Abstract
Purpose
To examine the functional outcomes of children with osteogenesis imperfecta (OI) following initial Fassier–Duval (FD) rodding to the femur at 1 year, and to determine which factors are associated with change in gross motor function, ambulation, and functional performance.
Methods
Approval from our Institutional Review Board was obtained. A retrospective chart review identified 60 children (28 males, 32 females) with OI who underwent initial FD femoral rodding (101 rods) and who were receiving bisphosphonates. The mean age of the children was 3 years, 11 months at the initial femoral FD rodding. Two had type I OI, 30 type III, 27 type IV, and one type VI. The maximum length of follow-up was 4 years. Telescoping FD rods were used for the femurs, with surgeries performed one leg at a time, with a 1-week interval. The active range of motion (AROM) of the hips and knees in flexion was measured 4–5 weeks post-initial rodding. Outcomes on the Gillette Functional Assessment Questionnaire (FAQ) Ambulation Scale, the Gross Motor Function Measure (GMFM), and the Pediatric Evaluation of Disability Inventory (PEDI) were compared pre-operatively and at 1 year post-surgery using t-tests and multivariate linear regression.
Results
Pre-operatively, the mean FAQ score was 2.0, and this increased to 5.8 at 1 year post-surgery. Statistically significant improvements (P ≤ 0.05) were found on the FAQ, crawling, standing, walking and running, and total domains of the GMFM, and PEDI mobility and self-care from baseline to 1 year. The results from the multivariate linear regression indicate that older age (P = 0.0045) and higher weight (P = 0.0164) are significantly associated with lower scores in the self-care domain of the PEDI, and that OI type III compared to type IV is significantly associated (P = 0.0457) with greater improvement on the crawling domain of the GMFM. Higher weight was also associated (P = 0.0289) with lower scores in the standing domain of the GMFM, as well as with the total GMFM score (P = 0.0398).
Conclusions
Our findings indicate that initial FD femoral rodding resulted in benefits in ambulation, gross motor function, self-care, and mobility for children with OI beyond physiological expectations due to developmental growth. FD rodding is a procedure which can improve the overall mobility in children with OI with significant femoral deformities.
Keywords: Fassier–Duval rodding, Lower extremities, Functional outcomes, Bisphosphonates, Osteogenesis imperfecta
Introduction
Osteogenesis imperfecta (OI) is a genetic disorder of connective tissue which, in 70% of individuals, is caused by mutations of one of two genes (COL1A1 and COL1A2) that encode for the type I collagen chains [1–4]. The major clinical features of OI are bone fragility, osteopenia, varying degrees of short stature, and progressive skeletal deformity. Other clinical features include joint hypermobility, hypotonia, and delayed or arrested developmental milestones in the more severely affected children. Its reported incidence ranges from approximately 1 in 10,000 [1] to 1 in 20,000 [2] births.
The most widely accepted classification, which was developed by Sillence et al. [3], is based on modes of inheritance and radiological and clinical findings, and includes OI types I, II, III, and IV. This classification has been expanded by Glorieux and Rauch [5–7] to include individuals who have a clinical diagnosis of OI but are negative for collagen type I mutations; hence, the addition of types V, VI, and VII [4].
The current medical treatment with bisphosphonates, in particular, pamidronate, has been shown to increase bone density, reduce the fracture rate and pain, as well as to improve function, including ambulation in children with OI [8]. While pamidronate increases the bone density, it does not have an effect on the femoral and tibial bowing present in the more severely involved children. Intramedullary rodding (IM) of these long bones is the current orthopedic approach. The goal of orthopedic surgery is twofold: correct long bone deformity and reduce the incidence of fractures [9].
Correction of deformity combined with bone stabilization has been used for many years, notably since Sofield and Millar [10] reported their technique of bone fragmentation and subsequent fixation with solid small-diameter rods in 1959. Others, such as Williams [11] and Middleton [12], have also described the correction of deformity for children with OI. However, the problem of bone overgrowing the rods with those methods remained, and some rods became completely incorporated in the diaphysis, which could affect future rod retrieval.
