Abstract
Purpose
Angular deformities of the knee resulting from idiopathic, congenital, or acquired causes are commonly encountered in pediatric orthopedics. Whereas physiological deformities should be treated expectantly, the remaining often progress enough to warrant operative treatment, despite attempted bracing. Historical methods of surgical treatment (e.g., epiphysiodesis and stapling) have yielded to the increasingly popular method of reversible guided growth using the eight-Plate.
Methods
We studied 58 patients with knee angular deformities managed with eight-Plate guided growth. All etiologies except physiological deformities and those with very slow growth rate were included. Each patient was under appropriate medical management during the entire duration of treatment and after plate removal.
Results
In the dysplasia/syndrome group, we noted complete correction in 22 patients (78.5%), partial correction in 5 (17.9%), and no correction in 1 patient (3.6%). All cases of idiopathic deformities resolved. Patients with osteochondral dysplasias and genetic syndromes underwent earlier intervention and slower correction than those with idiopathic genu varum or valgum. The time difference in reaching a neutral mechanical axis between the two groups (11 months in idiopathic versus 18 months in pathological physis) could be explained by a significant difference in growth speeds (P = 0.003).
Conclusion
Results indicate that early intervention is advisable for patients with osteochondral dysplasias/syndromes as subsequent correction takes longer. If rebound growth causing recurrent deformity occurs, guided growth can be safely repeated. Additionally, complications reported with other techniques such as hardware failure, physeal violation by the implant, premature physeal closure, and overcorrection were not reported while using the eight-Plate.
Keywords: Eight-Plate, Guided growth, Hemiepiphysiodesis, Knee angular deformities
Introduction
Angular deformities of the knee, a common problem encountered in pediatric orthopedics, may often be managed expectantly and with benign neglect, requiring only parental reassurance [1]. Most physiological deformities peak between 1 and 3 years (varus) or between 3 and 6 years (valgus) and resolve spontaneously [2]. Pathological angular deformities can be either idiopathic or due to congenital syndromes such as skeletal dysplasia. In contrast to physiological deformities, pathological deformities manifest as the underlying disease progresses and acts on skeletal growth, leading to a gradual mechanical axis displacement. Valgus deformities in excess of 10° can cause anterior knee pain, circumduction gait, and occasionally patellofemoral instability [3]. Varus deformities may result in lateral thrust, ligamentous laxity, and a waddling gait. Regardless of whether the etiology is idiopathic, dysplastic, or related to an endocrinopathy, the common goal of surgical treatment is to restore and maintain a neutral mechanical axis [4].
Several methods have been described to attain normal alignment. Although osteotomy has been considered the gold standard for correcting angular deformities, it is fraught with potential complications [5, 6]. Continued growth may result in recurrent deformities and the need for revision osteotomies [1]. The associated discomfort, immobilization, aggregate hospitalization, recovery time, costs, and risks make osteotomy, in our opinion, the last resort for angular corrections while the physis is still open.
Stapling [7] has waxed and waned in popularity since its introduction by Blount [7], but its use has decreased in recent times because of unpredictability and the fear of permanent iatrogenic physeal arrest [8–10]. Although stapling can work well, occasional breakage or migration of staples can necessitate the revision of hardware or premature abandonment of this method of treatment [7, 11–13]. The next advance in reversible hemiepiphyseal arrest was proposed by Métaizeau [14], who suggested the use of transphyseal screws. However, it is unclear whether this epiphysiodesis truly is reversible [15, 16]. To avoid possible complications, a different hardware construct was adopted, employing a nonlocking extraperiosteal plate and two screws (eight-Plate); this construct guides the physis growth with few complications and high efficacy [4, 16–18].
In this retrospective study, we analyzed 58 cases of angular deformity in children who were subjected to correction with the eight-Plate (Orthofix Srl, Verona, Italy). The children were divided into two groups according to etiology: idiopathic group and pathological group (osteochondral dysplasia or endocrinopathy). Regardless of etiology, the common goal of this treatment method was to obviate, or at least postpone, osteotomy. We sought to compare the efficacy and rate of correction in each group.
