Abstract
The functional significance of newly formed granule neurons in the adult mammalian hippocampus remains a mystery. Recently, it was demonstrated that wheel running increases new neuron survival and c-Fos expression in new and pre-existing granule cells in an activity-dependent manner. It is currently unknown whether other immediate early genes (IEGs) become expressed in granule neurons from running. Further, it is unknown whether locomotor activity in home cages without wheels can influence neurogenesis and IEG expression similar to running. The purpose of this study was three fold: 1) to determine if Arc and Zif268 expression are also induced from wheel running in both pre-existing and newly formed neurons 2) to determine if neurogenesis and IEG induction is related to horizontal distance traveled in home cages without wheels and 3) to determine whether IEG induction is related to acute bouts of running or chronic effects. Adult C57BL/6J female mice were placed in cages with or without running wheels for 31 days. The first 10 days, mice received daily injections of 5-Bromo-2′-deoxyuridine (BrdU) to label dividing cells. On day 31, running and non-running animals were euthanized either 2 hours after peak activity, or during a period of relative inactivity.
Immunohistochemistry was performed on hippocampal sections with antibodies against BrdU, mature neuron marker NeuN, c-Fos, Arc, and Zif268. Results demonstrate that Arc, Zif268, and c-Fos are induced from wheel running but not movement in cages without wheels. All IEGs were expressed in new neurons from running. Further, IEGs were induced acutely by running, as increased expression did not continue into the light cycle, a period of relative inactivity. The results suggest that robust movements, like running, are necessary to stimulate IEG expression and neurogenesis. Moreover, results suggest new neurons from running may be processing information about running behavior itself.
Keywords: Immediate early gene, adult hippocampal neurogenesis, Arc, Zif268, c-Fos, exercise, wheel running, C57BL/6J mice
Introduction
Aerobic running, either voluntary or forced, stimulates the formation of granule neurons in the adult rodent hippocampus (van Praag et al., 1999b, Uda et al., 2006). The number of new neurons generated is strongly related to distance run in mice housed with exercise wheels (Rhodes et al., 2003b, Bednarczyk et al., 2009, Clark et al., 2009, Clark et al., 2011). Moreover, exercise-induced neurogenesis can increase the volume of the entire granule cell layer by adding to the total number of granule neurons (Rhodes et al., 2003b, Clark et al., 2008). These discoveries have drawn much interest, as they provide evidence that the generation of new nervous tissue in the adult mammalian brain is possible and can be stimulated naturally by physical activity. However, very little is known about the function of new neurons from wheel running (Clark et al., 2008, Clark et al., 2009). Moreover, how new neurons function during hippocampal-dependent tasks remains a hotly debated topic (Kempermann, 2002, Kempermann et al., 2004, Schinder and Gage, 2004, van Praag, 2009, Deng et al.).
One way to assess the function of new neurons in the adult hippocampus is to determine whether they become selectively activated while performing a hippocampal-dependent task. Immediate early gene (IEG) expression has been widely accepted as a marker for neuron activation, although the physiological mechanisms underlying their expression are not well understood (Guzowski et al., 2005). Recent studies have used the expression of IEGs such as c-Fos, Zif268, and Arc to demonstrate that new granule neurons become activated during performance on hippocampal-dependent cognitive tasks (Jessberger and Kempermann, 2003, Ramirez-Amaya et al., 2006, Kee et al., 2007, Tashiro et al., 2007, Snyder et al., 2009c, Trouche et al., 2009). It has been hypothesized that this activation indicates a role for new neurons in hippocampal-dependent learning and memory retrieval.
The assessment that new neurons are activated during hippocampal-dependent learning or memory retrieval can be problematic as many recent studies have not properly controlled for the effects of acute bouts of locomotor activity required to perform the cognitive task on inducing IEGs. These studies typically compared IEG expression of animals performing a behavioral task to animals that remained housed in standard laboratory cages, or did not equate the relationship between IEG expression and distance traveled while performing the task (Jessberger and Kempermann, 2003, Ramirez-Amaya et al., 2006, Kee et al., 2007, Tashiro et al., 2007, Trouche et al., 2009).
There is reason to speculate that IEG expression in the granule cells may be induced by the physical activity necessary to complete hippocampal-dependent task. One not widely known or underappreciated feature of hippocampus granule neurons is that they are quantitatively activated by acute bouts of voluntary movement. For example, wheel running increases c-Fos expression in the granule cell layer which is strongly related (r > 0.88) to the distance the animal traveled 90–120 minutes before euthanasia (Rhodes et al., 2003a, Clark et al., 2009, Clark et al.). The acute c-Fos response from running does not habituate and the induction continues at a similar level even after 50 days of continuous access to running wheels (Lee et al., 2003, Clark et al.). Our lab has recently reported that new granule neurons preferentially display c-Fos in response to acute bouts of running as compared to pre-existing granule neurons (Clark et al., 2009). While it is well established that c-Fos is up-regulated from running in the dentate gyrus, to the best of our knowledge, whether other IEGs become up-regulated from acute bouts of running in new and pre-existing granule neurons is unknown. It is also unknown if IEG expression in the granule cell layer can be induced in an activity dependent manner during home cage activity, as opposed to more robust repetitive movements specific to running.
The purpose of this study is three-fold. The first objective of this study is to determine which IEGs are most influenced by acute locomotor activity in new and pre-existing granule neurons. Arc and Zif268 were chosen because these IEGs, as well as c-Fos, are commonly used as markers of new neuron activation in association with hippocampal-dependent learning and memory task performance (Jessberger and Kempermann, 2003, Ramirez-Amaya et al., 2006, Kee et al., 2007, Tashiro et al., 2007, Snyder et al., 2009c, Trouche et al., 2009). Further, these IEGs are also well characterized for their expression following learning tasks and the induction of long-term potentiation (as reviewed in Abraham et al., 1991, Dragunow, 1996, Knapska and Kaczmarek, 2004, Bramham et al., 2008). Occasionally, these IEGs can be regulated differently by the same stimulus (Douglas et al., 1988, Cole et al., 1989, Dragunow et al., 1989, Wisden et al., 1990, Kaczmarek et al., 1999, Guzowski et al., 2001). Identifying IEGs that can distinguish between neuronal activation related to cognitive function as opposed to locomotor activity is crucial for understanding the functional contribution of new neurons in cognition.
The second objective of this study was to determine whether IEG activation and neurogenesis is related to distance traveled in home cages without running wheels. One possibility is that only traveling distances at fast speeds, sufficient to tax aerobic capacity and activate the stress response, can induce IEG expression in the granule cell layer. If this is the case, then normal locomotion in home cages would not be enough to stimulate IEG expression or new neuron formation in an activity-dependent manner. The outcome of this experiment is important for determining whether acute bouts of movement, similar to those necessary to complete a small number of trials on less physically taxing cognitive tests can induce IEGs, or whether robust repetitive movements are necessary to induce IEG expression in granule cells.
The third objective of this study was to determine whether an elevation of IEGs from running is a result of a long-term increase in basal IEG expression, or the reflection of acutely induced cellular activity. IEGs have been most commonly used as markers for acute neural activity. However, IEGs have a baseline expression in the brain that varies between regions and is maintained by ongoing synaptic or hormonal signaling (Beckmann and Wilce, 1997, Herdegen and Leah, 1998, Lee et al., 1998, Shirayama et al., 1999, Guzowski et al., 2001). Fluctuations from basal IEG concentrations can be readily detected following application or removal of a stimulus (Lee et al., 1998, McGahan et al., 1998,, French et al., 2001). The hippocampal granule cell layer is a unique brain area in that basal levels or c-Fos, Zif268, and Arc are all low (Gass et al., 1992, French et al., 2001). However, a possibility exists that running may induce a long-term upward shift in the basal IEG protein expression.