Bailey–Dubow rods, introduced in 1963, can elongate telescopically during bone growth, thus, reducing the need for replacement. Marafioti and Westin [13] reviewed 153 rod-fixations in 20 patients using both the Sofield–Millar procedure of multiple osteotomies, realignment and intramedullary rodding, as well as the Bailey–Dubow elongating type of intramedullary rodding. The non-elongating rods had a 3.5 times greater rate of re-operation than the elongating-type rods, and the time period during which the non-elongating rods remained in place was statistically significantly shorter than the elongating-type rods. Modifications to the original technique were made to decrease the risk for complications [14–17]. Despite being the standard for several decades, a high incidence of mechanical complications (migration, disconnection of T-parts) remain with the Bailey–Dubow telescopic rod [18].
A new concept was developed by Fassier and Duval [19] to protect joints, reduce rod migration, offer less invasive surgery, and to reduce the mechanical complications. The advantages of such a method are fewer surgical scars, reduced blood loss, decreased time of operation, less pain, better post-operative mobility, and small scars [20]. The Fassier–Duval (FD) rod also has the advantage of eliminating the need for knee arthrotomy in femoral surgery as well as ankle arthrotomy for tibial surgery, as rodding is done through the greater trochanter, as in adult fractures or through the pre-spinal area of the proximal tibia. A major advantage of this technique is that multiple bones may be treated during the same surgical procedures and this reduces the rehabilitation time required [21]. This procedure does involve meticulous technique and experience to ensure proper correction of all the deformity and for appropriate placement of the rods, and involves a steep learning curve.
Possible complications of all rods include joint intrusion, inability to telescope, migration, and epiphysiodesis (cessation of growth). A 35% complication rate with the FD rod was reported and this compares favorably with a 55% complication rate for the Bailey–Dubow rod [22]. Furthermore, the rate of re-operation has been shown to be lower with this surgical technique, and was found to be 14.3% for the femur in a multicenter study on 112 children with OI [22] and 18.0% for the tibia in 2010 [23]. These findings reflect our experience, which underscores a well-defined medical protocol at our institution. Another communication [24] reported a higher complication rate with FD rods, but the protocol used (dosage of bisphosphonates) varied, which may explain these reported differences. Post-rodding fractures occur through the osteotomy site 20–25% of the time.
Despite having straighter and stronger bones following rodding and bisphosphonate treatment, children with OI must have adequate muscle strength in order to ensure good functional outcome [25]. FD rodding was also reported to significantly improve both the global quality of life and walking in nine patients (14 rods), and presented a safe and useful implant for the treatment of lower limb deformities in a recent study on patients with OI [26].
Although most studies report the efficacy and safety of different types of rodding methods for children with OI, there is conflicting opinion as to whether this surgical approach improves motor development or follows the natural course [27–29], and little is known on the functional outcomes of these patients following such procedures.
The objectives of this retrospective observational study were to examine the functional outcomes of children with OI following initial FD rodding to the femora at 1 year, and to determine which factors are associated with change in gross motor function, ambulation, and functional performance in these children.
Methods
Approval from the Director of Professional Services at the Shriners Hospital was obtained to conduct the chart review, in accordance with the guidelines provided from our Institutional Review Board. A retrospective chart review was conducted on all patients with OI who had an initial femoral intramedullary rodding using the FD rod in 2000–2007 by the same surgeon (FF).