Materials and methods
Patient selection
From September 2005 to June 2008, a total of 58 patients with angular deformities managed with an eight-Plate were observed. On preoperative anteroposterior (AP) radiographs, we evaluated mechanical axis displacement as well as the widths of the physis at the femoral and tibial ends. When in doubt, we assessed skeletal maturity with wrist films in order to ascertain that at least 12 months of predicted growth were remaining. Only patients with an angular deformity greater than 10° were subjected to surgery. Patients with disease conditions associated with slow growth rates, for example, dwarfism, osteochondromatosis, and others, were not included in the study.
Surgery
The surgery was performed on an outpatient basis under general or regional anesthesia. In younger patients, the use of a general anesthetic was preferred, whereas in older or obese patients, regional anesthesia was used. A single eight-Plate was used per physis selected (femur, tibia, or both). In patients nearing skeletal maturity, the plates were used in both the femur and the tibia to expedite the correction.
Postoperative management
No immobilization was required after surgery, and early weight-bearing was encouraged along with a rapid return to normal activities. Follow-up evaluations were performed to assess the deformity correction approximately every 3 months, until reaching the neutralization of the axis, whereupon the hardware was removed by a quick surgical procedure. Following plate removal, periodic follow-up (until bone maturity) was performed for all patients to assess if rebound growth, limb length discrepancy, or premature physeal closure had occurred. Monitoring was important, especially for patients with dysplasia or endocrinopathy, as they had been treated from an early stage. Patients with idiopathic deformities were usually treated until they almost reached skeletal maturity, so the predicted growth period subsequent to plate removal was short, and the risk of rebound was low.
Data collection
Clinical and radiological (AP full-length weight-bearing) evaluation was performed pre-surgery, immediately post-surgery, before and immediately after plate removal. In order to decrease unnecessary exposure of the children to radiation, only clinical evaluation was performed during follow-up visits, which included measuring of the intercondylar or intermalleolar distance. If these values were anomalous, or if axial deviations were observed, X-rays were performed. If the patients did not show deformities in the lateral plane pre-operatively, X-rays in the lateral view were not taken during the follow-up visits, except in the cases where knee range of movement decreased. The anatomical tibial femoral angle (TFA) was measured on a radiograph at the time of implantation and removal of the plates. The TFA was used to calculate deformities because the quality of X-rays does not always allow clear visualization of the hip; the use of TFA to calculate deformities following similar treatments has been reported in the literature [19, 20]. All the measurements were made by the same orthopedic surgeon to avoid interobserver error, and were then reviewed by a second senior surgeon.
Statistical analysis
Statistical analysis was performed using SAS® System (version 9.2). Continuous variables were summarized using mean, standard deviation (SD), and range, whereas categorical variables were determined using frequency distribution and percentages. Comparison between the idiopathic and pathological groups regarding the speed of correction (corrected degrees per month) was carried out by means of an analysis of covariance (ANCOVA) that included terms for disease effect, age, implant site, and baseline deviation angle as covariates. The difference between least-square means and relative 95% confidence interval was calculated for the disease effect, adjusting for age, implant site, and baseline deviation angle. Correlations between corrected degrees per month and age of patients were investigated within each group by means of Pearson’s correlation coefficient.
Results
Between September 2005 and June 2008, 162 plates were implanted in 58 patients. We evaluated the patients as a single group to assess the effectiveness of the hardware, and as two groups divided according to the etiology (idiopathic and pathological) to investigate the different approaches to treatment. There were 30 patients (52%) with idiopathic deformities and 28 patients (48%) with pathological deformities; 30 patients (52%) were male and 28 patients (48%) were female.
