro, ex vivo
Level of Evidence
N/A
Methods
Collagen secretion by human vocal fold fibroblasts (HVFF) was assayed in response to TGF-β1, PGE2, and specific EP receptor agonists. Basal HVFF migratory rate was also quantified in response to PGE2. TGF-β1 induced COX-2 mRNA expression/PGE2 secretion. Excised vocal folds were subjected to exogenous IL-1β; PGE2 secretion into the supernatant was then assayed.
Results
TGF-β1-induced collagen secretion was blunted in a dose-dependent manner in response to PGE2. This effect appears to be mediated primarily through the EP1 and EP2 receptors. TGF-β1 induced COX-2 mRNA expression and PGE2 secretion. In our organ culture model, IL-1β stimulated PGE2 secretion in a dose-dependent manner.
Conclusions
PGE2 is anti-fibrotic; this finding suggests that the upper airway response to this inflammatory mediator differs significantly from the lower airway. These data have important clinical implications for a variety of pathological processes. Furthermore, exogenous TGF-β1 elicits induction of COX-2, suggesting inherent complexity regarding these processes and PGE2, signaling specifically. In addition, our organ culture model may prove useful as a means to quantify biological phenomena in the vocal folds.
Keywords: Vocal fold, inflammation, cyclooxygenase, prostaglandin
INTRODUCTION
The complexity of interactions between multiple cell types, matrix, and soluble mediators of wound healing following injury are substantial. As evidenced by data regarding the pathological processes involved in pulmonary fibrosis, the events following injury are also organ-specific. These observations not only confound our collective insight regarding a generic tissue response following injury, but also impede the translational generalizability of data from other systems to the vocal folds, an inherently unique organ from a biomechanical perspective. Furthermore, relatively small regions of fibrosis appear to have disproportionately detrimental effects on phonatory physiology. In general, little is known regarding the events following injury in the vocal folds, beyond global histological outcomes. To address these pervasive issues which likely contribute to the lack of standardized, efficacious therapies for vocal fold fibrosis, we have attempted to continue to describe specific pathways as a means to potentially provide targets for therapeutic interventions with the ultimate goal of directing wound healing toward a more regenerative, less fibrotic outcome.
Specifically, our laboratory has long posited that interventions which target the inflammatory or early events following injury have the potential to coordinate a more favorable wound outcome. Generally, the response following injury involves a series of coordinated, overlapping phases including inflammation, proliferation, and remodeling, in adults, yielding an overproduction of poorly organized collagen fibers.1 In contrast, wounding during the first and second trimester of fetal gestation heal quickly and without scar.1-4 The hallmark of scarless fetal healing appears to be the absence of a robust inflammatory response.5-11 The development of anti-fibrotic therapeutics based on the inherent biochemical switches responsible for the transition from scarless to adult healing along with their signaling pathways should prove to be clinically valuable.
Cyclooxygenase (COX)-2, the rate-limiting enzyme in prostaglandin synthesis via the conversion of arachadonic acid, is induced during the acute response to injuy12 and may be implicated in this transition as prostaglandins, and particularly prostaglandin (PG)E2, are multifunctional; active in both the acute inflammatory response and the subsequent fibroblastic events including alteration of cytoskeletal dynamics as well as fibroblast migration, contraction, proliferation, and extracellular matrix metabolism.13-15 In most cases, PGE2 is considered a potent proinflammatory mediator implicated in the pathogenesis of several processes including periodontitis, UVB-mediated cutaneous inflammation, rheumatoid arthritis, cancer growth, and keloid formation.16-19 Interestingly, in most cases, inhibition of COX-2 is effective at diminishing disease progression. Furthermore, topical inhibition of COX-2 has been shown to be anti-fibrotic in full thickness skin wounds.20 In contrast, however, the lung may serve as a privileged site for PGE221 whereby increased PGE2 concentrations may be therapeutic in the context of pulmonary fibrosis.22
The vocal folds provide an interesting model for investigating these phenomena given their proximity to the lungs. Both COX-2 and PGE2 have been shown to be upregulated following acute vocal fold injury,23-24 and our laboratory previously characterized COX-2 signaling in human vocal fold fibroblasts.25 We now seek to further elucidate the regulatory role of PGE2 on these cells with a specific interest in the interaction between transforming growth factor (TGF)-β1, a putative profibrotic member of the TGF superfamily which implicated in a variety of fibroproliferative processes including vocal fold fibrosis,26-29 and PGE2. In addition, acknowledging that current models of investigation in the vocal folds are relatively limited, we sought to develop an ex vivo, organ culture system to quantify the tissue response to exogenous mediators of healing. Ultimately, this model may prove to be a critical link between in vitro data and in vivo trials.