Subjects
The study population consisted of patients with a diagnosis of OI followed at the Shriners Hospital for Children Canada (SHC) in Montreal. This hospital is an elective pediatric orthopedic facility with a catchment area that covers all Canadian provinces, the New England states, and selected cases from abroad. Children from other areas of the USA, South America, Europe, and the Middle East also formed part of the patient population. Between January 2000 and May 2006, 60 children with OI underwent initial FD femoral roddings, including 101 rods (19 unilateral and 41 bilateral femoral FD rods) consisting of the study sample. The children ranged in age between 1 year and 2 months and 11 years and 8 months (mean: 3 years and 11 months, standard deviation [SD]: 2 years and 3 months) at the time of the initial femoral FD rodding. The older children were from South America and the Middle East, where early surgical management was unavailable. There were 28 males and 32 females. Two had type I OI, 30 type III, 27 type IV, and one type VI. There were no children with types V or VII. The maximum length of follow-up extended to 4 years post-initial FD rodding. All patients were receiving bisphosphonate treatment according to our protocol [4]. Fifty-nine children were receiving pamidronate treatment, a form of bisphosphonates, and started this treatment at a mean age of 1 year and 9 months, SD: 2 years and 1 month, with the youngest child starting pamidronate treatment at 8 days of age. The other child was using another type of bisphosphonate treatment, zoledronate, and started this treatment at 3 years and 7 months. See Table 1.
Table 1.
Demographic and clinical data
| Variable | N = 60 |
|---|---|
| Age at surgery, mean (SD), years | 4.0 (2.3) |
| Weight, mean (SD), kg | 11.2 (4.6) |
| Gender, n (%) | |
| Male | 28 (46.7) |
| Female | 32 (53.3) |
| OI type, n (%) | |
| Type I | 2 (3.3) |
| Type III | 30 (50.0) |
| Type IV | 27 (45.0) |
| Type VI | 1 (1.7) |
| Bisphosphonate treatment | |
| Pamidronate, n (%) | 59 (98.3) |
| Zoledronate, n (%) | 1 (1.7) |
Procedures
Rodding was performed on children with the following criteria: had experienced multiple lower extremity fractures, had femoral bowing greater than 20°, demonstrated the potential for ambulation appropriate for chronological age, and had never been rodded in the past. Telescoping FD rods were used for the femurs, while in most cases, regular non-telescoping rods were used for the tibiae due to the small size of these bones. The surgeries were performed on one leg at a time, with a 1-week interval between the sides. At the end of the second surgery, while still sedated, the patients were molded for knee–ankle–foot orthoses (KAFOs). The children were then immobilized with posterior slabs and rotational control was maintained by taping the legs together. Three weeks following the second surgery, the back slabs were removed, braces fitted, and antero-posterior femoral and tibial radiographs were taken to verify bony healing. The children were then referred for 1 week of intensive rehabilitation as outpatients. The KAFOs were locked in extension using solid ankle–foot orthoses (AFOs) and anterior shin panels.
Rehabilitation consisted of active range of motion (AROM), strengthening exercises, and hydrotherapy in chest-high warm water. Weight-bearing with the locked KAFOs was graduated on a tilt table and, in most cases, progressed to stepping with a rollator walker during that week. Over the subsequent months, the KAFOs were replaced by AFOs when the quadriceps were at least a 4 in muscle grade (0–5). When the AFOs needed to be replaced due to growth, articulating AFOs with anterior panels were then prescribed to limit possible bowing due to growth beyond the non-elongating tibial rods which were used at the time of surgery. Children were re-evaluated over a 3-year period following rodding with progression of the home program and orthotic requirements as deemed necessary.
Outcome measures
AROM of the hips and knees in flexion was measured in this study with a goniometer 4–5 weeks post-initial rodding. It is the range of movement through which a patient can actively (without assistance) move a joint using the adjacent muscles.
The Gillette Functional Assessment Questionnaire (FAQ) Ambulation Scale [30] was used to measure ambulation. It is a 10-level parent report scale which describes a range of walking abilities from non-ambulatory to ambulatory in various community settings and terrains with or without assistive devices. It has been shown to be a reliable and valid scale for walking and can detect functional change in children with chronic neuromuscular conditions [30].