There were 45 patients (78%) with genu valgum and 12 patients (21%) with genu varum; 1 patient (1.7%) exhibited windswept deformity. The deformities were bilateral in 50 patients (86%) and unilateral in 8 patients (14%). None of the patients had any deformity in the sagittal plane. The average age at the time of surgery was 10 years 10 months (range 2 years 3 months to 14 years 11 months). The age distribution at the time of surgery is shown in Fig. 1. We employed a different approach to the treatment of each group. Compared to patients with idiopathic deformities, patients with a pathological physis were treated at a younger age, and the treatment was spread over a longer time period. The plates were removed on average 14 months after implantation (range 2–37 months). At the time of data collection, 54 of 58 patients (93.1%) had undergone the plate removal procedure. The plates were not removed in the remaining 4 patients because of various reasons: (1) one hypochondroplasia patient with corrected deformity was awaiting plate removal at the time of data collection (plate removed in May 2010); (2) the second patient with dysplasia and with corrected deformity was awaiting plate removal and concomitant application of an external fixator to correct a 6-cm limb discrepancy; (3) the plate was not removed in the third patient who had osteopetrosis because of his systemic health and local bone condition; and (4) the parents of the fourth patient (idiopathic group) with corrected deformity refused to give permission for plate removal.
Fig. 1.
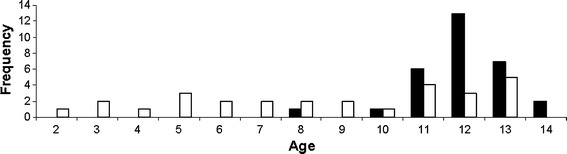
Distribution of age at time of surgery. White bar patients with pathological physis; black bar patients with idiopathic physis
The mean degree of correction was 11 ± 4.9° for all patients (range 0–25°), and the mean degree of correction per month was 0.93 ± 0.82° (range 0–6° per month). No difference between genu valgum and genu varum patients was detected. We noted restoration of the TFA to within the physiological range in 52 of 58 patients (89.7%). Complete deformity correction did not occur in 6 patients, and they were classified as “partially corrected” or “not corrected.” One patient, after 1 year of treatment, had no degree of correction, and he was considered “not corrected” (1.7%) (Table 1).
Table 1.
Summary of clinical outcomes after treatment with eight-Plate
| Outcome | Number of patients, n (%) | |||
|---|---|---|---|---|
| Idiopathic (n = 30) | Pathological (n = 28) | Total (n = 58) | ||
| Completely corrected | 30 (100) | 20 (71.4) | 50 (86.2) | 89.7%b |
| Completely corrected, awaiting removal | 0 | 2 (7.1)a | 2 (3.5) | |
| Partially corrected | 0 | 5 (17.9) | 5 (8.6) | |
| Not corrected | 0 | 1 (3.6) | 1 (1.7) | |
aOne dysplasia patient showed deformity correction at 14 months after implantation and was awaiting plate removal and concomitant application of an external fixator to correct a 6-cm limb discrepancy; the second patient with hypochondroplasia was awaiting plate removal after the deformity corrected at 28 months after implantation (plate removed in May 2010). These 2 patients were close to skeletal maturity; their growth plates were closed although visible, and rebound was unlikely to occur
bThe total percentage of patients with complete correction of deformity
Idiopathic group
This group included 30 patients with bilateral genu valgum. The mean TFA at the time of surgery was 14 ± 2.1° (range 10–20°), and the mean age at time of surgery was 12 years 5 months (range 8 years 8 months to 14 years 11 months). The plates were removed in 29 of 30 patients (96.6%); in 1 case, as per the decision of the parents, the plates were not removed at skeletal maturity. The average treatment duration was 11 months (range 2–16 months). The mean angle after correction was 5 ± 1.2° (range 4–8°) (Table 2). Therefore, in this group, 30 patients (100%) experienced complete correction of deformities. Figure 2 shows an example of an idiopathic patient who was corrected 11 months after the plates were placed in both the femurs and tibias.
Table 2.