MATERIALS AND METHODS
Cell Model and Reagents
The HVOX human vocal fold fibroblast cell line developed by our laboratory was employed for each in vitro experiment described.29 Interleukin-1β and PGE2 were purchased from Sigma-Aldrich (St. Louis, MO) and Cayman Chemical (Ann Arbor, MI), respectively. EP receptor agonists, 17-phenyl trinor Prostaglandin E2 (EP1), butaprost (EP2), sulprostone (EP3), and 11-deoxy Prostaglandin E1 (EP4) were purchased from Cayman Chemical (Ann Arbor, MI). TGF-β1 was purchased from R&D Systems (Minneapolis, MN).
Fibroblast Collagen Secretion
Total secreted collagen in the supernatant media was quantified using the Sircol Soluble Collagen Assay (Biocolor, Ltd, Ireland). Near-confluent cells grown in 6-well plates were treated for 24 hours and the post-culture medium (supernatant) was collected. We first sought to describe the effects of PGE2 on TGF-β1-mediated collagen secretion. For these experiments, physiological concentrations of both PGE2 (1.0 and 10.0uM) and TGF-β1 (10ng/mL) were applied to the HVOX cultures, concurrently. Secondly, we sought to determine the relevant EP receptors mediating this effect. As such, we concurrently treated HVOX with TGF-β1 (10ng/mL) and the individual EP receptor agonists described below. Each agonist was employed at a concentration of 10uM, as suggested by the manufacturer. Sircol Dye Reagent was added to 200μL of supernatant media and vortexed every 5 minutes for 30 minutes, then centrifuged at 12,000rpm for 10 minutes. The unbound dye in the supernatant was then removed by inversion and the pellet incubated for 10 minutes with 1mL of alkali reagent to dissolve bound dye. The samples were then read at 550nm using a multi-well plate reader. Absorbance values were extrapolated to collagen concentration via standard curve. All data were standardized to total protein content using the bicinchoninic acid assay.
Fibroblast Migration
HVOX migratory rate was assessed using an in vitro scratch assay previously described by our laboratory and others.29 Confluent HVOX in pre-marked 24-well plates were serum-starved overnight. The monolayers were interrupted by scratching along the longitudinal axis of each well using a 200μL pipette tip, washed twice with PBS, and then incubated for eight hours in serum-free medium containing 1.0 and 10.0uM PGE2. Digital images were captured of each well immediately following the scratch procedure and again, eight hours later, using the markings on the plates as landmarks to ensure identically positioned photographs were obtained at both time points. The difference in the relative area (pixels) of the scratch over time was assessed using ImageJ (National Institutes of Health). The following equation was employed to calculate HVOX migration over the period of observation: initial area-final area=Δarea/8 hours.
Polymerase Chain Reaction
In order to determine the effects of exogenous TGF-β1 on COX-2 mRNA expression, we isolated total RNA from HVOX following 24 hours of treatment with TGF-β1 (0.1, 1, and 10ng/mL). The OneStep RT-PCR Kit (Qiagen Inc., Santa Clara, CA) was then employed to reverse transcribe and amplify the RNA, following the manufacturer's protocol. Briefly, 13.0 μL of OneStep PCR Mix was added to 10ng RNA with 2.0μL of COX-2 primer, incubated at 37° for one hour, heated to 95° for six minutes, and then subjected to 28 cycles of 30 seconds at 37°, 30 seconds at 60°, and one minute at 72°. PCR products were electrophoresed on a 1.5% agarose gel containing ethidium bromide.