The Gross Motor Function Measure (GMFM) [31] consists of 88 items which have been grouped into five dimensions: lying and rolling; sitting; crawling and kneeling; standing; walking, running, and jumping. It represents abilities obtained by typically developing children by 5 years of age. Each item is scored on a four-point ordinal scale. It has been shown to have good reliability and validity in children with cerebral palsy [32]. The GMFM-88 showed good interrater reliability in children with OI [33] and has been used in children with OI in Italy [34].
The Pediatric Evaluation of Disability Inventory (PEDI) [35] was designed to serve as a descriptive measure of the child’s functional performance in self-care, mobility, and social function. The PEDI self-care and mobility domains are routinely collected as a measure of activity functioning and can be used to identify client-centered goals with the families of children with OI. This tool has been previously used in children with OI [36]. The scaled scores were used for the self-care and mobility domains in the present study. Scoring was done with the use of mobility aids to reflect the child’s true performance within the environment.
Data analysis
Analyses were performed using the SPSS 14.0 statistical software. Descriptive statistics and score distributions were examined. Differences over time (pre-operative to 1 year) were evaluated using t-tests. Because many children were not always present at the different follow-up time points, especially those from other countries, only those with data at both the pre-operative and 12-month time points for each outcome measure were analyzed. Indeed, the length of visits of patients from abroad was short and included consultations with many specialties (including dentistry and orthotics), often precluding the administration of all of the outcome measures. Many of the children from South America and the Middle East had never been seen at our institution prior to surgery. When they arrived, they often had long bone fractures which prevented administration of the observational outcome measures (GMFM, FAQ, and ROM). The mean change for each of the outcome measures (Table 2) is presented for those children who had data at both the pre-operative and 12-month time points.
Table 2.
Comparisons between pre-operative and 12-month outcomes
| Outcome measure: mean (SD) [n] | Pre-operative | 12 month | Mean change between pre-operative and 12 months |
|---|---|---|---|
| FAQ | 2.0 (2.0) [45] | 5.8 (2.4) [39] | 3.6 (2.3) [29]* |
| GMFM C | 39.9 (37.8) [42] | 74.9 (28.7) [39] | 30.2 (31.2) [29]* |
| GMFM D | 16.7 (27.5) [42] | 54.6 (31.6) [39] | 30.4 (28.2) [29]* |
| GMFM E | 9.4 (19.3) [42] | 36.1 (29.4) [39] | 19.4 (18.7) [29]* |
| GMFM total | 43.4 (23.9) [43] | 69.3 (20.6) [39] | 21.7 (17.1) [30]* |
| PEDI mobility | 45.4 (13.1) [50] | 59.1 (14.0) [45] | 12.5 (8.3) [39]* |
| PEDI self-care | 57.3 (17.7) [49] | 66.3 (14.2) [45] | 8.9 (7.1) [39]* |
* Statistically significant at P ≤ 0.05
( ): standard deviation
[ ]: sample size
Multivariate linear regression analyses were conducted for each of the FAQ, GMFM, and PEDI change scores in order to determine which variables were predictive of changes from pre-operative to 1 year. Data from children who were assessed at both the pre-operative and 12-month time points for each of the nine outcome scores (GMFM A, B, C, D, E, total, FAQ, PEDI self-care, and PEDI mobility scores) were included in the regression analyses. The forward stepwise regression procedure used variable entry/removal at α = 0.15. The final regression equation incorporated all predictors that attained a P-value <0.10. The adjusted R2 indicated how much variation was explained by regression. Predictors tested included: age at initial FD rodding, weight at initial FD rodding, gender, and OI types (III and IV). Only patients with data at both time points were included, and those with OI types I (n = 2) and VI (n = 1) were excluded from the linear regression modeling due to the small sample size.
Results
The mean AROM post-initial FD femoral rodding were 97.9° and 102.6° for left and right hip flexion, respectively, and 100.4° and 106.1° for left and right knee flexion, respectively. At this time point, the proportion of children with active flexion equal to or over 90° was 32/34 for the right hip, 32/38 for the left hip, 32/36 for the right knee, and 28/40 for the left knee.