The results of this study provide insight into the function of new neurons generated from running. Further, results provide useful information for future studies using IEGs as markers of neuronal activation in cognitive tests assessing the function of new neurons.
2. Materials and Methods
Animals and husbandry
Cohorts of female C57BL/6J mice arrived at the Beckman Institute Animal facility from The Jackson Laboratory at 5 weeks of age. Females were used because they run more than males (Clark et al., 2011). Upon arrival, mice were housed four per standard polycarbonate shoebox cages with corncob bedding, 7097 ¼ (Harlan Teklad, Madison, Wisconsin, USA) for 2 weeks. The mice were then individually housed either in standard shoe box cages, custom home cages (without wheels) used for video tracking, or cages with wheels as described below. Rooms were controlled for temperature (21 °C) and photo-period (12-h L:D; lights on at 7 am and off at 7 pm). Food (Harlan Teklad 7012) and water were provided ad libitum. The Beckman Institute Animal Facility is AAALAC approved. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines. All efforts were made to minimize the number of animals used and their suffering.
Dimensions of running wheel cages were 36×20×14 cm (LxWxH) with a 23 cm diameter wheel mounted in the cage top (Respironics, Bend, OR). Wheel rotations were monitored continuously in 1 min increments throughout the experiments via magnetic switches interfaced to a computer. Dimensions of home cages without wheels for video tracking were 34×18×16 cm (LxWxH). Video tracking cages were constructed out of clear plastic with food and water access mounted on the side and clear plastic lids. Dimensions of standard polycarbonate shoebox cages without wheels were 29×19×13 cm (LxWxH).
Experimental Design
Experiment 1: IEG induction from wheel running in new and pre-existing granule neurons (n=16)
The primary objective of this experiment was to determine whether Arc, Zif268, and c-Fos become up-regulated from wheel running in new and pre-existing granule neurons. The secondary objective of this experiment was to determine whether 21–31 day old new neurons show a greater IEG expression from running as compared to pre-existing neurons.
Mice (7 weeks old) were placed individually in cages either without (n = 6; Sedentary) or with running wheels (n = 10 Runners) for 31 days. The first 10 days all mice received daily injections of 50 mg/kg 5-Bromo-2′-deoxyuridine (BrdU) to label dividing cells. Note that mice were deliberately not housed in cages with locked wheels because mice climb in locked wheels and we wanted to keep physical activity to a minimum in the sedentary group. On day 32, mice were euthanized 2 hours after the height of their active period, approximately 3–5 hours after the lights shut off in the animal room.
Experiment 2: Neurogenesis and IEG induction in home cages without wheels (n=8)
The objective was to determine if IEG activation and new neuron formation is related to distance moved in home cages without running wheels.
Mice (7 weeks old) were individually housed in custom-made home cages for video tracking for 31 days. Starting on day 3, all mice received daily injections of 50 mg/kg BrdU for 10 days, to label dividing cells. Horizontal distance traveled per day in the home cages was recorded for each sedentary mouse by overhead video cameras and analyzed using TopScan 2.0 (Clever Sys, Vienna, VA, USA) video tracking software (Zombeck et al., 2008). On day 32, mice were euthanized 2 hrs after the height of their most active period, approximately 3–5 hrs after the lights shut off in the animal room.
Experiment 3: IEG induction during the inactive light phase (n=24)
The objective of this experiment was to determine whether an elevation of IEGs from running reflects a long-term increase in basal IEG expression or acute cellular activity. If IEG expression in the granule cell layer from running is a result of a long-term increase in basal expression, then we reasoned that IEG expression will remain elevated during a period of relative inactivity following several hours of running. Number of wheel revolutions were monitored during the light cycle, a period during which running is minimal. If IEG expression decreases in correlation with decreased distance traveled or is no different from sedentary mice, this would constitute evidence that IEG induction from running is a reflection of acute cellular activity. On the other hand, if IEG induction remains elevated even though running has decreased, this would indicate that running increases basal IEG expression in granule cells.
Mice (7 weeks old) were individually housed in cages with (n = 18; Runners) or without (n=6; sedentary) running wheels for 31 days. The first 10 days all mice received daily injections of 50 mg/kg BrdU to label dividing cells. On day 32, runner mice were euthanized in three groups (n = 6 per group) at 3, 6, or 9 hours after the onset of the light cycle in the animal rooms (a period of relative inactivity for mice). In addition, 2 sedentary mice were euthanized at each respective time point.
Immunohistochemistry
Following Clark et al. (2008), mice were anesthetized with 150 mg/kg sodium pentobarbital (ip) and then perfused transcardially with 4% paraformaldehyde in a phosphate buffer solution (PBS). Brains were post-fixed overnight, and transferred to 30% sucrose in PBS. Brains were sectioned using a cryostat into 40 micron coronal sections and stored in tissue cryoprotectant at −20°C. Separate 1-in-8 series of these sections (i.e., series of sections throughout the rostro-caudal extent of the brain with 320 micron increments separating each section) were stained in each of the following ways.
1) BrdU-DAB. Purpose: To detect BrdU-positive (newly divided) cells in the dentate gyrus. Free floating sections were washed in TRIS-buffered solution (TBS) and then treated with 0.6% hydrogen peroxide. To denature DNA, sections were treated with 50% de-ionized formamide, 10% 20XSCC buffer, 2N hydrochloric acid, and 0.1 M Boric acid. Sections were then treated with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus), and then incubated in primary antibody against BrdU made in rat (Accurate, Westbury, NY) at a dilution of 1:100 in TBS-X plus for 72 hrs at 4 °C. Sections were then washed in TBS, treated with TBS-X plus for 30 min and then incubated in secondary antibody against rat made in goat at 1:250 in TBS-X plus for 100 min at room temperature. Sections were then treated using the ABC system (Vector, Burlingame, CA) and stained using a diaminobenzidine kit (Sigma, St. Louis, MO).
2) Arc, c-Fos, Zif268 -DAB. Purpose: To detect the presence of IEGs in the granule cell layer. Free floating sections were washed in Phosphate-buffered solution (PBS) and then treated with 0.6% hydrogen peroxide. Sections were then treated with a solution of 0.2% Triton-X and 5% goat serum in PBS (PBS-X plus) for 1 hour, and then incubated in primary antibody against c-Fos (Calbiochem, San Diego, CA), Arc (SySy, Goettingen, Germany), or Zif268 (Santa Cruz Biotech, Santa Cruz, CA) made in rabbit at a dilution of 1:18,000, 1:10,000, or 12:000 respectively in PBS-X plus for 48 hrs at 4 °C. Sections were then washed in PBS, treated with PBS-X plus for 60 min and then incubated in secondary antibody against rabbit made in goat at 1:250 in PBS-X plus for 90 min at room temperature. Sections were then treated using the ABC system (Vector, Burlingame, CA) and stained using a diaminobenzidine kit (Sigma, St. Louis, MO).
3) Triple-fluorescent label. To determine the proportion of BrdU+ and BrdU-negative (BrdU-) neurons in the dentate gyrus that expressed an IEG. The procedure for BrdU-DAB was repeated except for the following. A cocktail was used for the primary antibody step that included rat anti-BrdU (1:100; Accurate, Westbury, NY), mouse anti-NeuN (1:50; Chemicon, Billerica, MA), and either rabbit anti-c-Fos (1:7000; Calbiochem, San Diego, CA), anti-Arc (1:1000; SySy, Goettingen, Germany), or anti-Zif268 (1:2000; Santa Cruz Biotech, Santa Cruz, CA). Secondary antibodies made in goat were conjugated with fluorescent markers (Cy2 anti-rabbit, Cy3 anti-rat, Cy5-anti mouse; Jackson ImmunoResearch, West Grove, PA) at dilution 1:200 and also delivered as a cocktail.