Clinical data regarding deformities in patients stratified by etiology
| Idiopathic | Pathological | |
|---|---|---|
| Number of patients | 30 | 28 |
| Age at time of surgery | 12 years 5 months (8 years 8 months to 14 years 11 months) | 9 years 1 month (2 years 3 months to 13 years 11 months) |
| TFA at time of surgery (°) | 14 ± 2.1 | 22 ± 4.9 |
| TFA at time of plate removal (°) | 5 ± 1.2 | 9 ± 5.9 |
| Time of treatment (months) | 11 ± 4.0 | 18 ± 6.9 |
| Degrees corrected (°) | 9 ± 1.9 | 13 ± 6.2 |
Data reported are expressed as mean ± SD or range
TFA tibial femoral angle
Fig. 2.
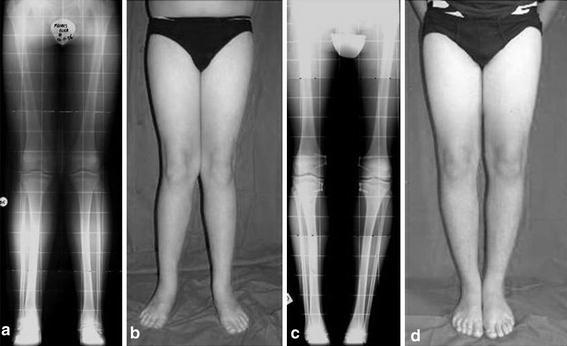
A 13-year-old with idiopathic genu valgum. a, b Before treatment, the patient shows bilateral genu valgum. c, d The neutralization of the mechanical axis was reached 11 months after placing plates in both the femurs and tibias
Pathological group
The pathological group included 28 patients: 15 patients (53.6%) with genu valgum, 12 patients (42.8%) with genu varum, and 1 patient (3.6%) with windswept deformity. The primary etiology of angular deformities included dysplasias (7), exostoses (4), pseudoachondroplasia (3), rickets (3), Schmidt’s syndrome (3), congenital syndrome (3), Ellis-van Creveld syndrome (1), hypochondroplasia (1), Blount’s disease (1), osteopetrosis (1), and Ollier’s syndrome (1). Bilateral deformities were present in 20 patients, and unilateral deformities in 8 patients. The mean TFA at the time of surgery was 22 ± 4.9° (range 14–30°). The mean age at the time of surgery was 9 years 1 month (range 2 years 3 months to 13 years 11 months). After an average of 18 months (range 9–37 months), the degrees corrected were 13 ± 6.2° (range 0–25°). The mean angle after correction was 9 ± 5.9° (range 5–25°) (Table 2).
In this group, deformities were completely corrected in 22 patients (78.6%), partially corrected in 5 patients (17.9%), and remained uncorrected in only 1 patient (3.6%). Figure 3 shows an example of a pathological patient who was corrected 12 months after the plates were placed in both the femurs and tibias. The 6 patients who did not achieve complete correction underwent a subsequent osteotomy using internal fixators and plaster of Paris (1 patient), or external fixators (5 patients). All patients who underwent osteotomies experienced subsequent bone consolidation and neutralization of the mechanical axis. In case of torsional deformity (present occasionally in patients with Blount’s disease and other skeletal dysplasias), the torsion corrected through the physis as the mechanical axis was restored to neutral. Although scientific evidence for this may be lacking, our results indicate that rotational osteotomies are never required.
Fig. 3.