Pre-operatively, the mean FAQ was 2.0, demonstrating that, at best, the children could take some steps with the assistance of another person. At 1 year post-operatively, the mean was 5.8, indicating that the children, on average, were starting to walk short distances outside the home.
When looking at the changes in the GMFM crawling (C), standing (D), and walking and running (E) domains from baseline to 1 year, statistically significant improvements are observed. For the total GMFM scores, there was a 21.7% improvement at 1 year.
The PEDI mean-scaled scores improved in the mobility domain from 45.4 at baseline to 59.1 at 1 year, and from 57.3 at baseline to 66.3 at 1 year for the self-care domain.
Changes from baseline to 1 year post-operatively on the FAQ, GMFM C, D, E, and total domains, and PEDI mobility and self-care domains were all statistically significant (Table 2). We did not analyze the data at the 2-, 3-, and 4-year intervals due to the small sample size. Figures 1, 2, and 3 demonstrate that the gains made in ambulation, gross motor function, mobility, and self-care at 1 year are at least maintained at 4 years post-operatively.
Fig. 1.
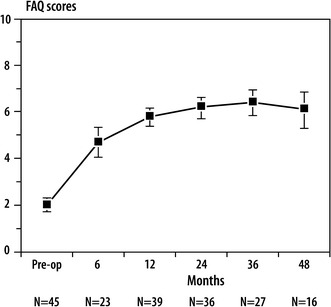
Scores on the Functional Assessment Questionnaire (FAQ) Ambulation Scale over time
Fig. 2.
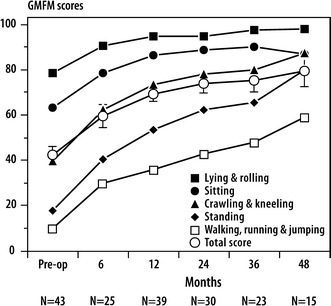
Scores on the Gross Motor Function Measure (GMFM) over time
Fig. 3.
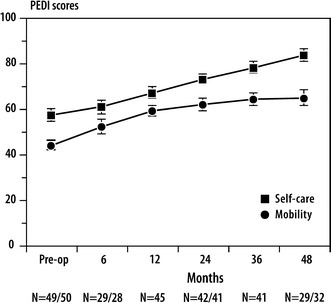
Scores on the Pediatric Evaluation of Disability Inventory (PEDI) over time
Results from the multivariate linear regression to identify which factors are associated with change from baseline to 1 year in children with OI types III and IV indicate that older age (P = 0.0045) and higher weight (P = 0.0164) are significantly associated with lower scores in the self-care domain of the PEDI. For the crawling (C) domain of the GMFM, OI type III compared to type IV is significantly associated (P = 0.0457) with greater improvement. Higher weight was also associated (P = 0.0289) with lower scores in the standing (D) domain of the GMFM, as well as with the total GMFM score (P = 0.0398) (Table 3).
Table 3.
Factors significantly associated with change from pre-operative to 12 months
| Outcome measure | Beta estimate | P-value |
|---|---|---|
| PEDI self-care (n = 38) | ||
| Age | −1.34 | 0.0045 |
| Weight | −0.53 | 0.0164 |
| GMFM C (n = 27) | ||
| OI type III | 26.59 | 0.0457 |
| OI type IV | ||
| GMFM D (n = 27) | ||
| Weight | −3.22 | 0.0289 |
| GMFM Total (n = 27) | ||
| Weight | −1.57 | 0.0398 |
This analysis was conducted with the exclusion of children with OI types I and VI (n = 3)
The following factors were included in the multivariate linear regression: age, gender, weight, OI types III and IV
Discussion
Previous reports indicated that FD rodding of the femur in children with OI involves a lower complication rate as compared to the Bailey–Dubow rod [25]. FD rodding was also reported to significantly improve both the global quality of life and walking in nine patients (14 rods), and presented a safe and useful implant for the treatment of lower limb deformities in patients with OI [26].