2.4. Image analysis
1) BrdU-DAB, Arc-DAB, c-Fos-DAB, and Zif268-DAB. Following Clark et al., 2008, the entire granule layer (bilateral), represented in the 1-in-8 series was photographed by systematically advancing the field of view of the Zeiss brightfield light microscope, and taking multiple photographs, via camera interfaced to computer, under 10X (total 100X) magnification. Positively labeled cells in these photographs were counted to generate unbiased estimates of total number of labeled cells (BrdU, Arc, c-Fos, or Zif268). In 6 runners, IEG cells counts were conducted separately for the supra- or infra-pyramidal blades of the granule cell layer for comparison of counts between locations. Positively labeled cells that were predicted to be in the top plane of the sections were not included in cell count estimates. In addition, the total volume of the dentate gyrus represented in the series was measured so that the IEG counts could be expressed per cubic micrometer dentate gyrus sampled. Area per section was obtained by outlining the entire bilateral granule cell layer. Volume was obtained by multiplying average area per section by the number of sections and then by the space between sections for each animal.
2) Triple label. Following Clark et al. (2009), a confocal Leica SP2 laser scanning confocal microscope (using a 40X oil HCX PL APO C5 objective with 1.25 numerical aperture, pinhole size 81.35 μm, 1-Airy Unit) was used to determine the proportion of BrdU cells (BrdU+) that differentiated into neurons (NeuN+) and to determine the proportion of neurons (BrdU+/NeuN+, or BrdU-/NeuN+) displaying an IEG (Arc, c-Fos, or Zif268). Each image sequentially captured a part of the dentate gyrus on a single plane (randomly selected on the z-axis) that was just out of view from previous image. This sequential imaging was done until the entire medial to lateral extent of the each bilateral granule cell layer was captured. The bi-lateral granule cell layer was imaged in 3 – 4 hippocampal sections (spanning the rostral, medial, and caudal extent) of 3 – 4 sedentary and farthest running mice. All the new (BrdU+/NeuN+) and pre-existing (BrdU-/NeuN+) granular neurons, as well as the number of neurons co-labeled with an IEG (BrdU-/NeuN+/IEG+, or BrdU+/NeuN+/IEG+) in that plane were counted for each image.
IEG-expressing new neurons were also analyzed for the location within the granule cell layer. The number of IEG expressing new neurons that shared a border directly adjacent to the hilus of the granule cell layer was compared with the proportion of IEG expressing new neurons that had migrated into the granule cell layer (as defined by not having a border adjacent to the hilus). This analysis was conducted to determine whether the probability of IEG expression in new neurons differs depending on the location of the new neurons within the granule cell layer.
2.5. Statistical Analysis
Data were analyzed using SAS, or R statistical software. In all analyses, P < 0.05 was considered statistically significant.
The proportion of BrdU labeled cells in the granule cell layer that also expressed NeuN was compared in sedentary and runner conditions by logistic regression. To determine whether new neurons preferentially display an IEG from running, the proportions of NeuN+/BrdU− and NeuN+/BrdU+ neurons expressing an IEG (Arc, c-Fos, or Zif268) were also compared by logistic regression. For these analyses, the deviance is reported in place of an F statistic.
The number of IEG cells per mm3 and total number of new neurons in the granule cell layer was compared between running and sedentary animals using a two sample T-test assuming equal variance. Pearson’s correlations between amount of running (e.g., km/hr or km/day) and number of IEG positive cells or number of new neurons were analyzed using simple linear regression.
In experiment 3, the sedentary average was obtained by collapsing data across pairs of mice sampled 3, 6, or 9 hrs after the onset of the light cycle. The number of IEG cells per mm3 was compared between sedentary and runners sampled 3, 6, or 9 hrs after lights on using a 1-way ANOVA. T-tests with Tukey correction were used for pair-wise group comparisons on statistically significant ANOVAs.
Results
Experiment 1: IEG induction from wheel running in new and pre-existing granule neurons
Wheel Running
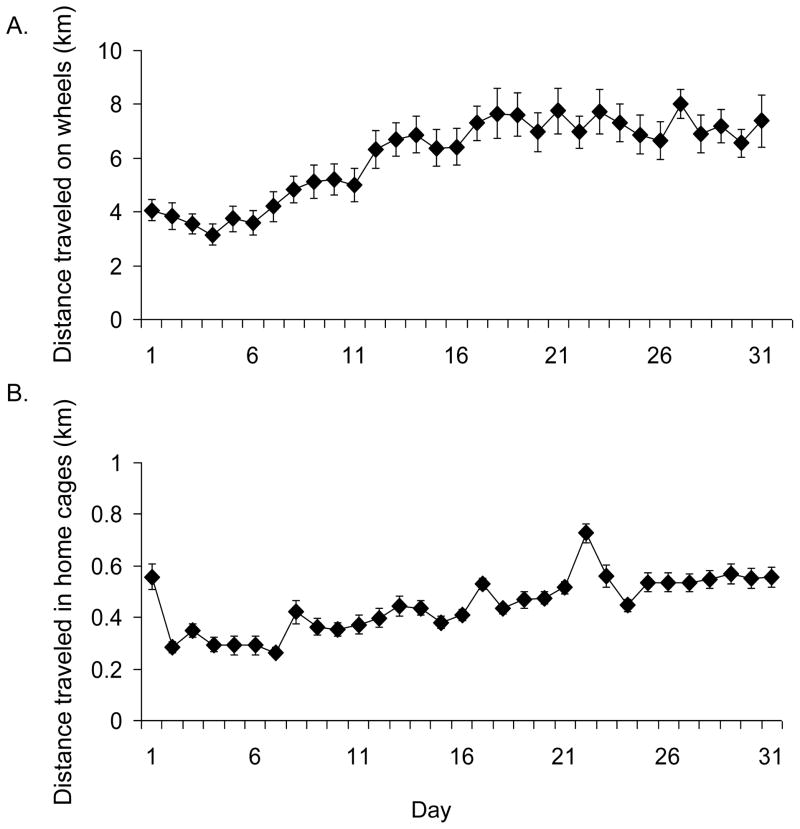
Inspection of Figure 1A shows that wheel running distance increased steadily for the first 19 days and thereafter maintained a plateau. Average amount of wheel running over the entire experiment was 6.1 km/day (± 0.29 S.E.).
Figure 1.
Average wheel running and home cage activity. A) Average daily running distance in km/day (±SE) over 31 days of access to wheels for experiment 1. B) Average daily distance traveled in home cages without wheels in km/day (±SE) over 31 days as recorded by video tracking in experiment 2.
Neurogenesis from wheel running
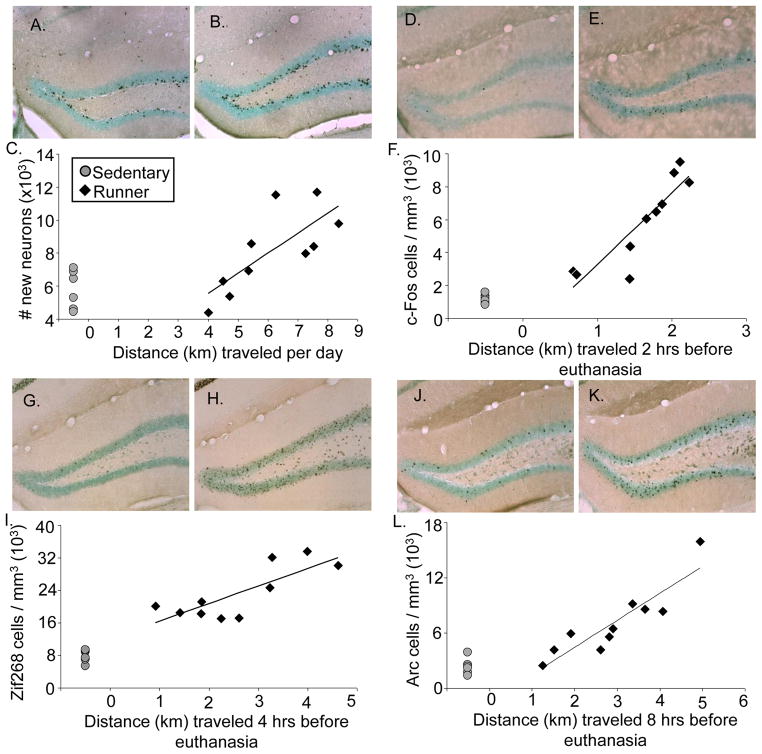
Wheel running increased neurogenesis approximately 2-fold (Fig. 2A & B) [t(14)=3.2, P<0.006]. Average daily running distance was significantly correlated (r = 0.75) with number of new neurons among individuals (Fig. 2C) [p = 0.012]. The percentage of BrdU cells double labeled with NeuN was 94% (±1.0) in runners and 85% (± 1.8) in sedentary mice [Deviance=8.6, P=0.003]. Running caused the volume of the granule cell layer to increase by 13% [t(14) = 3.6, P=0.002].
