Abstract
Purpose
We aimed to investigate the effects on post-operative pain of local anaesthetic administration via a catheter placed into the operation site in patients who were undergoing upper and lower extremity paediatric orthopaedic surgery.
Methods
In this randomised, double-blind and placebo study, 40 ASA I–II patients aged between 1 and 12 years were randomly allocated into two groups: study group (Group S: 0.2 ml/kg, 0.5% bupivacaine, n = 20) and control group (Group C: 0.2 ml/kg, serum physiologic, n = 20). Before the fascia was closed by the surgical team, the solution previously prepared by the chief nurse was injected into the subfascial soft tissue with the syringe as the “injected dose” of serum physiologic or bupivacaine. After the closure, 0.2 ml/kg (1 mg/kg) bupivacaine or saline was instillated as the “first instillated dose” into the surgical area via the catheter. Pain scores were recorded at 0, 1, 2, 4, 8, 12, 24 and 48 h post-operatively. Patients were administered 0.75 mg/kg meperidine intramuscularly post-operatively to equalise the pain scores.
Results
No statistically significant difference was found between Group S and Group C in terms of demographic and other data and pain scores in the post-anaesthesia care unit, while a statistically significant decrease was found at 2, 4, 8, 12, 24 and 48 h in Group S and at 1, 2 and 4 h in Group C based on pain scores in the post-anaesthesia care unit (P < 0.05). A statistically significant decreasing pain score was found at 4, 8, 12, 24 and 48 h in Group S (P < 0.05).
Conclusion
The local anaesthetic administered via a catheter implanted in the surgical field may provide long-term and efficient post-operative analgesia.
Keywords: Post-operative pain, Paediatric, Local anaesthetic
Introduction
Post-operative pain is an inflammatory and acute pain which begins with surgical incision and ends when there is complete recovery of the tissue. Currently, in paediatric surgery, the management of post-operative pain is as important as the management of post-operative pain in adult patients. Therefore, recently, regional anaesthetic techniques have also been used in addition to general anaesthesia in paediatric patients [1, 2]. Thereby, regional anaesthesia techniques have provided effective analgesia and allowed early mobilisation in the post-operative period without causing respiratory depression [3]. However, for many years, there was little interest in regional anaesthesia in paediatric patients because of difficulties in cooperation, such as belonephobia (fear of needles), technical difficulties during practice and inexperienced anaesthesiologists. Recently, the combination of regional techniques with general anaesthesia, innovations in needles and cannulas, and the development of new local anaesthetics have increased the application of regional anaesthesia in paediatric patients [4, 5].
In initial pain management, a multi-modal analgesia protocol, which consists of regional anaesthesia combined with pre-operative administered analgesic drugs in order to control the pain arising from nociceptive and central stimuli, has been used. These methods minimise adverse effects, while the effect of each method provides maximum benefit.
However, more data is needed for this protocol in paediatric patients. This study aimed to investigate the efficiency of local anaesthetic injections in post-operative pain management when they are applied to the surgical wound directly or by a catheter in paediatric patients undergoing orthopaedic extremity surgery.
Methods
This double-blind, randomised controlled study was applied to ASA I–II status patients aged between 1 and 12 years undergoing elective lower or upper extremity surgery at the Orthopaedic and Traumatology Department of the Medical Faculty Hospital of Uludag University, Bursa, Turkey. Consent was obtained from the Local Ethics Committee and from the parents of the patients.
Patients whose parents did not give consent, those who had bleeding or coagulation abnormalities, mental retardation or cerebral palsy, neurological diseases involving pain sensitivity, patients in need of revision surgery or urgent surgery, and those of ASA status III and above were excluded from the study.
The study group comprised 20 patients, 11 male and 9 female with a mean age of 6.3 ± 3.8 years. The control group comprised 19 patients, 12 male and 7 female with a mean age of 7.16 ± 3.3 years. All of the patients had pre-operative fasting for 6 h and no pre-medications.