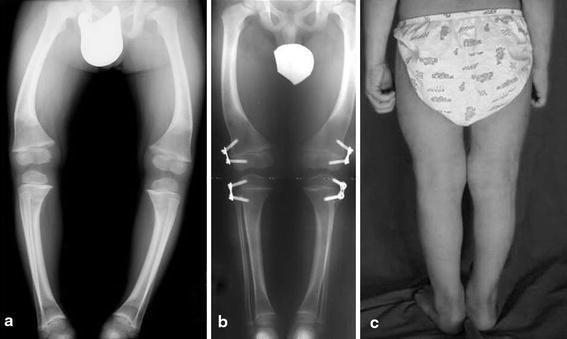
A 4-year-old male with Schmidt’s syndrome. a Before treatment, the patient shows bilateral genu varum and mild coxa vara. b, c The neutralization of the mechanical axis was reached 12 months after placing plates in both the femurs and tibias
Speed of correction
In order to assess the influence of pathology on the speed of correction, we present two cases with similar baseline characteristics, one with a pathological knee deformity and the other with an idiopathic deformity. The pathological case is of a male child aged 11 years and 10 months who had rickets with bilateral genu valgum with a pre-correction TFA of 15°. The duration of treatment from implant to removal was 28 months, with a final TFA of 6° at removal. The speed of correction was 0.3°/month. At the last follow-up, the patient was 16 years of age and had not yet reached skeletal maturity (Fig. 4). The idiopathic case is of a male child aged 11 years and 10 months who had bilateral genu valgum with a pre-correction TFA of 13°. The duration of treatment from implant to removal was 14 months with a final TFA of 4° at removal and a correction speed of 0.6°/month. At the last follow-up, the patient was aged 14 years and had not yet reached skeletal maturity (Fig. 5). Thus, the speed of correction was faster in idiopathic deformity than in pathological deformity.
Fig. 4.
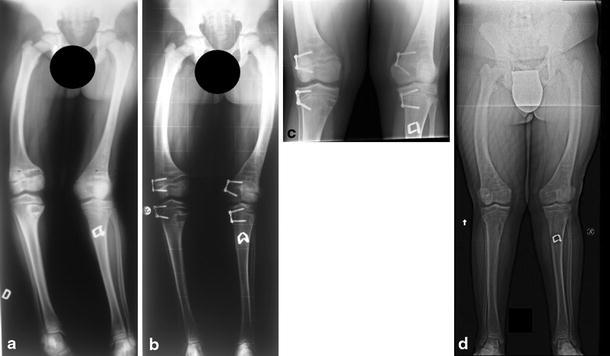
Pathological case: An 11-year-old male with rickets and windswept deformity. a Pre-surgery (TFA = 15°), b follow-up at 8 months after implantation, c immediately prior to plate removal (TFA = 6°), d follow-up at 20 months after plate removal
Fig. 5.
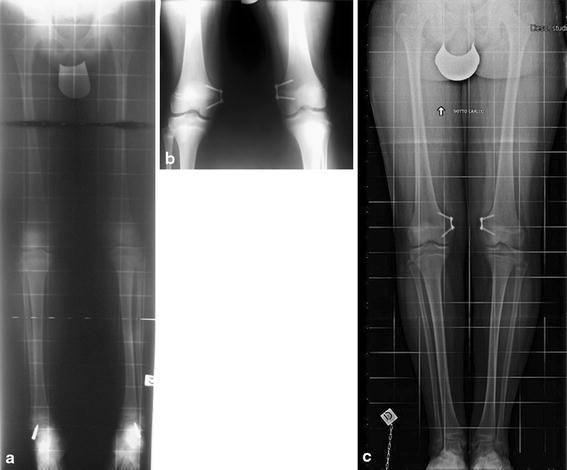
Idiopathic case: An 11-year old male with bilateral genu valgum. a Pre-surgery (TFA = 13°), b immediately post-surgery, c follow-up during treatment, 8 months after plate implantation
To assess the difference in speed of correction between the idiopathic and pathological groups, we used an ANCOVA, considering age, implant site, and baseline deviation angle as covariates. This method helped us to analyze the speed of correction without the influence of age, implant site, and baseline deviation angle. The speed of correction per month estimated from this model was significantly higher in the idiopathic group (adjusted mean 1.43) than in the pathological group (adjusted mean 0.38) (P = 0.003). This, in turn, resulted in a longer time to correction in the pathological group than in the idiopathic group. In both groups, there was a tendency toward decreasing speeds of correction with increasing age, as indicated by Pearson’s correlation coefficient (Pearson’s correlation coefficient for idiopathic patients −0.113; Pearson’s correlation coefficient for pathological group −0.251). This indicates that early intervention in pathological physis patients is warranted.