PGE2 Immunoassay
PGE2 synthesis was assayed via a commercially-available ELISA kit (R&D Systems, Minneapolis, MN). Concentrations were standardized to total cellular protein.
Vocal Fold Organ Culture
We then sought to provide preliminary data regarding the viability of ex vivo methods to quantify biological phenoema in the vocal folds, primarily the secretion of mediators of wound healing. As such, adult, female New Zealand rabbits employed by other investigators were sacrificed and subjected to total laryngectomy. The membranous vocal folds were then dissected from the anterior commissure to the vocal process in the anterior-posterior dimension and from the inferior border of the fold to the superior surface within the ventricle under magnification. These specimens included epithelium, lamina propria, and vocalis muscle. The specimens were weighed, washed in phosphate buffered saline (PBS) three times, and placed in serum-free Dulbecco's Modified Eagle Medium (DMEM) supplemented with 1% antibiotic/antimycotic cocktail for 4 hours. The media was then changed to include supplementation with various concentrations of IL-1β and left at 37°C for 24 hours. The media was then harvested, diluted appropriately, and subjected to immunoassay for PGE2. Immunoassay data were standardized to tissue weight. Data are presented as pg/mL/gram of tissue.
Statistical Analyses
One way Analyses of Variance were performed using SPSS (v12.0). Pending a significant main effect at p=0.05, post hoc comparisons were employed using the Tukey method.
RESULTS
PGE2 limited TGF-β1-induced HVOX collagen synthesis
As shown in Figure 1A, exogenous TGF-β1 elicited a significant increase collagen secretion by HVOX (p<0.05), consistent with our previous findings.29 PGE2 abrogated this effect at concentrations of 1.0 and 10.0μM (p<0.05). This effect was amplified at increased concentrations; 10μM of PGE2 decreased TGF-β1 mediated collagen synthesis to below baseline levels. We then employed specific agonists of the four cell surface prostaglandin receptors to further investigate the mechanism of this effect. At baseline, treatment with each EP receptor agonists decreased collagen secretion slightly, and in a consistent manner. This effect did not achieve statistical significance (Figure 2). Treatment with each agonist decreased TGF-β1-induced collagen secretion, but in a more heterogeneous fashion. This effect, however, only achieved significance when HVOX were treated with agonists of EP1 and EP2 (p=0.003 and 0.001, respectively).
Figure 1.
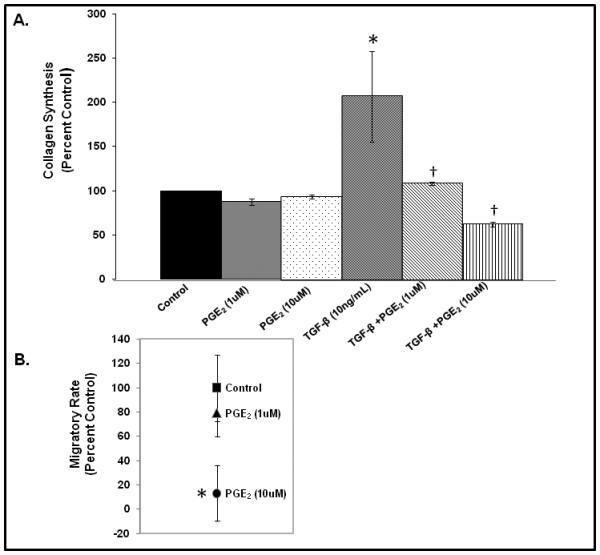
Collagen secretion by HVOX in response to treatment with exogenous PGE2 +/− TGF-β1 (A; *<0.05 from baseline, δ<0.05 from TGF-β1 treatment) and relative changes in basal migratory rate in response to PGE2 (B; *<0.05).
Figure 2.