The solutions which would be administered to the surgical area were prepared by the clinic chief nurse: 0.2 ml/kg, saline (control group, n = 20) and 0.2 ml/kg 0.5% bupivacaine (study group, n = 20). The solutions were sent to the operation room with the patients. Only the clinic chief nurse knew the group states of the patients. The electrocardiogram, heart rate, peripheral oxygen saturation and blood pressure of the patients were monitored. Isolyte P or saline solution (0.9% NaCl) were administered intravenously as required via 20–22 G catheters and maintenance liquid requirement appropriate to their ages. Anaesthesia induction was provided with propofol 1 mg/kg, rocuronium 0.6 mg/kg and fentanyl 1 mcg/kg. Twelve patients in whom an intravenous catheter could not be placed had inhalation induction with sevoflurane, then the intravenous route was applied and the same procedure was administered. After 3 min of ventilation, patients were intubated with an appropriate tube. Anaesthesia was maintained with 2% sevoflurane.
Before the fascia was closed by the surgical team, the solution previously prepared by the chief nurse was injected into the subfascial soft tissue with the syringe as the “injected dose” of serum physiologic or bupivacaine. Then, an epidural catheter (Egemen® Epifix Standard 3 lateral holed, 20G epidural catheter, Turkey) was placed under the fascia by the surgical team (Fig. 1).
Fig. 1.

Schematic representation of the catheter placed subfascially
The catheter was stabilised on the skin using 2.0 silk suture material. After the closure, 0.2 ml/kg (1 mg/kg) bupivacaine or saline was instillated as the “first instillated dose” into the surgical area via the catheter. The same amount of bupivacaine or saline was administered via the wound catheter every 6 h after the “first instillated dose”. A drain was not inserted in the surgical area.
At the end of the operation, sevoflurane inhalation was terminated and patients were ventilated with 100% oxygen. For the antagonism of neuromuscular blockage, 0.05 mg/kg neostigmine and 0.02 mg/kg atropine sulphate were administered intravenously.
The patient was extubated when spontaneous respiration occurred and oxygen saturation was 97% or above and the respiratory function of the patient was adequate. Patients were transferred to the post-operative care unit accompanied by an anaesthesiologist. As a component of multi-modal analgesia and to equalise the pain scores of fully awakened and active patients, meperidine (0.75 mg/kg intramuscularly) was administered in the post-operative care unit.
On the clinical ward, solutions were delivered in 10 s by the wound catheter every 6 h with a sterile syringe after the “first instillated dose” according to the groups known by the chief nurse. Meperidine (0.75 mg/kg intramuscularly) was additionally administered to the patients whose pain scores were measured at 5 or above.
The catheter was withdrawn before the patient was discharged. If there was a cast application following surgery, the same post-operative anaesthesia procedure was carried out and the catheter was removed from the wound by opening a small window in the cast.
The FLACC (Face, Legs, Activity, Cry and Consolability) pain scale was used for the pain evaluation of patients aged 1–7 years (Table 1) and the Faces Pain Scale was used for evaluating 8–12-year-old patients (Fig. 2) [6, 7]. Taking arrival time in the post-operative care unit as zero, pain scores were evaluated at 0, 1, 2, 4, 8, 12, 24 and 48 h and recorded by an anaesthesiologist who did not know which drug had been used for which patient. It was also recorded whether any additional analgesic was used and the time at which it had been given. Furthermore, post-operative complications (e.g. nausea, vomiting, sedation, itching, respiratory depression) were recorded.
Table 1.
FLACC (Face, Legs, Activity, Cry and Consolability) pain scale [6] (used for 1–7-year-old children; if the FLACC point of pain ≥5, treatment is necessary)
| Category | Scoring | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Face | No particular expression or smile | Occasional grimace or frown, withdrawn, disinterested | Frequent to constantly quivering chin, clenched jaw |
| Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking or legs drawn up |
| Activity | Laying quietly, normal position, moves easily | Squirming, shifting back and forth, tense | Arched, rigid or jerking |
| Cry | No crying (awake or asleep) | Moans or whimpers, occasional complaint | Crying steadily, screams or sobs, frequent complaints |
| Consolability | Content, relaxed | Reassured by occasional touching, hugging or being talked to, distractible | Difficult to console or comfort |
Fig. 2.