The objectives of the current study were to examine the functional outcomes of children with OI at 1 year following initial FD femoral rodding to the femora, and to determine which factors are predictive of change in gross motor function, ambulation, and functional performance. The functional outcomes at 1 year following initial FD femoral rodding in children with OI have not yet been reported in the literature.
The results indicated that most children obtained at least 90° AROM in the flexion of both hips and knees at 5 weeks post-initial FD rodding of the femora. Twelve out of 40 had less than 90° of left knee flexion; seven out of 40 had a minimum of 80°. Ninety degrees of knee flexion is needed to climb and descend stairs alternately. Our findings indicate that, following removal of the back slabs and 5 days of rehabilitation, a large majority of the children regained AROM ≥90° of the knees and hips. Our experience suggests that the absence of a knee arthrotomy in the FD rodding system favors this high degree of AROM 5 weeks after surgery.
Ambulation status pre-operatively was not functional, as a score of 2 on the FAQ represents “some stepping on his/her own with the help of another person. Does not take full weight on feet; does not walk on a routine basis [30].” The biggest transition in terms of ambulation is at 1 year, when the mean FAQ is close to 6, which is the first of five community ambulation levels (6–10) of the walking scale. At this stage, the child is able to walk more than 15–20 feet outside the home, but usually uses a wheelchair or stroller for community distances or in congested areas.
As for gross motor function, total and individual domain scores increased from pre-operatively to 1 year. The greatest gains were observed in the crawling (C) and standing (D) domains of the GMFM, where we would anticipate changes from baseline to 1 year. The goal of the FD femoral rodding is to facilitate standing and ambulation with a lower risk of fracture. It would be expected that the greatest improvement would be in the standing domain, especially for the children with type III OI, as these children do not always achieve independent ambulation. The gains in the walking, running, and jumping (E) domain demonstrate the child’s ability to ambulate independently. The mean total GMFM score at 1 year is 69.3, with an increase of 21.7% from baseline. An improvement of 7% over 1 year has been determined to be a clinically significant change [37]. All items on the GMFM could usually be accomplished by a 5-year-old with normal motor abilities [37].
The gains in the mobility scores of the PEDI may reflect the impact that the FD rodding had on the correction of the lower limb deformity in our sample of children with OI, thus, resulting in better weight-bearing and ambulation and, consequently, in better performance in daily mobility activities, such as transferring to a car or taking a bath and climbing partial or full flights of stairs. Gains were also observed in the self-care domain; however, these gains may be attributable to developmental achievements, as well as increased skill performance following FD femoral rodding.
It has been our experience that children with OI who have been rodded in the lower extremities participate in more activities than prior to the surgery. As a result, they increase their risk for more falls and possible fractures. It is reassuring to observe that the gains made at 1 year are maintained up until 4 years. The younger the child, the higher the incidence of re-rodding due to growth. Even in the older children who were not able to walk prior to surgery, their level of ambulation, gross motor skills, and independence in self-care improved. In 11 children aged 4.6–11.7 years at the time of initial FD rodding, significant improvement in the FAQ ambulation score were noted at 1 year and ranged from 2 to 9 points. It is our conclusion that these gains were primarily due to the surgical intervention, as children aged 4.5 and upwards are already full community ambulators.
We looked at the factors which were associated with change at 1 year. Our findings indicate that increasing age and weight is associated with lower scores in the self-care areas. Our clinical experience has demonstrated that children with physical limitations and increased weight are at higher risk for lower performance in daily life activities. Children with increased weight also had lower scores in the GMFM standing (D) domain and total scores. We conclude that children with OI should be rodded as soon as they meet the criteria established. In our study, the children from South America and the Middle East would have benefited from earlier rodding. The children with type III OI have the greatest impairments. At the time of surgery, their scores in the crawling (C) domain were lower.