Figure 2.
IEG induction and neurogenesis from wheel running. A) Representative coronal section stained for BrdU-DAB (combined with a light Nissl stain to highlight the dentate gyrus) of an animal euthanized in the sedentary condition (50X total magnification) B) same as A except representing the runner condition. C) Total number of new neurons shown for individual mice plotted against average daily running distance in km. D) Representative coronal section stained for c-Fos-DAB (combined with a light Nissl stain to highlight the dentate gyrus) of an animal euthanized in the sedentary condition (50X total magnification) E) same as D except representing the runner condition F) Number of c-Fos positive cells per cubic mm shown for individual mice plotted against distance run in km accumulated within 2 hrs before euthanasia. G) Representative coronal section stained for Zif268-DAB (combined with a light Nissl stain to highlight the dentate gyrus) of an animal euthanized in the sedentary condition (50X total magnification) H) same as G except representing the runner condition I) Number of Zif268 positive cells per cubic mm shown for individual mice plotted against distance run in km accumulated within 4 hrs before euthanasia. J) Representative coronal section stained for Arc-DAB (combined with a light Nissl stain to highlight the dentate gyrus) of an animal euthanized in the sedentary condition (50X total magnification) K) same as J except representing the runner condition L) Number of Arc positive cells per cubic mm shown for individual mice plotted against distance run in km accumulated within 8 hrs before euthanasia. Sedentary mice are represented as grey circles and runners as black diamonds.
IEG induction from wheel running
c-Fos
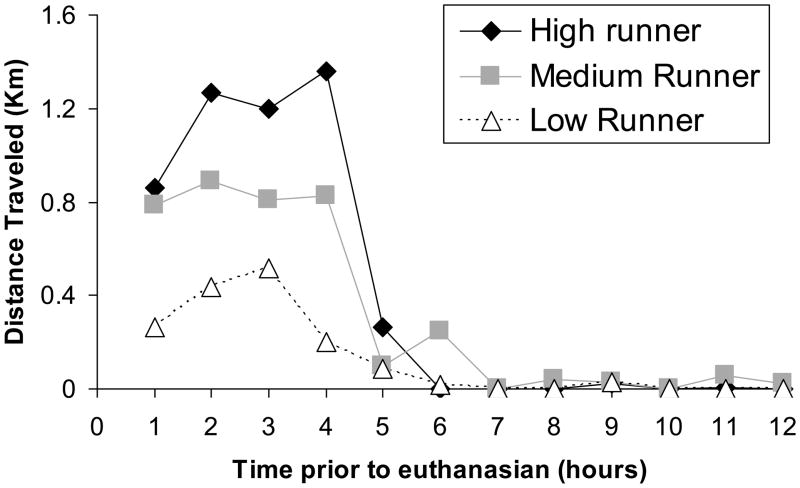
Running caused approximately a 5-fold increase in the number of c-Fos positive cells in the dentate gyrus (Fig. 2D & E) [t(14)=4.3, P=0.0007]. Previous literature has established that c-Fos protein is present at 30 mins and peaks at approximately 2 hrs post cell activation then sharply diminishes (Zangenehpour and Chaudhuri, 2002). The Pearson’s correlation (r) between level of running accumulated within 2 hrs before euthanasia and number of c-Fos positive cells among the 10 individuals was 0.89 (Fig. 2F) [p = 0.0006]. See Figure 3 for a representative plot of running levels at hourly intervals before euthanasia for a representative low, medium and high running mouse. c-Fos expression did not differ between the supra- and infra-pyramidal blades.
Figure 3.
Distance traveled on wheels 12 hours before euthanasia for a representative low, medium, and high running mouse.
Zif268
Running caused approximately a 3-fold increase in the number of Zif268 positive cells in the dentate gyrus (Fig. 2G & H) [t(14)=5.7, P<0.0001]. Previous literature has established that Zif268 protein peaks at approximately 2 hrs following cell activation, but displays a more broad bell shaped expression curve than c-Fos (Richardson et al., 1992, Zangenehpour and Chaudhuri, 2002). The Pearson’s correlation (r) between level of running 2 hrs before euthanasia and number of Zif268 positive cells among the 10 individuals was 0.65 [p = 0.04]. However, the correlation was even stronger (r = 0.79) for distance run 4 hours preceding euthanasia (Fig. 2I) [P=0.006]. Given the large range in Zif268 cell stain intensity (light grey to dark black cells) as compared to c-Fos and Arc, as well as the broad expression curve, it is likely we were able to detect protein expression over a longer period of time. Zif268 expression did not differ between the supra- and infra-pyramidal blades.
Arc
Running caused approximately a 3-fold increase in the number of Arc positive cells in the dentate gyrus (Fig. 2J & K) [t(10)=3.0, P=0.009]. Previous literature has established that Arc protein peaks at approximately 30 min after cellular activation, but remains elevated for at least 8 hours in the granule cell layer (Ramirez-Amaya et al., 2005). Thus, Arc expressed in the granule cell layer may likely represent an accumulation of the previous 8 hours of running. Our data supported this finding, as the relationship between distances traveled before euthanasia and Arc induction continued to improve over time, before peaking at 8 hours. The Pearson’s correlation (r) between level of running 2 hours before euthanasia and number of Arc positive cells was 0.63 [P < 0.05]. However, the Pearson’s correlation (r) between level of running 8 hours before euthanasia and number of Arc positive cells among the 10 individuals was 0.90 (Fig. 2L) [P=0.0004]. Arc expression did not differ between the supra- and infra-pyramidal blades.