Faces Pain Scale [7]
Statistical analysis was performed by using the SPSS for Windows 13.0 (Chicago, IL) software package. Continuous variable factors in the study were given as mean, standard deviation and max–min values. The Shapiro–Wilk test was used for the normality test of the continuous variable factors. When continuous variable factors had a normal distribution, inter-group comparisons were made by the independent samples t-test as one of the parametric tests. Wilcoxon’s test was used to compare groups of dependent variable factors. For inter-group comparison of these variable factors, firstly, the percentage changes of these factors were calculated according to the first value and then the two groups were compared with the Mann–Whitney U-test. Pearson’s Chi-square and Fisher’s Chi-square tests were used for inter-group comparison of the variable factors categorically. A P-value of <0.05 was accepted as being statistically significant.
Results
There were no statistically significant differences between the control group (Group C) and the study group (Group S) with respect to the demographic data, duration of surgery, perioperative opioid use and location of surgery (Tables 2 and 3).
Table 2.
Demographic data, duration of surgery, perioperative opioid used, location and diagnosis of surgical procedure
| Group C (n = 19) | Group S (n = 20) | P-value | |
|---|---|---|---|
| Sex (M/F) | 12/7 | 11/9 | 0.605 |
| Age (years) | 7.16 ± 3.30 | 6.30 ± 3.80 | 0.513 |
| Weight (kg) | 24.20 ± 13.60 | 21.40 ± 11.50 | 0.120 |
| Duration of surgery (min) | 190.00 ± 62.40 | 178.00 ± 57.00 | 0.070 |
| Perioperative opioid used (mcg) | 37.62 ± 11.63 | 30.75 ± 10.77 | 0.405 |
Mean ± standard deviation
Table 3.
Types of surgical interventions in the two study groups
| Group C (n = 19) | Group S (n = 20) | |
|---|---|---|
| Fractures (femur, humerus, forearm) | ||
| Intramedullary nailing | 4 | 3 |
| Open reduction and internal fixation | 2 | 2 |
| DDH, LCP | ||
| Anterior open reduction | – | 2 |
| Pelvic osteotomy | 1 | 6 |
| Femoral osteotomy | 2 | – |
| Foot deformity | ||
| Soft-tissue release, tendon transfers | 2 | 1 |
| Osteotomy | 1 | 1 |
| Tumour | ||
| Wide resection | 2 | 1 |
| Implant removal | ||
| Pelvis | 3 | 1 |
| Femur | 1 | |
| Humerus | 1 | 1 |
| Forearm | 1 | 1 |
DDH developmental dysplasia of the hip, LCP Legg–Calve–Perthes disease
One patient was excluded from the control group as the wound catheter spontaneously detached from the wound area.
Comparing Group C with Group S, there was no significant difference between pain scores in the post-operative care unit. All patients reached the ward in 1 h. Group S post-operative pain scores showed a significant reduction at 2, 4, 8, 12, 24 and 48 h (P < 0.05), while Group C post-operative pain scores showed a significant reduction at 1, 2 and 4 h. When the percentages of the post-operative pain scores were compared between the groups, a significant reduction was recorded in Group S compared to Group C at 4, 8, 12, 24 and 48 h (Fig. 3).
Fig. 3.
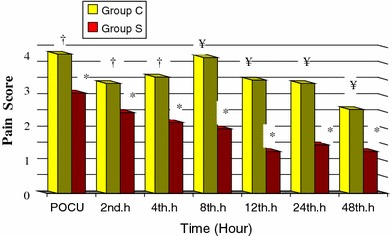
Distribution of the pain scores of the patients in the two groups (mean ± standard deviation). Pain score 0: post-operative care unit (POCU) pain score value. *P < 0.05 comparison of Group S post-operative care unit pain score values. †P < 0.05 comparison of Group C post-operative care unit pain score values. ¥P < 0.05 comparison of Group S and Group C
When comparing post-operative opioid requirements of Group S and Group C, five patients in Group S and 14 patients in Group C required additional analgesia. This difference was determined to be statistically significant (P < 0.05). The additional analgesic requirement was administered at a mean of 258.00 ± 165.70 min in Group C and at a mean of 508.93 ± 338.50 min in Group S. This result was found to be statistically significant (P = 0.013) (Table 4).
Table 4.