Our sample included children from local communities (younger children), as well as children from abroad (older children), with younger children probably having better prospects for developmental outcome. This may have attenuated the improvements for all patients at 12 months. The sample size of this study precluded analyses at the 2-, 3-, and 4-year time points, as many children came from abroad and returned on a needs basis. This study did not include a control group as withholding surgery for appropriate candidates would be deemed unethical. The outcome measures used in this study did not demonstrate a ceiling effect, especially in children with more severe OI types. Multisite studies with a longitudinal follow-up would strengthen our findings. The findings of this study are generalizable to children with OI who have undergone bisphosphonate treatment prior to intramedullary femoral rodding using the FD rod.
Conclusions
This study represents an examination of the functional outcomes 1 year post-initial Fassier–Duval (FD) rodding to the femora in children with osteogenesis imperfecta (OI) receiving bisphosphonates. The current study revealed that initial FD femoral rodding resulted in benefits in ambulation, gross motor function, self-care, and mobility for children with OI beyond physiological expectations due to developmental growth. The gains obtained persist for up to 4 years. This study provides useful information for clinicians on the post-operative course for children with OI. It is our opinion that FD rodding is a procedure which can improve the overall mobility in children with OI with significant femoral deformities.
Acknowledgments
The authors wish to thank Xun Zhang for statistical support, Guylaine Bédart for graphic design, Stephanie Gould, Corrine Mercier, Rita Yap, Rochelle Rein, Chantal Damas, and Gloria Thevasagayam for their contribution to this project, and Andréa Poupart, Carla Briffault, and Nancy Cyr for their assistance with the patient database. Special thanks go to the entire medical, nursing, and rehabilitation team at the Shriners Hospital for Children for their devoted care of children with osteogenesis imperfecta.
References
- 1.Glorieux FH. Osteogenesis imperfecta. Best Pract Res Clin Rheumatol. 2008;22:85–100. doi: 10.1016/j.berh.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Michell C, Patel V, Amirfeyz R, et al. Osteogenesis imperfecta. Curr Orthop. 2007;21:236–241. doi: 10.1016/j.cuor.2007.04.003. [DOI] [Google Scholar]
- 3.Sillence D, Senn A, Danks D. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–1385. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 5.Glorieux FH, Rauch F, Plotkin H, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res. 2000;15:1650–1658. doi: 10.1359/jbmr.2000.15.9.1650. [DOI] [PubMed] [Google Scholar]
- 6.Glorieux FH, Ward LM, Rauch F, et al. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res. 2002;17:30–38. doi: 10.1359/jbmr.2002.17.1.30. [DOI] [PubMed] [Google Scholar]
- 7.Labuda M, Morissette J, Ward LM, et al. Osteogenesis imperfecta type VII maps to the short arm of chromosome 3. Bone. 2002;31:19–25. doi: 10.1016/S8756-3282(02)00808-6. [DOI] [PubMed] [Google Scholar]
- 8.Rauch F, Travers R, Glorieux FH. Pamidronate in children with osteogenesis imperfecta: histomorphometric effects of long-term therapy. J Clin Endocrinol Metab. 2006;91:511–516. doi: 10.1210/jc.2005-2036. [DOI] [PubMed] [Google Scholar]
- 9.Enright W, Noonan K. Bone plating in patients with type III osteogenesis imperfecta: results and complications. Iowa Orthop J. 2006;26:37–40. [PMC free article] [PubMed] [Google Scholar]