Running-induced IEG expression in new neurons
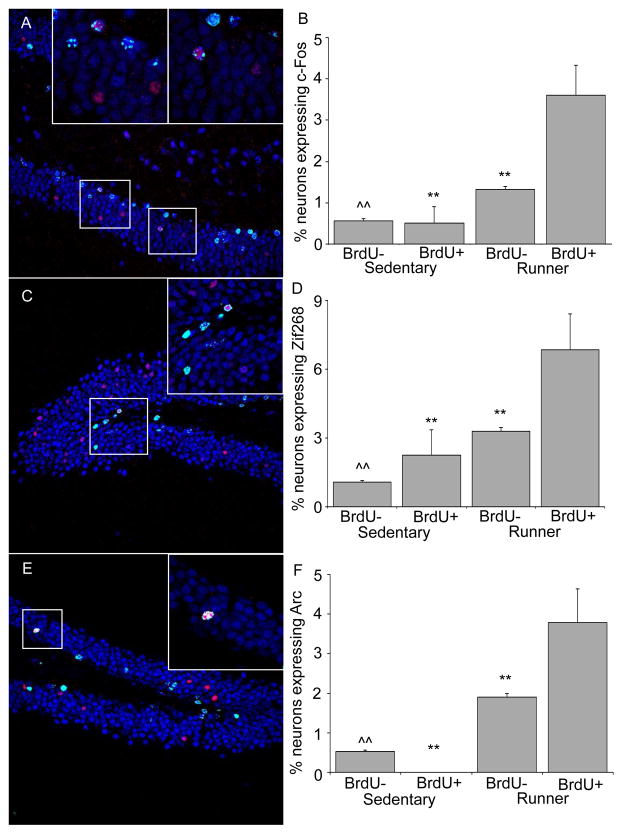
Proportions of BrdU+ and BrdU− neurons expressing c-Fos (Figs. 4A & B)
Figure 4.
c-Fos, Zif268, and Arc expression in new neurons. A) Representative coronal section of the dentate gyrus stained for BrdU (green) and NeuN (blue), and c-Fos (red). The white boxes contain zoomed in images of two new neurons within the section that express c-Fos. B) The proportion of new and pre-existing granule neurons expressing c-Fos in both sedentary and runners. C) Representative coronal section of the dentate gyrus stained for BrdU (green) and NeuN (blue), and Zif268 (red). The white box contains a zoomed in image of a new neuron within the section that expresses Zif268. D) The proportion of new and pre-existing granule neurons expressing Zif268 in both sedentary and runners. E) Representative coronal section of the dentate gyrus stained for BrdU (green) and NeuN (blue), and Arc (red). The white box contains a zoomed in image of a new neuron within the section that expresses Arc. F) The proportion of new and pre-existing granule neurons expressing Arc in both sedentary and runners. ** P < 0.001 different from BrdU+ in runners. ^^ P < 0.001 different from either BrdU+ or BrdU− in runners.
In sedentary mice, a total of 25,420 neurons (NeuN+) unlabeled with BrdU (BrdU-) and 343 new neurons labeled with BrdU (BrdU+) were analyzed for the expression of c-Fos. In runners, a total of 21,117 BrdU− and 666 BrdU+ neurons were analyzed for the expression of c-Fos. The number of neurons expressing c-Fos was greater in the runners as compared to sedentary animals [Deviance=97.6, P<0.0001]. In sedentary mice, the percentage of BrdU− and BrdU+ neurons expressing c-Fos was low and not statistically different from each other. In BrdU− neurons, the percentage of c-Fos was 0.5% ± 0.04 S.E., and in BrdU+ neurons the percentage was also 0.5% ± 0.40 S.E. In runners, the percentage of neurons expressing c-Fos was greater in BrdU+ neurons than BrdU− neurons [Deviance=17.4, P < 0.0001]. In BrdU+ neurons the percentage was 3.6 % (± 0.72 S.E.) whereas in BrdU− neurons the percentage was 1.3 % (± 0.08 S.E.).
Proportions of BrdU+ and BrdU− neurons expressing Zif268 (Figs. 4C & D)
In sedentary mice, a total of 15,674 BrdU− and 178 BrdU+ neurons were analyzed for the expression of Zif268. In runners, a total of 11,332 BrdU− and 263 BrdU+ neurons were analyzed for the expression of Zif268. The basal and running-induced Zif268 levels in the dentate gyrus was noticeably greater than c-Fos in the granule cells (see Fig. 2D–I). This resulted in a greater overall proportion of neurons expressing Zif268 than c-Fos [Deviance=168.5, P<0.0001]. The percentage of neurons expressing Zif268 was greater in runners than sedentary animals [Deviance=167.9, p<0.0001]. The percentage of BrdU− neurons expressing Zif268 was approximately 1.1% (± 0.08 S.E.) and 3.3 % (± 0.17 S.E.) in sedentary and runners, respectively. The percentage of BrdU+ neurons expressing Zif268 was approximately double that of BrdU− neurons for both sedentary at 2.3% (± 1.11 S.E.) and runners at 6.8 % (± 1.56 S.E.) [Deviance=13.9, P = 0.0002 ], however post hoc analysis revealed this difference only reached significance for the runners [Deviance= 7.8, P = 0.005]. A qualitative difference in the intensity of the Zif268 stain was observed between sedentary and runners under the confocal microscope. Zif268 fluoresced noticeably dimmer in sedentary mice.
Proportions of BrdU+ and BrdU− neurons expressing Arc (Figs. 4E & F)
A total of 30,769 BrdU− and 191 BrdU+ neurons were analyzed for the expression of Arc in sedentary mice. In runners, a total of 21,573 BrdU− and 502 BrdU+ neurons were analyzed for the expression of Arc. In sedentary mice, the percentage of Arc expressing BrdU-neurons was approximately 0.5% (± 0.04 S.E.). No Arc expressing BrdU+ cells were identified in sedentary mice. The overall percentages of Arc expressing neurons was greater in the runners as compared to sedentary mice [Deviance= 227.1, P<0.0001]. In runners, the percentage of neurons expressing Arc was 1.9% (± 0.09 S.E.) in BrdU− neurons which was significantly different from the 3.8 % (± 0.85 S.E.) in BrdU+ neurons [Deviance=7.3, P = 0.007].
Localization of IEG-expressing new neurons within the granule layer
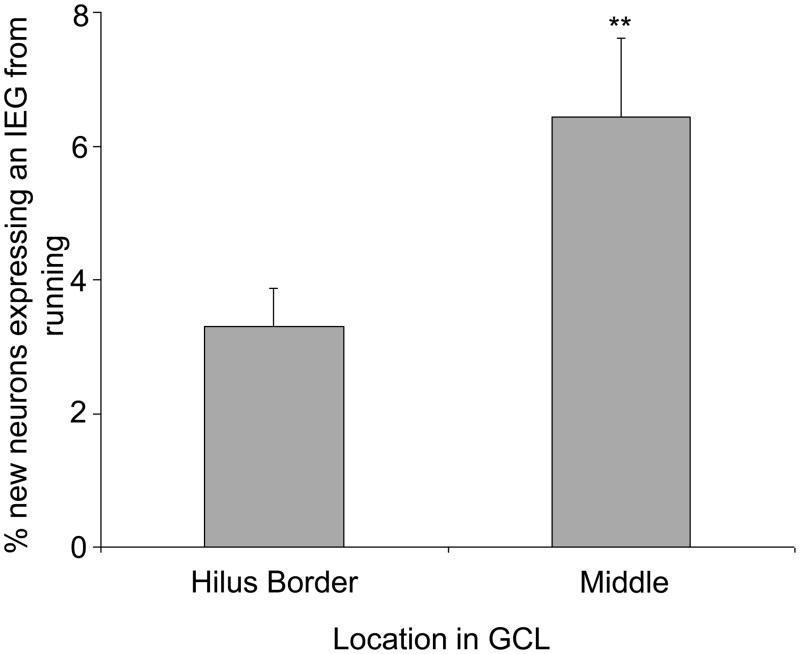
Collapsing data across all analyzed IEGs, a combined total of 2,197 BrdU+ and 125,885 BrdU− neurons were examined in two sub-regions of the granule cell layer of the dentate gyrus, the inside layer adjacent to the hilus versus the remaining layers, to determine the proportion expressing an IEG (either c-Fos, Arc, or Zif268). The BrdU+ neurons were concentrated in the inside layer adjacent to the hilus where 70% of the 1,433 neurons were located for runners and 81% of the 764 were located for sedentary animals. Thus, runners thus had a larger proportion of new neurons located away from the hilus than sedentary mice [Deviance= 32.6, P<0.0001]. In contrast, IEG-labeled cells were evenly distributed from the inside layer adjacent to the hilus to the outer edge of the granular layer (away from the hilus). In runners, the percentage of new neurons displaying an IEG was 2-fold greater if the new neurons were located in the middle of the granule cell layer as compared to adjacent to the hilus (Fig. 5) [Deviance=6.8, P=0.009]. The same comparison could not be made in sedentary mice due to the low number of IEG expressing new neurons (2, 4, & 0 for c-Fos, Zif268, & Arc respectively).