Additional post-operative analgesia requirements and time of use
| Group C (n = 19) |
Group S (n = 20) |
P-value | |
|---|---|---|---|
| Analgesic requirement (n)* | 14 (73.6%) | 5 (25.0%) | 0.020 |
| Time of additional analgesic administration (min)* | 258.00 ± 165.70 | 508.93 ± 338.50 | 0.013 |
*P < 0.05 Group S compared to Group C
With respect to post-operative complications, nausea and vomiting were observed in two patients in Group S and in four patients in Group C. No other complications were observed.
Discussion
The International Association for the Study of Pain (IASP) has defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage, related to an individual’s experience” [7].
Post-operative pain is the result of a harmful stimulus which can lead to organ dysfunction and, thus, causes severe neuroendocrine stress [7].
It is important to treat the pain effectively for the comfort of the patient in the post-operative period. Effective analgesia can relieve much of the discomfort.
Preventative pain treatment has been recommended, thus, reducing stress, morbidity, hospitalisation duration and financial cost [8].
Several factors play a part in inappropriate pain treatment during the post-operative period, such as inexperienced or ill-trained health providers, personal differences in regard to analgesic requirements, fears about addiction to opioids and insufficient follow-up of the pain. However, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) accepted pain as the fifth vital parameter and indicated that the pain has to be monitored and treated as a team [9]. This study aimed to provide post-operative pain control with a team concept comprised of a surgeon, an anaesthetist and a clinic nurse.
Non-steroidal anti-inflammatory drugs (NSAIDs) and paracetamol-like analgesics used in paediatric orthopaedic surgery cannot provide adequate and effective analgesia. These drugs can only reduce opioid requirements and pain scores when they are used together with opioids [10]. The post-operative use of opioids causes severe side effects.
As there are excessive side effects such as nausea, vomiting, urinary retention, itching, sedation and respiratory depression, opioid use is avoided for children and, consequently, adequate analgesia cannot be provided. Various post-operative regional anaesthesia techniques together with multi-modal analgesia techniques have been attempted in order to be able to provide adequate analgesia, whilst reducing unwanted side effects and opioid dosage [11].
Central and peripheral nerve blocks have been used to provide optimal pain control in the post-operative period [12–16]. Many analgesia techniques which are used for adults are also valid for children. To prevent complications, a standard dose range must be used and attention be paid to general safety rules. However, it has been established that 50% of the complications arise in respect to the techniques and equipment used [17].
Central nerve blocks such as spinal analgesia, epidural analgesia and caudal analgesia and peripheral nerve blocks such as brachial plexus block, femoral nerve block, sciatica nerve block, ilioinguinal/iliohypogastric nerve block and penile block are used in paediatric patients. These techniques can be applied using a single dose of anaesthetic or with catheter placement to provide continuous post-operative analgesia. All of these treatments must be administered by an experienced paediatric anaesthesiologist.
Llewellyn and Moriarty [18] found severe adverse effect incidence with respect to epidural catheterisation to be 0.01%. This incidence is low; however, more studies are needed in order to investigate wound catheterisations and routine use.
The post-operative efficacy of infiltrative treatment can show variations depending on several factors, such as the type of surgical procedure, the area of infiltration, the volume and concentration of local anaesthesia, adjuvant supplementation to the local anaesthesia and pain-measurement techniques [19, 20]. In a study of paediatric patients, local anaesthesia infiltration was administered to the peritonsillar area, but it remained limited. In another study of 6–18-year-old patients undergoing tonsillectomy, bupivacaine infiltration was reported to reduce post-operative pain by a significant amount [21, 22].
Local anaesthesia administered into the incision area reduces opioid requirement and can usually be administered as a single dose or as a continuous infusion via a wound catheter. Patel et al. [23] showed that effective post-operative analgesia was achieved by local anaesthesia using infiltration techniques. This practice has been used in minor and mid-size surgery, as it has been shown to be cost effective, simple and safe, and has reduced hospitalisation, additional complications and opioid requirements [24]. Post-operative analgesia can be provided by a single dose of local anaesthetic or via a wound catheter. Sufficient, prolonged analgesia can be provided via the wound catheter. The catheter used in this study provides more advantages, as it is cost-effective, can be applied easily without the need for great experience and has fewer complications.