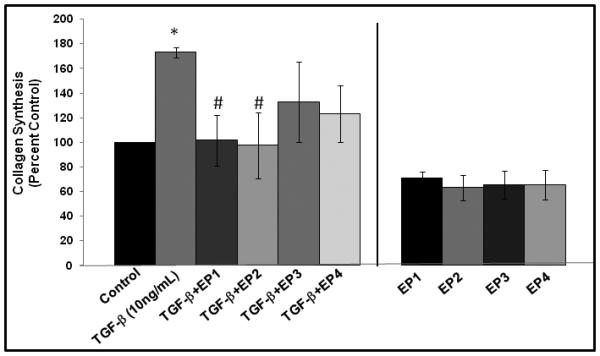
Collagen secretion by HVOX in response to treatment with exogenous PGE2 +/− specific EP receptor agonists (*<0.05 from baseline, #<0.05 from TGF-β1 treatment).
PGE2 decreased the basal migratory rate of HVOX
A dose-dependent effect in basal migratory rate was observed (Figure 1B); 1μM PGE2 decrease migratory rate by approximately 20%. This decrease did not achieve statistical significance. However, at 10.0μM, PGE2 decreased HVOX basal migratory rate by nearly 80% (p=0.038).
TGF-β1 induced COX-2 mRNA expression and PGE2 synthesis in HVOX
Given that PGE2 appears to have some inherent anti-fibrotic characteristics, we sought to determine if TGF-β1 induced COX-2 expression and PGE2 synthesis, potentially, as a component of an autocrine anti-fibrotic feedback loop. As shown in Figure 3A, COX-2 mRNA expression increased in a dose-dependent fashion following 24 hours of exposure to TGF-β1. PGE2 also increased over 24 hours of TGF-β1 exposure. However, only the highest concentration (10.0ng) of TGF-β1 induced a statistically-significant increase in PGE2 synthesis (p=0.039).
Figure 3.
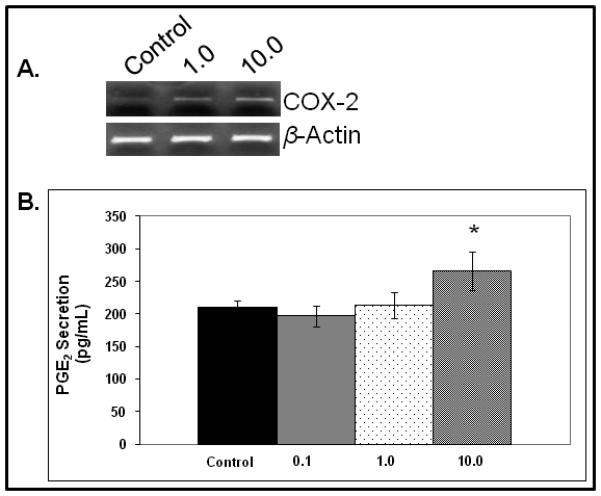
COX-2 mRNA expression (A) and PGE2 secretion (B) in response to exogenous TGF-β1 (*<0.05).
IL-1β induced PGE2 synthesis in an ex vivo organ culture model
Acknowledging that current models of vocal fold wound healing are limited, we sought to describe physiological phenomena in an ex vivo model. Specifically, we sought to determine if an inflammatory tissue phenotype could be elicited, in this case, characterized by increased PGE2 synthesis, by treating freshly-excised vocal fold tissue with exogenous IL-1β. Using this model, PGE2 synthesis into the supernatant increased by more than 60% (p=0.038) and approximately 100% (p=0.004) in response to 1.0 and 10.0ng/mL of IL-1β, respectively (Figure 4).
Figure 4.
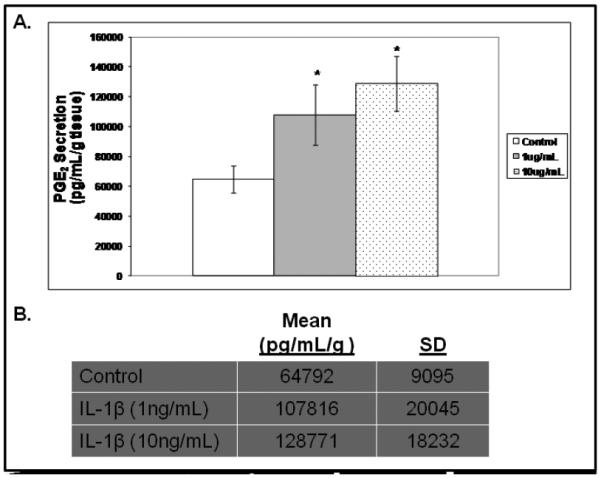
PGE2 secretion from excised rabbit vocal folds treated with IL-1β(A; *<0.05, raw data provided, B).