Figure 5.
Degree of IEG expression from running in new neurons depends on location within granule cell layer (GCL). The proportion of new neurons expressing an IEG located adjacent to the hilus compared to the middle of the GCL. Note that sedentary mice were not included in this comparison due to statistical limitations resulting from the small number of IEG expressing new neurons found (6 across all IEGs) despite an exhaustive search. ** indicates p<0.01
Experiment 2: Neurogenesis and IEG induction in home cages without wheels
Home cage activity
Inspection of Figure 1B shows distance traveled in home cages increased slightly each day, after habituation to the home environment by day 2. The average distance traveled over the entire experiment was 0.44 km/day (± 0.019 S.E.).
Correlation between home cage activity and neurogenesis
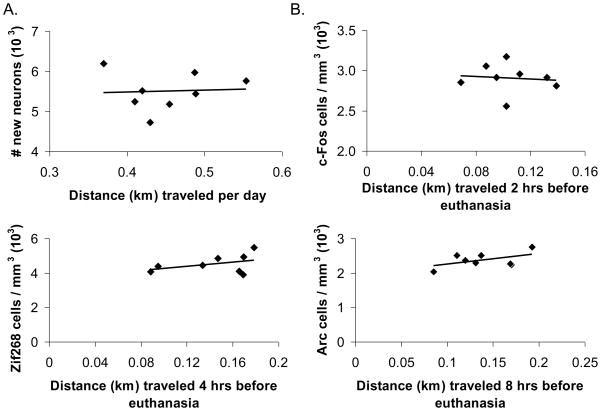
Average daily distance traveled in home cages was not significantly correlated with the number of new neurons formed for each animal (see Fig. 6A). Pearson’s r between average daily distance traveled and new neuron survival was 0.04 (P>0.05).
Figure 6.
IEG induction and neurogenesis from home cage activity. A) Total number of new neurons shown for individual mice plotted against average daily distance traveled in home cages without wheels (km). B) Total number of c-Fos positive cells per cubic mm plotted against distance traveled in home cages without wheels (km) accumulated within 2 hrs before euthanasia. C) Total number of Zif268 positive cells per cubic mm plotted against distance traveled in home cages without wheels (km) accumulated within 4 hrs before euthanasia. D) Total number of Arc positive cells per cubic mm shown for individual mice plotted against distance traveled in home cages without wheels (km) accumulated within 8 hrs before euthanasia.
IEG induction from home cage activity
No significant correlations were observed between c-Fos, Zif268, or Arc expression and distance traveled in home cages during the respective IEG induction periods (see Fig. 6B, C, & D). Pearson’s r for acute c-Fos, Zif268 and Arc induction and distance traveled was −0.13, 0.41, and 0.50 respectively (all P>0.05).
Experiment 3: IEG induction during the inactive light phase
c-Fos
Average distance traveled on running wheels 2 hrs before mice were euthanized was negligible and similar between groups sampled 3, 6, or 9 hrs after the onset of the light cycle. The collapsed average across all groups was 0.03 km (± 0.014 S.E.). c-Fos cell density did not differ between runners and sedentary mice. Average density for sedentary animals was 1.2 (± 0.36 S.E.) cells/mm3 (x 103). Average density of c-Fos cells for runners sampled at 3, 6, 9 hrs after lights on were 1.0 (± 0.25 S.E.), 1.3 (± 0.15 S.E.), 0.8 (± 0.17 S.E.), respectively.
Zif268
Average distance run 4 hrs before mice were euthanized was negligible and similar between groups sampled 3, 6, or 9 hrs after the onset of the light cycle. The collapsed average across all groups was 0.2 km (± 0.14 S.E.). Zif268 cell density did not differ between runners and sedentary mice. Average density of Zif268 cells in sedentary mice was 6.4 (± 1.11 S.E.) cells/mm3 (x 103). Average density for runners sampled at 3, 6, and 9 hrs after lights on was 8.0 (± 1.98 S.E.), 11.2 (± 1.10 S.E.), and 9.1 (± 0.55 S.E.), respectively.
Arc
Average distance run 8 hrs before mice were sampled either 3, 6, or 9 hrs after the onset of the light cycle was 2.2 km (± 0.62 S.E.), 0.6 km (± 0.07 S.E.), and 0.2 km (± 0.08 S.E.) respectively [F(2,15) = 8.54, P = 0.003]. Post hoc analysis revealed that mice sampled 3 hours after onset of the light cycle ran significantly more than those sampled at 6 [t(15)=1.9, P=0.012] and 9 hrs [t(15)=2.2, P=0.006]. However, mice sampled at 6 and 9 hours did not statistically differ from each other. Arc expressing cell density differed between groups [F(3, 20) = 4.5, P=0.01]. Post-hoc analysis revealed that runners sampled at 3 hrs had significantly more Arc cells than runners sampled at 6 hrs [t(20)=3.1, P=0.03] and 9 hrs [t(20)=3.3, P=0.02] after the onset of the light cycle. Sedentary mice were not significantly different from any running group. The average density of Arc cells for sedentary animals was 3.2 (± 0.36 S.E.) cells/mm3 (x 103). The average density of Arc cells for runners sampled at 3, 6, and 9 hrs after the onset of light cycle, was 5.1 (± 1.28 S.E.), 2.0 (± 0.28 S.E.), and 1.7 (± 0.52 S.E.), respectively.
4. Discussion
Results demonstrate that wheel running closely regulates the induction of three different IEGs in the hippocampal granule cell layer. The expression of c-Fos, Zif268, and Arc were all strongly correlated with the distance the animal traveled on wheels during the respective periods of induction for each IEG (see Fig. 2). The degree of IEG induction observed in the granule cell layer from wheel running is similar to previously reported inductions in mice performing spatial learning tasks or exploring novel environments (Stone et al.,, Ramirez-Amaya et al., 2005, Kee et al., 2007, Trouche et al., 2009, Tashiro, 2007 #17). Further, we report that an increased proportion of new dentate granule cells, 3–4 weeks old, were recruited into the neuronal c-Fos, Arc, and Zif268 induction from wheel running when compared to pre-existing or younger neurons (see Fig. 4). Arc, c-Fos, and Zif268 induction from running attenuated during periods of relative inactivity, suggesting these IEGs are induced from acute running and are not the result of long-term increases in basal concentrations within the granule cell layer. Taken together, these data suggest several IEGs are up-regulated in new and pre-existing neurons from acute bouts of physical activity.
While c-Fos, Zif268, Arc, and new neuron formation appear to be regulated by running (see Fig. 2), they are not strongly related to horizontal distance traveled in home cages without running wheels (see Fig. 6). The literature on the neurophysiology of home cage activity (without wheels) is scarce, however our lab recently reported no relationship when comparing distance traveled in home cages and number of new neurons across 12 different inbred strains of mice. This is in contrast to the positive relationship (r > 0.6) observed when comparing distance traveled on running wheels and amounts of neurogenesis in the same 12 strains (Clark et al., 2011). Little is also known about whether IEGs can be induced in an activity-dependent manner in the granule cell layer by other forms of less robust voluntary movement. The current study demonstrates that in C57BL/6J females, a strain that commonly displays a strong positive relationship between running distance, IEG induction and neurogenesis in the hippocampus, do not display a relationship between distance traveled in home cages and neurogenesis or IEG induction. It is possible that a threshold level of physical activity is required before IEG induction and neurogenesis can be enhanced, such as that which taxes aerobic capacity (Pereira et al., 2007, Erickson et al., 2009, Chaddock et al.).