In our study, compared to the control group, the study group, whose patients received multiple injections via a wound catheter, was seen to have significantly lower pain scores from the 4th hour to the 48th hour post-operatively. The absence of pain until the 4th hour may be explained by the opioid effects in the post-operative period and meperidine administered to equalise the pain scores in the post-operative care room.
To the best of our knowledge, there are no published studies on local anaesthesia administered via wound catheters in paediatric patients. In a study by Bültmann et al. [25], post-operative pain was sufficiently reduced by an infiltration of 0.2 ml/kg 0.5% bupivacaine infiltration. Machotta et al. [26] compared inguinal hernia patients who had received caudal block and those who had a single dose of wound infiltration. The application of 0.5% bupivacaine 0.2 ml/kg infiltration in the wound was reported to provide post-operative analgesia as effectively as a caudal block of 0.25% bupivacaine 1.0 ml/kg. In that study, patients were observed for 24 h and sufficient analgesia was provided with bupivacaine. In our study, the same amount of bupivacaine was applied by multiple injections into the wound throughout a 48-h period, providing adequate analgesia and reducing the need for opioids.
The immaturity of the nervous system results in fewer haemodynamic changes in children than adults from the use of regional anaesthesia. Local anaesthesia used in regional techniques in children has different pharmacokinetics because of the number and density of receptors in the anatomical and neuroaxial system. There are also particular factors with respect to the incomplete nervous system which play a part [27]. Myelinisation of the nerve structures is completed by 12 years of age. Therefore, local anaesthesia takes effect more rapidly in children and may be effective at low concentrations, but has a shorter duration.
Local anaesthesia blocks the transmission of impulses through the nerves and causes no damage to the nerves or neuron cells with which it is in contact.
Local anaesthesia to be used in children should be of low concentration, provide sufficient pre-operative and post-operative analgesia, have low toxicity, have no neurotoxicity, affect no motor functions and have a low financial cost [27, 28].
Local anaesthesia which will be applied via an infiltration anaesthetic should have longer term effects, a wide range toxicity limit and few side effects. Bupivacaine is a lipid-soluble high amide type local anaesthesia which has been used for this purpose for a long time.
Doses of different concentrations of bupivacaine infiltration have been tried in order to evaluate the efficacy of post-operative pain treatment. From doses between 0.125 and 0.75% having been studied, the maximum dose was reported to be 175 mg and successful analgesia was achieved in small-incision cases such as appendectomy [29]. Penile block using only bupivacaine has been reported to be more advantageous compared to penile block in combination with general anaesthesia [24].
In a study by Kastrissios et al. [30] of 12 patients following inguinal hernia surgery with a single dose of 0.5% bupivacaine administered in the incision area, the plasma bupivacaine concentration was found to be 0.07–1.4 mg/l (mean [standard deviation] 0.47 [0.33] mg/l). In that study, a 20-ml solution was used for each case and the plasma concentration was found to be below toxic levels. The dosage used in our study was much lower and there were no adverse effects related to the local anaesthesia.
The use of peripheral block together with local anaesthesia is not widely recommended because of opioid side effects such as nausea, vomiting, urine retention, respiratory depression and itching [31, 32]. In our study, opioids were not added to the local anaesthetic solution either.
One of the most frequent causes of post-operative mortality and morbidity is complications developing in the post-operative period. Reduced complications with lower costs increase patient satisfaction. In our study, the incidence of post-operative nausea and vomiting was found to be high in the control group. We think that this was due to the early need for additional analgesic requirement in this group and, thus, increased opioid consumption. Several studies have determined varying rates of nausea and vomiting after the administration of meperidine [33, 34].
In conclusion, the administration of local anaesthesia via a wound catheter demonstrated effective and prolonged post-operative analgesia with few complications, and no difficulties in using the technique, thus, not requiring much priori experience. For this technique to be accepted as an alternative in paediatric post-operative analgesia, further studies are necessary of comparisons with other regional anaesthesia techniques.