DISCUSSION
Treatment for vocal fold fibrosis remains suboptimal. Investigation regarding the sequence of events following vocal fold injury is likely to provide potential targets for intervention. We hypothesize that readily-available pharmacological agents targeting well-described pathways have been largely overlooked. One therapeutic option is targeting signaling that crosses the boundary between inflammation and fibrosis. In that regard, COX-2 and its downstream product PGE2 appear to be ideal. Our laboratory previously reported that PGE2 secretion can be maintained given that it is fibroblast-secreted.25 This finding is particularly relevant given that the effects are likely to be relatively long-term, compared to other shorter lived cytokines ad chemokines. We previously showed that PGE2 had a bimodal expression pattern with peak expression in the acute inflammatory phase and then increased concentrations approximately 1 to 2 weeks following injury.30 We hypothesized that this temporal pattern related primarily to the complexity of PGE2 as a regulator of both inflammation as well as the proliferative phase of healing, and its interactions with mesenchymal cells recruited into the wound bed.
Given the relationship between inflammation and fibrosis, many hypothesize that increased PGE2 is related to an aberrant inflammatory response resulting in hypertrophic scar or keloid formation. Furthermore, preliminary data suggested that topical inhibition of COX-2 limited dermal fibrosis.20 However, this response appears to be organ-specific as increased bleomycin-induced pulmonary fibrosis was observed in COX-2 deficient mice.31 In the current study, we attempted to characterize the regulatory effects of exogenous PGE2 on vocal fold fibroblasts in an attempt to reconcile some of these issues and potentially describe a reasonable pharmacological target for manipulation.
The development of fibrosis appears to be driven by cytokines like TGF-β1, which is a master regulator of wound healing. TGF-β1 is a multifunctional cytokine implicated in the pathogenesis of a variety of fibrotic diseases.32-33 Our laboratory and others have previously shown that TGF-β1 is associated with type I collagen synthesis related to fibrosis, among other cell activities.29 However, targeted anti-TGF-β1 therapeutics have largely failed. Recent data suggest that TGF-β1 may, in fact, induce an anti-fibrotic feedback loop via the regulation of COX-2, further complicating the putative role of the COX-2/PGE2 pathway as either a cornerstone of fibrosis or a potent antifibrotic agent. Our data suggest that PGE2 does, in fact, limit TGF-β1-induced collagen secretion and this phenomenon is largely mediated via the EP1 and EP2 receptors in HVFF. Furthermore, PGE2 limits basal migratory rates of HVFF migration, further contributing to its putative antifibrotic properties. In addition, TGF-β1 induced HVFF COX-2 mRNA and PGE2 secretion into the supernatant, suggesting the induction of a putative anti-fibrotic mechanism inherent to the fibrotic phenotype. These data also suggest some clinical value to altering this pathway. Pharmacological therapies have already been developed; we simply must determine the relevant temporal variables to optimize therapies related to altered PGE2 signaling.
Although traditional cell culture methods are critical to describing fundamental cell processes, the complexity of the wound healing processes are largely overlooked and oversimplified. Furthermore, anatomic factors related to both the size and location of the vocal folds often limit investigation regarding vocal fold dynamics. We, therefore, sought to compliment our in vitro data with ex vivo, organ culture data. This model, although not recapitulating the complexity of in vivo processes, attempts to close the gap between the quasi-artificial in vitro environment and the overly complex in vivo milieu. Our data suggest that freshly excised vocal folds do respond to exogenous mediators of healing. In this particular case, IL-1β induced significantly increased PGE2 secretion from the tissue. Clearly, further investigation is warranted to validate such techniques including immunolocalization to determine the cell source of these secreted mediators. Regardless, we are optimistic that this organotypic model is likely to yield critical information regarding the development of therapeutic trials.