Follow-up
Periodic follow-ups after plate removal were carried out for all patients who completed the treatment with eight-Plates (52 patients). The patients were followed up for an average of 21 months after plate removal (range 8–39 months) and the mean age at the last evaluation was 14 years (range 14–18 years). At the time of the latest follow-up visit in March 2010 during manuscript preparation, out of the 30 patients with complete deformity correction in the idiopathic group, 1 patient had reached skeletal maturity without any complications or rebound; the other patients will be followed up until skeletal maturity. In the pathological group, excluding the 6 patients who were partially corrected or not corrected and were undergoing different treatments, none of the 22 patients with full correction of deformity had reached skeletal maturity and will be followed up until maturity. During the follow-up visits, X-rays were not taken in lateral view because we did not notice any clinical deviation or loss in the range of motion in any patient that would indicate a procurvatum or recurvatum deformity.
Complications
In this series of patients treated with eight-Plates, we did not observe complications such as hardware failure over the duration of treatment, as is observed with other techniques. There was no need for repeat surgery other than routine planned hardware removal. The deformity remained uncorrected in only 1 patient in the pathological group. The reason for this is unknown, although it may be speculated that the patient was operated on too late, and the physes were nearing closure.
In the idiopathic group, no rebound deformity was observed. However, these patients will be followed up and monitored for any signs of rebound deformity until skeletal maturity. In the pathological group, rebound deformity was noticed in 2 patients (3.8%)—one affected by Schmidt’s syndrome and the other by vitamin d-resistant rickets, occurring at 34 and 11 months, respectively. Both deformities will be corrected during the lengthening procedures planned to treat short stature.
Discussion
Angular deformities of the knee alter the biomechanics of the knee by causing a distorted stress distribution on the weight-bearing surface of the knee joint, and various methods have been proposed to address this problem [1]. Osteotomy, still considered by some as the definitive solution for angular corrections, is associated with frequent and sometimes severe complications. Others have resorted to the less invasive method of hemiepiphysiodesis in order to restore alignment with lower cost and the fewest complications. The most recent technique involves guided growth with an eight-Plate. The only contraindication for guided growth is physeal closure due to damage or to skeletal maturity.
We have routinely employed this technique in patients exhibiting deformities due to both pathological and idiopathic causes, noting full correction in most cases. Some of the most severe deformities (5 patients) found in the pathological group did not achieve full correction, but as stated by Schroerlucke [21], in such cases, even partial correction is a positive result. This has also made subsequent osteotomies technically easier. Meanwhile, it is important to consider the different rates of growth associated with the spectrum of pathological conditions causing angular deformities of the knee, especially conditions such as diastrophic dwarfism, osteochondromatosis, and others that are associated with slow growth. However, we did not face any difficulties in this regard because the pathological group in our study comprised only patients with pathologies in which the epiphyseal plates have a good or discrete growth rate, e.g., rickets and exostosis disease.
We have adopted a different approach for treatment according to the etiology; patients with pathological deformities were treated earlier (2–13 years) than those with idiopathic deformities (8–14 years). Patients with idiopathic deformities were treated only after it was clear that physiological recovery had failed. Surgical treatment is not recommended before 8 years of age [22]. After the age of 8, an angular deformity of less than 10° can be considered cosmetic and does not warrant surgery [22–25]. However, surgical intervention should be considered for a deformity greater than 10° with a predicted remaining time for growth of at least 12 months. The timing of surgery should take into account the fact that the speed of correction seems to decrease with age, and as a patient approaches skeletal maturity, the physis grows at a slower rate. In some cases, plates were applied to both the femur and the tibia, in order to speed up the correction before reaching physeal closure (Figs. 2, 3).