Contributor Information
T. Bulut, Email: stolgabulut@gmail.com
A. Yilmazlar, FAX: +90-224-4428958, Email: ayyil@uludag.edu.tr
B. Yavascaoglu, Email: belcan@uludag.edu.tr
B. Sarisozen, Email: bartu@uludag.edu.tr
References
- 1.Yaster M, Maxwell LG. Pediatric regional anesthesia. Anesthesiology. 1989;70:324–338. doi: 10.1097/00000542-198903000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Ross AK, Eck JB, Tobias JD. Pediatric regional anesthesia: beyond the caudal. Anesth Analg. 2000;91:16–26. doi: 10.1097/00000539-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Semsroth M, Gabriel A, Sauberer A, Wuppinger G. Regional anesthetic procedures in pediatric anesthesia. Anaesthesist. 1994;43:55–72. doi: 10.1007/s001010050033. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Tellería A, Cano Serrano ME, Martínez-Tellería MJ, Castejón Casado J. Analysis of regional anesthetic efficacy in pediatric postop pain. Cir Pediatr. 1997;10:18–20. [PubMed] [Google Scholar]
- 5.Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 6.Gregory GA. Paediatric anaesthesia. 3. New York: Churchill Livingstone; 1994. pp. 743–771. [Google Scholar]
- 7.Cousins M. Acute and postoperative pain. In: Wall PD, Melzack R, editors. Textbook of pain. 3. New York: Churchill Livingstone; 1994. pp. 357–385. [Google Scholar]
- 8.Rawal N, Axelsson K, Hylander J, Allvin R, Amilon A, Lidegran G, Hallén J. Postoperative patient-controlled local anesthetic administration at home. Anesth Analg. 1998;86:86–89. doi: 10.1097/00000539-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Sinatra RS, Torres J, Bustos AM. Pain management after major orthopaedic surgery: current strategies and new concepts. J Am Acad Orthop Surg. 2002;10:117–129. doi: 10.5435/00124635-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Carney DE, Nicolette LA, Ratner MH, Minerd A, Baesl TJ. Ketorolac reduces postoperative narcotic requirements. J Pediatr Surg. 2001;36:76–79. doi: 10.1053/jpsu.2001.20011. [DOI] [PubMed] [Google Scholar]
- 11.Lönnqvist PA, Morton NS. Postoperative analgesia in infants and children. Br J Anaesth. 2005;95:59–68. doi: 10.1093/bja/aei065. [DOI] [PubMed] [Google Scholar]
- 12.Kay B. Caudal block for post-operative pain relief in children. Anaesthesia. 1974;29:610–611. doi: 10.1111/j.1365-2044.1974.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 13.McGown RG. Caudal analgesia in children. Five hundred cases for procedures below the diaphragm. Anaesthesia. 1982;37:806–818. doi: 10.1111/j.1365-2044.1982.tb01812.x. [DOI] [PubMed] [Google Scholar]
- 14.Broadman LM, Hannallah RS, Norden JM, McGill WA. ‘Kiddie caudals’: experience with 1154 consecutive cases without complications. Anesth Analg. 1987;66:S18. doi: 10.1213/00000539-198702001-00018. [DOI] [Google Scholar]
- 15.Dalens B, Hasnaoui A. Caudal anesthesia in pediatric surgery: success rate and adverse effects in 750 consecutive patients. Anesth Analg. 1989;68:83–89. doi: 10.1213/00000539-198902000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Busoni P, Andreuccetti T. The spread of caudal analgesia in children: a mathematical model. Anaesth Intensive Care. 1986;14:140–144. doi: 10.1177/0310057X8601400207. [DOI] [PubMed] [Google Scholar]
- 17.Dalens B. Anesthésie locorégionale en pédiatrie. In: Samii K, editor. Anesthésie-réanimation chirurgicale. 2. Paris: Flammarion Médecines-Sciences; 1995. pp. 634–648. [Google Scholar]
- 18.Llewellyn N, Moriarty A. The national paediatric epidural audit. Paediatr Anaesth. 2007;17:520–533. doi: 10.1111/j.1460-9592.2007.02230.x. [DOI] [PubMed] [Google Scholar]
- 19.Ross AK. Pediatric regional anesthesia. In: Motoyama EK, Davis PJ, editors. Smith’s anesthesia for infants and children. 7. Philadelphia: Mosby; 2006. pp. 459–506. [Google Scholar]
