Abstract
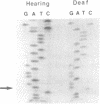
We have investigated the distribution of mitochondrial DNA polymorphisms in a rare maternally transmitted genetic trait that causes hypersensitivity to aminoglycoside antibiotics, in the hope that a characterization of its molecular basis might provide a molecular and cellular understanding of aminoglycoside-induced deafness (AGD). Here we report that the frequency of a particular mitochondrial DNA polymorphism, 1555G, is associated nonrandomly with aminoglycoside-induced deafness in two Japanese pedigrees, bringing the frequency of this polymorphism to 5 occurrences in 5 pedigrees of AGD, and in 4 of 78 sporadic cases in which deafness was thought to be the result of aminoglycoside exposure; both frequencies are significantly different from the occurrence of this mutation in the hearing population, which was 0 in 414 individuals surveyed. The 1555G polymorphism occurred in none of 34 aminoglycoside-resistant individuals. We propose a specific molecular mechanism for aminoglycoside hypersensitivity in individuals carrying the 1555G polymorphism, based on the three-dimensional structure of the ribosome, in which the 1555G polymorphism favors aminoglycoside binding sterically, by increasing access to the the ribosome cleft.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R. L., Greenamyre J. T. Alternative excitotoxic hypotheses. Neurology. 1992 Apr;42(4):733–738. doi: 10.1212/wnl.42.4.733. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Appel G. B., Neu H. C. The nephrotoxicity of antimicrobial agents (second of three parts). N Engl J Med. 1977 Mar 31;296(13):722–728. doi: 10.1056/NEJM197703312961305. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Hyman B. T., Koroshetz W. Do defects in mitochondrial energy metabolism underlie the pathology of neurodegenerative diseases? Trends Neurosci. 1993 Apr;16(4):125–131. doi: 10.1016/0166-2236(93)90117-5. [DOI] [PubMed] [Google Scholar]
- Blanc H., Adams C. W., Wallace D. C. Different nucleotide changes in the large rRNA gene of the mitochondrial DNA confer chloramphenicol resistance on two human cell lines. Nucleic Acids Res. 1981 Nov 11;9(21):5785–5795. doi: 10.1093/nar/9.21.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Cundliffe E. On the nature of antibiotic binding sites in ribosomes. Biochimie. 1987 Aug;69(8):863–869. doi: 10.1016/0300-9084(87)90213-6. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Bonilla E., Zeviani M., Nakagawa M., DeVivo D. C. Mitochondrial myopathies. Ann Neurol. 1985 Jun;17(6):521–538. doi: 10.1002/ana.410170602. [DOI] [PubMed] [Google Scholar]
- Gray M. W., Sankoff D., Cedergren R. J. On the evolutionary descent of organisms and organelles: a global phylogeny based on a highly conserved structural core in small subunit ribosomal RNA. Nucleic Acids Res. 1984 Jul 25;12(14):5837–5852. doi: 10.1093/nar/12.14.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell R. R., Weiser B., Woese C. R., Noller H. F. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1985;32:155–216. doi: 10.1016/s0079-6603(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Harpur E. S. The pharmacology of ototoxic drugs. Br J Audiol. 1982 May;16(2):81–93. doi: 10.3109/03005368209081452. [DOI] [PubMed] [Google Scholar]
- Higashi K. Unique inheritance of streptomycin-induced deafness. Clin Genet. 1989 Jun;35(6):433–436. [PubMed] [Google Scholar]
- Hornig H., Woolley P., Lührmann R. Decoding at the ribosomal A site: antibiotics, misreading and energy of aminoacyl-tRNA binding. Biochimie. 1987 Aug;69(8):803–813. doi: 10.1016/0300-9084(87)90207-0. [DOI] [PubMed] [Google Scholar]
- Hu D. N., Qui W. Q., Wu B. T., Fang L. Z., Zhou F., Gu Y. P., Zhang Q. H., Yan J. H., Ding Y. Q., Wong H. Genetic aspects of antibiotic induced deafness: mitochondrial inheritance. J Med Genet. 1991 Feb;28(2):79–83. doi: 10.1136/jmg.28.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui A. S., Eaton D. H., de Boer H. A. Mutagenesis at the mRNA decoding site in the 16S ribosomal RNA using the specialized ribosome system in Escherichia coli. EMBO J. 1988 Dec 20;7(13):4383–4388. doi: 10.1002/j.1460-2075.1988.tb03337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Transfer RNA shields specific nucleotides in 16S ribosomal RNA from attack by chemical probes. Cell. 1986 Dec 26;47(6):985–994. doi: 10.1016/0092-8674(86)90813-5. [DOI] [PubMed] [Google Scholar]
- Moazed D., Van Stolk B. J., Douthwaite S., Noller H. F. Interconversion of active and inactive 30 S ribosomal subunits is accompanied by a conformational change in the decoding region of 16 S rRNA. J Mol Biol. 1986 Oct 5;191(3):483–493. doi: 10.1016/0022-2836(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Munro E., Webb M., Kearsey S. E., Craig I. W. The isolation of an antimycin A-resistant human cell line. Exp Cell Res. 1983 Sep;147(2):329–339. doi: 10.1016/0014-4827(83)90215-x. [DOI] [PubMed] [Google Scholar]
- Oakes M. I., Kahan L., Lake J. A. DNA-hybridization electron microscopy tertiary structure of 16 S rRNA. J Mol Biol. 1990 Feb 20;211(4):907–918. doi: 10.1016/0022-2836(90)90083-X. [DOI] [PubMed] [Google Scholar]
- Olney J. W., Ho O. L., Rhee V. Cytotoxic effects of acidic and sulphur containing amino acids on the infant mouse central nervous system. Exp Brain Res. 1971;14(1):61–76. doi: 10.1007/BF00234911. [DOI] [PubMed] [Google Scholar]
- Petty R. K., Harding A. E., Morgan-Hughes J. A. The clinical features of mitochondrial myopathy. Brain. 1986 Oct;109(Pt 5):915–938. doi: 10.1093/brain/109.5.915. [DOI] [PubMed] [Google Scholar]
- Stern S., Weiser B., Noller H. F. Model for the three-dimensional folding of 16 S ribosomal RNA. J Mol Biol. 1988 Nov 20;204(2):447–481. doi: 10.1016/0022-2836(88)90588-8. [DOI] [PubMed] [Google Scholar]
- Weiss-Brummer B., Hüttenhofer A. The paromomycin resistance mutation (parr-454) in the 15 S rRNA gene of the yeast Saccharomyces cerevisiae is involved in ribosomal frameshifting. Mol Gen Genet. 1989 Jun;217(2-3):362–369. doi: 10.1007/BF02464905. [DOI] [PubMed] [Google Scholar]