Some proponents of stapling have advocated waiting until adolescence for fear of inducing premature physeal closure [9, 10, 15]. However, for pathological conditions that are likely to progress, we have adopted early intervention for guided growth. This was done on the premise that a flexible tension band that does not compress the physis is unlikely to have an adverse effect on physeal closure [4, 12, 18]. We observed that the rate of correction was slower in the pathological group (18 months) than in the idiopathic group (11 months). The degree per month of correction was also slower in the pathological group than in the idiopathic group. This, in all likelihood, is a reflection of physeal compromise that is characteristic of dysplasias and endocrinopathies. Considering this, we recommend early intervention for deformities of pathological etiology, even if the process has to be repeated during continued growth. There may be secondary benefits noted at the hip and ankle [4], and osteotomies may be postponed or perhaps even prevented.
Permanent physeal closure was not observed in this study. Rebound growth was rare (3 of 58 patients); all rebound cases were observed in the pathological group and considered part of the natural history of progression that may continue following eight-Plate removal. The majority of studies focusing on this treatment have reported results at the implant removal stage [15, 17] or before skeletal maturity [19–21]. Waiting for the complete skeletal maturity in patients, especially of those in the pathological group, would mean waiting for 10–15 years from the first surgery to publish these results. Although we are reporting these data now, we hope to report updated results at skeletal maturity in all cases in a future manuscript. We believe that a large, long-term, prospective, multicenter study would be required to monitor the real incidence of rebound from plate removal to skeletal maturity. Repeating the technique is a simple process, and it is well tolerated by patients. With the flexible tension band construct, the physis is spared from compressive forces, the screws are free to diverge with the growth, and the problem of implant migration or breaking does not occur. No hardware failure was observed in this study. This information is consistent with three other studies [1, 4, 16] but differs from the data from one study [21]. Perhaps patient selection and attention to technical details would explain the difference. We did not experience long-term problems with the positioning of screws after implantation. In case the screws become completely divergent and a considerable amount of deformity remains to be corrected, a change of screws can be considered by minimally invasive surgery.
We consider the eight-Plate the best solution for the treatment of pediatric angular deformities, be they idiopathic or due to an underlying pathological condition. The results of this study indicate that treatment of idiopathic patients during adolescence is only advised for deformities greater than 10° because they can lead to functional impairment. For patients with pathological physes, it is prudent to start treatment at a very young age, when the deformities are not severe and the speed of correction is rapid. This would subsequently prevent or slow the development of secondary deformities and simplify the surgical management of this challenging population, without putting the patients at risk of physeal closure. Rebound deformity, although uncommon and unpredictable, can safely be managed by repeated guided growth with the eight-Plate.
Acknowledgments
The authors thank Elena Carzana for her assistance with this project.
References
- 1.Stevens P. Guided growth: 1933 to the present. Strategies Trauma Limb Reconstr. 2006;1(1):29–35. doi: 10.1007/s11751-006-0003-3. [DOI] [Google Scholar]
- 2.Brooks WC, Gross RH. Genu varum in children: diagnosis and treatment. J Am Acad Orthop Surg. 1995;3(6):326–335. doi: 10.5435/00124635-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Fabry G, MacEwen GD, Shands AR., Jr Torsion of the femur. A follow-up study in normal and abnormal conditions. J Bone Joint Surg Am. 1973;55(8):1726–1738. [PubMed] [Google Scholar]