In summary, these data implicate the intricate balance of positive and negative signaling pathways in the regulation of basal physiological activities. Increased insight regarding this balance and the sequelae associated with an imbalance of these processes is critical to understanding pathological process, and the development of novel therapies. Our longstanding hypothesis is that modulating or attenuating the acute inflammatory response following vocal fold injury is likely to yield a less fibrotic outcome to the wound healing process. We also hypothesize that the COX-2 pathway provides an ideal opportunity for pharmacological intervention as it appears to be relatively ubiquitous during the wound healing process. However, the complexity of this particular signaling pathway is significant and as our data suggest, there is likely critical temporal variable that must be considered moving forward.
Acknowledgments
This work was funded by the NIH/NIDCD (RO3 DC010267), Hackers for Hope, The Langeloth Foundation, and the Garban Fund.
Footnotes
Portions of this manuscript were submitted for presentation at the 2010 American Laryngological Association meeting/Combined Otolaryngology Spring Meetings.
Conflicts of interest: NONE
REFERENCES
- 1.Ehrlich H. In: Scarless wound healing. Garg H, Longaker MT, editors. Marcel Dekker; 2000. pp. 99–114. [Google Scholar]
- 2.Chin GS, Stelnicki EJ, Gittes GK, Longaker MT. In: Scarless wound healing. Garg HG, Longaker MT, editors. Marcel Dekker; 2000. pp. 239–257. [Google Scholar]
- 3.Longaker MT, et al. Studies in fetal wound healing: VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25:63–68. doi: 10.1016/s0022-3468(05)80165-4. [DOI] [PubMed] [Google Scholar]
- 4.Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol. 1979;381:353–361. doi: 10.1007/BF00432477. [DOI] [PubMed] [Google Scholar]
- 5.Krummel TM, et al. Fetal response to injury in the rabbit. J Pediatr Surg. 1987;22:640–644. doi: 10.1016/s0022-3468(87)80117-3. [DOI] [PubMed] [Google Scholar]
- 6.Morykwas MJ, et al. Cellular inflammation of fetal excisional wounds: effects of amniotic fluid exclusion. Inflammation. 1991;15:173–180. doi: 10.1007/BF00918644. [DOI] [PubMed] [Google Scholar]
- 7.Frantz FW, et al. Biology of fetal repair: the presence of bacteria in fetal wounds induces an adult-like healing response. J Pediatr Surg. 1993;28:428–433. doi: 10.1016/0022-3468(93)90243-e. [DOI] [PubMed] [Google Scholar]
- 8.Kumta S, et al. Acute inflammation in foetal and adult sheep: the response to subcutaneous injection of turpentine and carrageenan. Br J Plast Surg. 1994;47:360–368. doi: 10.1016/0007-1226(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 9.Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998;77:80–84. doi: 10.1006/jsre.1998.5345. [DOI] [PubMed] [Google Scholar]
- 10.Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35:866–872. doi: 10.1053/jpsu.2000.6868. [DOI] [PubMed] [Google Scholar]
- 11.Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine. 2000;12:671–676. doi: 10.1006/cyto.1999.0598. [DOI] [PubMed] [Google Scholar]
- 12.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Ann Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 13.Sandulache VC, Parekh A, Li-Korotsky HS, Dohar JE, Hebda PA. Prostaglandin E2 inhibition of keloid fibroblast migration, contraction, and transforming growth factor (TGF)-beta1-induced collagen synthesis. Wound Rep Reg. 2007;15:122–133. doi: 10.1111/j.1524-475X.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 14.Sandulache VC, Parekh A, Li-Korotsky HS, Dohar JE, Hebda PA. Prostaglandin E2 differentially modulates human fetal and adult dermal fibroblast migration and contraction: implication for wound healing. Wound Repair Regen. 2006;14:633–643. doi: 10.1111/j.1743-6109.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 15.Sandulache VC, et al. Prostaglandin E2 is activated by airway injury and regulates fibroblast cytoskeletal dynamics. The Laryngoscope. 2009;119:1365–1373. doi: 10.1002/lary.20173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 17.Wilgus TA, et al. Inhibition of ultraviolet light B-induced cutaneous inflammation by a specific cyclooxygenase-2 inhibitor. Adv Exp Med Biol. 2002;507:85–92. doi: 10.1007/978-1-4615-0193-0_14. [DOI] [PubMed] [Google Scholar]