The discovery that Arc is induced in neurons directly proportional to running intensity is particularly interesting because Arc expression in the hippocampus has largely been attributed to its role in learning and memory (Tzingounis and Nicoll, 2006, Bramham et al., 2008). The current study revealed that Arc is induced in the dentate gyrus of mice running on a fixed running wheel for over 30 days (see Fig. 2L). Thus, it is unlikely that learning was responsible for the Arc induction, as the animals were not experiencing any novel stimuli. To the best of our knowledge, this is the first example of Arc induction in the hippocampus from a voluntary behavior that appears to be absent of learning.
Evidence suggests that young neurons at a critical age, approximately between 1 and 6 weeks, become preferentially incorporated into behavior involving the hippocampus. Young adult-generated granule neurons display unique electrophysiological properties such as deceased threshold for LTP induction (Wang et al., 2000, Snyder et al., 2001, Schmidt-Hieber et al., 2004, Ge et al., 2007) suggesting new neurons may be more likely than older neurons to display an action potential during an event that activates the granule cell layer (Snyder et al., 2009a). Three recent reports using IEGs as neural activity makers following animal behavior might support this hypothesis, as they found IEGs were twice as likely to be induced in new neurons when compared to younger and pre-existing granule cells (Ramirez-Amaya et al., 2006, Kee et al., 2007, Clark et al., 2009). The current study extends those findings, as Arc, Zif68, and c-Fos from wheel running were each more likely to become expressed in 3–4 week old new neurons, as compared to non-BrdU labeled NeuN cells which include mostly older pre-existing cells (see Fig. 4). On the other hand, a recent study used three different markers of cell division to directly compare older labeled neurons to younger labeled neurons and found a similar proportion of c-Fos induction from a hippocampal task in all neuron cohorts (Stone et al.). The authors concluded that a comparison of BrdU labeled neurons to unlabeled neurons, as was done in our study and previous studies, may be confounded as unlabeled neurons also contain a small percentage of immature neurons that have not yet become incorporated into hippocampal circuitry (Brandt et al., 2003, Kempermann et al., 2003, Clark et al., 2010). Hence, recruitment rates of mature neurons into IEG responses will be underestimated by sampling unlabeled cells because the sample also includes a small proportion of young non-functional NeuN cells. Given the recent Stone et al. findings, we cannot strongly conclude that new neurons are preferentially recruited into IEG responses to running. However, we can conclude that new neurons are recruited at a high rate into the IEG response.
New hippocampal neurons have been primarily studied for their potential role in learning. By reducing levels of neurogenesis or measuring the expression of IEGs in new neurons, studies have suggested that adult-born neurons are involved in several hippocampal-required learning tasks including spatial navigation (Snyder et al., 2005), contextual learning (Hernandez-Rabaza et al., 2009), novel object/place learning (Jessberger et al., 2009), pattern separation (Clelland et al., 2009), and trace conditioning (Shors et al., 2002). Moreover, other studies have suggested that new neurons from running play an important role in extending plasticity for spatial learning and memory (van Praag et al., 1999a, Clark et al., 2008). Under this context, wheel running appears to uniquely activate new neurons because it is seemingly unrelated to hippocampal-learning, since mice are habituated to running wheels and no new spatial information is being acquired.
It is possible that new neurons, characterized by their high degree of plasticity, become incorporated into whatever task the hippocampus is engaged in at any particular moment. Bland postulated an additional function of the hippocampus and related limbic structures is the processing of sensory or motor information (Bland, 1986, Oddie and Bland, 1998). The second major class of cells in the rodent hippocampus is the theta cell, which fires action potentials with a frequency and amplitude proportional to the velocity and magnitude of voluntary movement (O’Keefe, 2007). For instance, theta-rhythm recorded in the hippocampus of wheel running rats displays a greater frequency and amplitude as the velocity of running increases (Oddie et al., 1996). Theta-rhythm induced from movement has been proposed to coordinate ensembles of cell activity involved in processing sensory cues necessary for maintenance of motor activity (Oddie and Bland, 1998, Bland and Oddie, 2001). Therefore, new neurons may be recruited into the function of the hippocampus during running by passively processing ongoing sensory information or actively modulating motor behavior (i.e. current intensity of activity). Recent studies using irradiation to reduce neurogenesis from running did not report any impairments in wheel running or motor task performance, so new neurons may not be critical for sensory or motor processing (Clark et al., 2008, Wojtowicz et al., 2008). However, neither study reported a complete ablation of neurogenesis. As technology improves for blocking neuron formation in the hippocampus, it would be interesting to test if a near complete removal of neurogenesis impairs running performance.
Running appears to increase the maturation and integration of new neurons into hippocampal circuitry. Precursor cells located in the inside layer adjacent to the hilus can differentiate into young neurons, and it has been suggested that as new neurons mature, they continue to move, or are pushed outwards to the superficial edges of the granule cell layer. Neurons located near the outer layers display a more pronounced dendritic arborization than neurons in the inner layer (Green and Juraska, 1985, Redila and Christie, 2006), suggesting that an increase in complexity of neuron morphology occurs with neuron age (van Praag et al., 2002, Schmidt-Hieber et al., 2004). In the current study, running mice had a greater proportion of new neurons located deep in granule cell layer than sedentary mice. These new neurons located in the middle of the granule cell layer were twice as likely to express either c-Fos, Zif268, or Arc from running when compared to the neurons located near the hilus (see Fig. 5). One explanation for these observations is that running may alter the maturation of a subset of new neurons located deeper in the granule cell layer, and that these neurons may be more likely to become functionally integrated. Additional support for this hypothesis has been demonstrated in recent literature. Running increased the proportion of 21 day old neurons capable of expressing Arc following kainate-induced seizure, suggesting that a greater proportion of young neurons from running are integrated into hippocampal circuitry (Snyder et al., 2009b). Further, running increased the proportion of young neurons that expressed the protein NeuN, a commonly used marker for mature neurons (Clark et al.). Our data in conjunction with previous reports suggest that a subset of neurons formed from running may develop more rapidly and incorporate into granule cell circuitry.
IEG expression from running-induced neural activity may be important for the increased survival and differentiation of new neurons from running. Several recent reports have revealed that neural precursor cells have activity sensing properties that are necessary for their differentiation into neurons (Deisseroth et al., 2004). We found wheel running increased the survival of granule cells in a manner strongly correlated with average distance the animal traveled each day (see Fig. 2C). Moreover, Arc, Zif268 and c-Fos were all induced from running in an activity-dependent manner (see Fig. 2F, I, & L). These relationships suggest that neural activity from wheel running may directly influence the survival of cells. Indeed, the expression of several genes that are transcribed by activity-induced IEG proteins also regulate hippocampal neurogenesis [e.g. neuropeptide Y by Zif268 (Wernersson et al., 1998), nerve growth factor by c-Fos (Herdegen and Leah, 1998, Kovacs, 1998), ect.]. Moreover, the expression of the brain derived neurotrophic factor (BDNF), which is necessary for the survival of newly formed neurons (Li et al., 2008), appears to regulate activity-dependant expression of the effector gene Arc (Bramham, 2008). It seems possible that IEGs are translating neural activity from wheel running into factors that promote the survival and differentiation of new neurons.
In conclusion, we report that acute bouts of wheel running induce the expression of three different IEGs, c-Fos, Zif268, and Arc, in the granule cell layer of the hippocampus. IEG induction and neurogenesis is specific for wheel running and does not occur for lower levels of physical activity such as that displayed in the home cage without running wheels. Moreover, new adult-born neurons are more likely to express c-Fos, Arc, and Zif268 from running than the population of pre-existing or younger neurons. These data, in conjunction with other reports, suggest that the granule cell layer may play a role in passively processing ongoing sensory information or actively modulating motor behavior, and new neurons can be incorporated into this response. Whether new neurons generated from running are critical for processing sensory-motor information necessary for running, or whether they are also capable of being activated by hippocampus-dependant learning tasks are topics for future investigations.