- 4.Stevens PM, Klatt JB. Guided growth for pathological physes: radiographic improvement during realignment. J Pediatr Orthop. 2008;28(6):632–639. doi: 10.1097/BPO.0b013e3181841fda. [DOI] [PubMed] [Google Scholar]
- 5.Mycoskie P. Complication of osteotomies about the knee in children. Orthopedics. 1981;4:1005–1015. doi: 10.3928/0147-7447-19810901-04. [DOI] [PubMed] [Google Scholar]
- 6.Steel HH, Sandrow RE, Sullivan PD. Complications of tibial osteotomy in children for genu varum or valgum. Evidence that neurological changes are due to ischemia. J Bone Joint Surg Am. 1971;53(8):1629–1635. [PubMed] [Google Scholar]
- 7.Blount WP, Clarke GR. Control of bone growth by epiphyseal stapling; a preliminary report. J Bone Joint Surg Am. 1949;31A(3):464–478. [PubMed] [Google Scholar]
- 8.Bylski-Austrow DI, Wall EJ, Rupert MP, Roy DR, Crawford AH. Growth plate forces in the adolescent human knee: a radiographic and mechanical study of epiphyseal staples. J Pediatr Orthop. 2001;21(6):817–823. [PubMed] [Google Scholar]
- 9.Frantz CH. Epiphyseal stapling: a comprehensive review. Clin Orthop Relat Res. 1971;77:149–157. [PubMed] [Google Scholar]
- 10.Zuege RC, Kempken TG, Blount WP. Epiphyseal stapling for angular deformity at the knee. J Bone Joint Surg Am. 1979;61(3):320–329. [PubMed] [Google Scholar]
- 11.Brockway A, Craig WA, Cockreli BR., Jr End-result study of sixty-two stapling operations. J Bone Joint Surg Am. 1954;36(5):1063–1070. [PubMed] [Google Scholar]
- 12.Mielke CH, Stevens PM. Hemiepiphyseal stapling for knee deformities in children younger than 10 years: a preliminary report. J Pediatr Orthop. 1996;16(4):423–429. doi: 10.1097/01241398-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Stevens PM, Maguire M, Dales MD, Robins AJ. Physeal stapling for idiopathic genu valgum. J Pediatr Orthop. 1999;19(5):645–649. [PubMed] [Google Scholar]
- 14.Métaizeau J-P, Wong-Chung J, Bertrand H, Pasquier P. Percutaneous epiphysiodesis using transphyseal screws (PETS) J Pediatr Orthop. 1998;18(3):363–369. [PubMed] [Google Scholar]
- 15.Burghardt R, Herzenberg J, Standard S, Paley D. Temporary hemiepiphyseal arrest using a screw and plate device to treat knee and ankle deformities in children: a preliminary report. J Child Orthop. 2008;2(3):187–197. doi: 10.1007/s11832-008-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens PM, Pease F. Hemiepiphysiodesis for posttraumatic tibial valgus. J Pediatr Orthop. 2006;26(3):385–392. doi: 10.1097/01.bpo.0000206515.84577.70. [DOI] [PubMed] [Google Scholar]
- 17.Nouth F, Kuo LA. Percutaneous epiphysiodesis using transphyseal screws (PETS): prospective case study and review. J Pediatr Orthop. 2004;24(6):721–725. doi: 10.1097/01241398-200411000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Novais E, Stevens PM. Hypophosphatemic rickets: the role of hemiepiphysiodesis. J Pediatr Orthop. 2006;26(2):238–244. doi: 10.1097/01.bpo.0000218531.66856.b7. [DOI] [PubMed] [Google Scholar]
- 19.Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg Br. 2010;92(2):273–276. doi: 10.1302/0301-620X.92B2.22937. [DOI] [PubMed] [Google Scholar]
- 20.Stevens PM. Guided growth for angular correction: a preliminary series using a tension band plate. J Pediatr Orthop. 2007;27(3):253–259. doi: 10.1097/BPO.0b013e31803433a1. [DOI] [PubMed] [Google Scholar]
- 21.Schroerlucke S, Bertrand S, Clapp J, Bundy J, Gregg FO. Failure of Orthofix eight-Plate for the treatment of Blount disease. J Pediatr Orthop. 2009;29(1):57–60. doi: 10.1097/BPO.0b013e3181919b54. [DOI] [PubMed] [Google Scholar]
- 22.Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259–261. [PubMed] [Google Scholar]
- 23.Heath CH, Staheli LT. Normal limits of knee angle in white children-genu varum and genu valgum. J Pediatr Orthop. 1993;13(2):259–262. [PubMed] [Google Scholar]
- 24.Kling TF, Jr, Hensinger RN. Angular and torsional deformities of the lower limbs in children. Clin Orthop Relat Res. 1983;176:136–147. [PubMed] [Google Scholar]
- 25.Levine AM, Drennan JC. Physiological bowing and tibia vara. The metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg Am. 1982;64(8):1158–1163. [PubMed] [Google Scholar]