- 18.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossiello L, et al. Differential expression of cyclooxygenases in hypertrophic scar and keloid tissues. Wound Rep Reg. 2009;17:750–757. doi: 10.1111/j.1524-475X.2009.00530.x. [DOI] [PubMed] [Google Scholar]
- 20.Wilgus TA, Vodovotz Y, Vittadini E, Clubbs EA, Oberyszyn TM. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Rep Reg. 2003;11:25–34. doi: 10.1046/j.1524-475x.2003.11106.x. [DOI] [PubMed] [Google Scholar]
- 21.Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends in Immunology. 2004;24:40–46. doi: 10.1016/j.it.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Ozaki T, Rennard SI, Crystal RG. Cyclooxygenase metabolites are compartmentalized in the human lower respiratory tract. J Appl Physiol. 1987;62:219–222. doi: 10.1152/jappl.1987.62.1.219. [DOI] [PubMed] [Google Scholar]
- 23.Welham NV, Lim X, Tateya I, Bless DM. Inflammatory factor profiles one hour following vocal fold injury. Ann Otol Rhinol Laryngol. 2008;117:145–152. doi: 10.1177/000348940811700213. [DOI] [PubMed] [Google Scholar]
- 24.Branski R, Rosen C, Verdolini K, Hebda P. Biochemical markers associated with acute vocal fold wound healing: a rabbit model. Journal of Voice. 2005;19:283–289. doi: 10.1016/j.jvoice.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Branski RC, et al. Cyclooxygenase-2 signaling in vocal fold fibroblasts. Laryngoscope. 2010;120:1826–1831. doi: 10.1002/lary.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano S, Bless D, Heisey D, Ford C. Roles of hepatocyte growth factor and transforming growth factor B1 in production of extracellular matrix by canine vocal fold fibroblasts. Laryngoscope. 2003;113:144–148. doi: 10.1097/00005537-200301000-00027. [DOI] [PubMed] [Google Scholar]
- 27.Hirano S, Bless DM, Heisey D, Ford CN. Effect of growth factors on hyaluronan production by canine vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2003;112:617–624. doi: 10.1177/000348940311200708. [DOI] [PubMed] [Google Scholar]
- 28.Hirano S, Bless DM, Massey RJ, Hartig GK, Ford CN. Morphological and functional changes of human vocal fold fibroblasts with hepatocyte growth factor. Ann Otol Rhinol Laryngol. 2003;112:1026–1033. doi: 10.1177/000348940311201206. [DOI] [PubMed] [Google Scholar]
- 29.Branski RC, et al. The effects of Transforming Growth Factor-beta1 on human vocal fold fibroblasts. Annals of Otology, Rhinology, & Laryngology. 2009;118:218–226. doi: 10.1177/000348940911800310. [DOI] [PubMed] [Google Scholar]
- 30.Branski RC, Rosen CA, Hebda PA, Verdolini K. Cytokine analysis of acute wound healing in the larynx: A rabbit model. Journal of Voice. 2005;19:283–289. doi: 10.1016/j.jvoice.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Keerthisingam CB, et al. Cyclooxygenase-2 Deficiency Results in a Loss of the Anti-Prolferative Response to Transforming Growth Factor-beta in Human Fibrotic Lung Fibrobasts and Promotes Bleomycin-Induced Pulmonary Fibrosis in Mice. American Journal of Pathology. 2001;158:1411–1422. doi: 10.1016/s0002-9440(10)64092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verrecchia F, Mauviel A. Transforming growth factor-beta and fibrosis. World J Gastroentrol. 2007;13:3056–3052. doi: 10.3748/wjg.v13.i22.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochem Biophys Acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]