- 20.Zohar E, Fredman B, Phillipov A, Jedeikin R, Shapiro A. The analgesic efficacy of patient-controlled bupivacaine wound instillation after total abdominal hysterectomy with bilateral salpingo-oophorectomy. Anesth Analg. 2001;93:482–487. doi: 10.1097/00000539-200108000-00048. [DOI] [PubMed] [Google Scholar]
- 21.Schoem SR, Watkins GL, Kuhn JJ, Thompson DH. Control of early postoperative pain with bupivacaine in paediatric tonsillectomy. Ear Nose Throat J. 1993;72:560–563. [PubMed] [Google Scholar]
- 22.Stuart JC, MacGregor FB, Cairns CS, Chandrachud HR. Peritonsillar infiltration with bupivacaine for paediatric tonsillectomy. Anaesth Intensive Care. 1994;22:679–682. doi: 10.1177/0310057X9402200606. [DOI] [PubMed] [Google Scholar]
- 23.Patel JM, Lanzafame RJ, Williams JS, Mullen BV, Hinshaw JR. The effect of incisional infiltration of bupivacaine hydrochloride upon pulmonary functions, atelectasis and narcotic need following elective cholecystectomy. Surg Gynecol Obstet. 1983;157:338–340. [PubMed] [Google Scholar]
- 24.Serour F, Cohen A, Mandelberg A, Mori J, Ezra S. Dorsal penile nerve block in children undergoing circumcision in a day-care surgery. Can J Anaesth. 1996;43:954–958. doi: 10.1007/BF03011810. [DOI] [PubMed] [Google Scholar]
- 25.Bültmann M, Streich R, Risse A, Falke KJ, Pappert D. Postoperative analgesia in children after hernioplasty. Wound infiltration with different concentrations of bupivacaine: a pilot study. Anaesthesist. 1999;48:439–443. doi: 10.1007/s001010050727. [DOI] [PubMed] [Google Scholar]
- 26.Machotta A, Risse A, Bercker S, Streich R, Pappert D. Comparison between instillation of bupivacaine versus caudal analgesia for postoperative analgesia following inguinal herniotomy in children. Paediatr Anaesth. 2003;13:397–402. doi: 10.1046/j.1460-9592.2003.01080.x. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita M, Miyasaka K. Regional anaesthesia and postoperative pain. Curr Opin Anaesthesiol. 1991;4:384–388. doi: 10.1097/00001503-199106000-00013. [DOI] [Google Scholar]
- 28.Patel JM, Lanzafame RJ, Williams JS, Mullen BV, Hinshaw JR. The effect of incisional infiltration of bupivacaine hydrochloride upon pulmonary functions, atelectasis and narcotic need following elective cholecystectomy. Surg Gynecol Obstet. 1983;157:338–340. [PubMed] [Google Scholar]
- 29.Møiniche S, Mikkelsen S, Wetterslev J, Dahl JB. A qualitative systematic review of incisional local anaesthesia for postoperative pain relief after abdominal operations. Br J Anaesth. 1998;81:377–883. doi: 10.1093/bja/81.3.377. [DOI] [PubMed] [Google Scholar]
- 30.Kastrissios H, Triggs EJ, Sinclair F, Moran P, Smithers M. Plasma concentrations of bupivacaine after wound infiltration of an 0.5% solution after inguinal herniorrhaphy: a preliminary study. Eur J Clin Pharmacol. 1993;44:555–557. doi: 10.1007/BF02440858. [DOI] [PubMed] [Google Scholar]
- 31.Yaster M, Hardart RA. Paediatric pain management. In: Raj PP, editor. Textbook of regional anesthesia. New York: Churchill Livingstone; 2002. pp. 1009–1032. [Google Scholar]
- 32.Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203:914–932. doi: 10.1016/j.jamcollsurg.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg AA, Montoya-Pelaez LF, Halliday EM, Hassan I, Baloch MS. Analgesia for adenotonsillectomy in children and young adults: a comparison of tramadol, pethidine and nalbuphine. Eur J Anaesthesiol. 1999;16:186–194. doi: 10.1046/j.1365-2346.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- 34.Elbourne D, Wiseman RA (2000) Types of intra-muscular opioids for maternal pain relief in labour. Cochrane Database Syst Rev 2:CD001237 [DOI] [PubMed]