Acknowledgments
Special thanks to Beckman Institute Animal Care facility. This work was supported by grants from National Institutes of Health, MH083807 and DA027487.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Dragunow M, Tate WP. The role of immediate early genes in the stabilization of long-term potentiation. Mol Neurobiol. 1991;5:297–314. doi: 10.1007/BF02935553. [DOI] [PubMed] [Google Scholar]
- Beckmann AM, Wilce PA. Egr transcription factors in the nervous system. Neurochem Int. 1997;31:477–510. doi: 10.1016/s0197-0186(96)00136-2. discussion 517–476. [DOI] [PubMed] [Google Scholar]
- Bednarczyk MR, Aumont A, Decary S, Bergeron R, Fernandes KJ. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus. 2009;19:913–927. doi: 10.1002/hipo.20621. [DOI] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav Brain Res. 2001;127:119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr Opin Neurobiol. 2008;18:524–531. doi: 10.1016/j.conb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, von der Behrens W, Kempermann G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ, Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav. 2011 doi: 10.1111/j.1601-183X.2010.00674.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Haferkamp EH, Rhodes JS. Adult hippocampal neurogenesis and c-Fos induction during escalation of voluntary wheel running in C57BL/6J mice. Behav Brain Res. 2010;213:246–252. doi: 10.1016/j.bbr.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RM, Dragunow M, Robertson HA. High-frequency discharge of dentate granule cells, but not long-term potentiation, induces c-fos protein. Brain Res. 1988;464:259–262. doi: 10.1016/0169-328x(88)90033-2. [DOI] [PubMed] [Google Scholar]
- Dragunow M. A role for immediate-early transcription factors in learning and memory. Behav Genet. 1996;26:293–299. doi: 10.1007/BF02359385. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Abraham WC, Goulding M, Mason SE, Robertson HA, Faull RL. Long-term potentiation and the induction of c-fos mRNA and proteins in the dentate gyrus of unanesthetized rats. Neurosci Lett. 1989;101:274–280. doi: 10.1016/0304-3940(89)90545-4. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French PJ, O’Connor V, Jones MW, Davis S, Errington ML, Voss K, Truchet B, Wotjak C, Stean T, Doyere V, Maroun M, Laroche S, Bliss TV. Subfield-specific immediate early gene expression associated with hippocampal long-term potentiation in vivo. Eur J Neurosci. 2001;13:968–976. doi: 10.1046/j.0953-816x.2001.01467.x. [DOI] [PubMed] [Google Scholar]
- Gass P, Herdegen T, Bravo R, Kiessling M. Induction of immediate early gene encoded proteins in the rat hippocampus after bicuculline-induced seizures: differential expression of KROX-24, FOS and JUN proteins. Neuroscience. 1992;48:315–324. doi: 10.1016/0306-4522(92)90493-l. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EJ, Juraska JM. The dendritic morphology of hippocampal dentate granule cells varies with their position in the granule cell layer: a quantitative Golgi study. Exp Brain Res. 1985;59:582–586. doi: 10.1007/BF00261350. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG, Gomez-Pinedo U, Perez-Villalba A, Rosello J, Trejo JL, Barcia JA, Canales JJ. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L, Zangenehpour S, Chaudhuri A. Sensory regulation of immediate-early genes c-fos and zif268 in monkey visual cortex at birth and throughout the critical period. Cereb Cortex. 1999;9:179–187. doi: 10.1093/cercor/9.2.179. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Lee TH, Jang MH, Shin MC, Lim BV, Kim YP, Kim H, Choi HH, Lee KS, Kim EH, Kim CJ. Dependence of rat hippocampal c-Fos expression on intensity and duration of exercise. Life Sci. 2003;72:1421–1436. doi: 10.1016/s0024-3205(02)02406-2. [DOI] [PubMed] [Google Scholar]
- Lee YI, Park KH, Baik SH, Cha CI. Attenuation of c-Fos basal expression in the cerebral cortex of aged rat. Neuroreport. 1998;9:2733–2736. doi: 10.1097/00001756-199808240-00009. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahan L, Hakim AM, Nakabeppu Y, Robertson GS. Ischemia-induced CA1 neuronal death is preceded by elevated FosB and Jun expression and reduced NGFI-A and JunB levels. Brain Res Mol Brain Res. 1998;56:146–161. doi: 10.1016/s0169-328x(98)00039-4. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Oddie SD, Bland BH. Hippocampal formation theta activity and movement selection. Neurosci Biobehav Rev. 1998;22:221–231. doi: 10.1016/s0149-7634(97)00003-1. [DOI] [PubMed] [Google Scholar]
- Oddie SD, Stefanek W, Kirk IJ, Bland BH. Intraseptal procaine abolishes hypothalamic stimulation-induced wheel-running and hippocampal theta field activity in rats. J Neurosci. 1996;16:1948–1956. doi: 10.1523/JNEUROSCI.16-05-01948.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J. Hippocampal Neurophysiology in the Behaving Animal. In: Per Andersen RM, Amaral David, O’Keefe John, editors. The Hippocampus Book. Oxford University Press; 2007. p. 490. [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003a;117:1243–1256. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003b;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Richardson CL, Tate WP, Mason SE, Lawlor PA, Dragunow M, Abraham WC. Correlation between the induction of an immediate early gene, zif/268, and long-term potentiation in the dentate gyrus. Brain Res. 1992;580:147–154. doi: 10.1016/0006-8993(92)90938-6. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Gage FH. A hypothesis about the role of adult neurogenesis in hippocampal function. Physiology (Bethesda) 2004;19:253–261. doi: 10.1152/physiol.00012.2004. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K, Matsuki H, Tsunashima K, Iyo M, Higuchi T, Minabe Y. Increased expression of zif268 mRNA in rat retrosplenial cortex following administration of phencyclidine. Brain Res. 1999;839:180–185. doi: 10.1016/s0006-8993(99)01738-2. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009a;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009b;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Ramchand P, Rabbett S, Radik R, Wojtowicz JM, Cameron HA. Septo-temporal gradients of neurogenesis and activity in 13-month-old rats. Neurobiol Aging. 2009c doi: 10.1016/j.neurobiolaging.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SS, Teixeira CM, Zaslavsky K, Wheeler AL, Martinez-Canabal A, Wang AH, Sakaguchi M, Lozano AM, Frankland PW. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus. doi: 10.1002/hipo.20845. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci U S A. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Nicoll RA. Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron. 2006;52:403–407. doi: 10.1016/j.neuron.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006;1104:64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- Wernersson J, Johansson I, Larsson U, Minth-Worby C, Pahlman S, Andersson G. Activated transcription of the human neuropeptide Y gene in differentiating SH-SY5Y neuroblastoma cells is dependent on transcription factors AP-1, AP-2alpha, and NGFI. J Neurochem. 1998;70:1887–1897. doi: 10.1046/j.1471-4159.1998.70051887.x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Errington ML, Williams S, Dunnett SB, Waters C, Hitchcock D, Evan G, Bliss TV, Hunt SP. Differential expression of immediate early genes in the hippocampus and spinal cord. Neuron. 1990;4:603–614. doi: 10.1016/0896-6273(90)90118-y. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Zangenehpour S, Chaudhuri A. Differential induction and decay curves of c-fos and zif268 revealed through dual activity maps. Brain Res Mol Brain Res. 2002;109:221–225. doi: 10.1016/s0169-328x(02)00556-9. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS. Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol Behav. 2008;93:637–650. doi: 10.1016/j.physbeh.2007.11.004. [DOI] [PubMed] [Google Scholar]