- 10.Sofield H, Millar E. Fragmentation, realignment, and intramedullary rod fixation of deformities of the long bones in children: a ten-year appraisal. J Bone Joint Surg Am. 1959;41:1371–1391. [Google Scholar]
- 11.Williams P. Fragmentation and rodding in osteogenesis imperfecta. J Bone Joint Surg Br. 1965;47:23–31. [PubMed] [Google Scholar]
- 12.Middleton RW. Closed intramedullary rodding for osteogenesis imperfecta. J Bone Joint Surg Br. 1984;66:652–655. doi: 10.1302/0301-620X.66B5.6501356. [DOI] [PubMed] [Google Scholar]
- 13.Marafioti RL, Westin GW. Elongating intramedullary rods in the treatment of osteogenesis imperfecta. J Bone Joint Surg Am. 1977;59:467–472. [PubMed] [Google Scholar]
- 14.Harrison W, Rankin K. Osteogenesis imperfecta in Zimbabwe: a comparison between treatment with intramedullary rods of fixed-length and self-expanding rods. J R Coll Surg Edinb. 1998;43:328–332. [PubMed] [Google Scholar]
- 15.Bilsel N, Beyzadeoglu T, Kafadar A. Application of Bailey–Dubow rods in the treatment of Osteogenesis Imperfecta. Eur J Orthop Surg Traumatol. 2000;10:183–187. doi: 10.1007/BF01682313. [DOI] [Google Scholar]
- 16.Finidori G (1988) Osteogénèse imparfaite, indications thérapeutiques chez l’enfant (in French). In: Conférences d’enseignement 1988. Cahiers d’enseignement de la SOFCOT. Expansion Scientifique Française, Paris, pp 327–345
- 17.Wilkinson J, Scott B, Clarke A, et al. Surgical stabilisation of the lower limb in osteogenesis imperfecta using the Sheffield Telescopic Intramedullary Rod System. J Bone Joint Surg Br. 1998;80:999–1004. doi: 10.1302/0301-620X.80B6.8667. [DOI] [PubMed] [Google Scholar]
- 18.Cho T-J, Choi IH, Chung CY, et al. Interlocking telescopic rod for patients with osteogenesis imperfecta. J Bone Joint Surg Am. 2007;89:1028–1035. doi: 10.2106/JBJS.F.00814. [DOI] [PubMed] [Google Scholar]
- 19.Fassier F, Duval P (2001) New concept for telescoping rodding in osteogenesis imperfecta: preliminary results. In: Proceedings of the Annual Meeting of the Pediatric Orthopaedic Society of North America (POSNA), Cancun, Mexico, May 2001, p 101
- 20.Zeitlin L, Fassier F, Glorieux FH. Modern approach to children with osteogenesis imperfecta. J Pediatr Orthop B. 2003;12:77–87. doi: 10.1097/01.bpb.0000049567.52224.fa. [DOI] [PubMed] [Google Scholar]
- 21.Esposito P, Plotkin H. Surgical treatment of osteogenesis imperfecta: current concepts. Curr Opin Pediatr. 2008;20:52–57. doi: 10.1097/MOP.0b013e3282f35f03. [DOI] [PubMed] [Google Scholar]
- 22.Fassier F, Esposito P, Sponseller P et al (2006) Multicenter radiological assessment of the Fassier–Duval femoral rodding. In: Proceedings of the Annual Meeting of the Pediatric Orthopaedic Society of North America (POSNA), San Diego, California, May 2006
- 23.Halloran J, Fassier F, Alam N (2010) Radiological assessment of Fassier–Duval tibial rodding in patients with Osteogenesis Imperfecta. In: Proceedings of the 29th Annual Meeting of the European Paediatric Orthopaedic Society (EPOS), Zagreb, Croatia, April 2010
- 24.Larson T, Brighton B, Esposito P et al (2010) High reoperation rate and failed expansion in lower extremity expandable rods in osteogenesis imperfecta. In: Proceedings of the Annual Meeting of the Pediatric Orthopaedic Society of North America (POSNA), Waikoloa, Hawaii, May 2010
- 25.Osteogenesis Imperfecta Foundation (2003) New research strategies in osteogenesis imperfecta. Osteogenesis Imperfecta Foundation, Gaithersburg, MD. Available online at: http://www.oif.org/site/PageServer?pagename=04ResearchStrategies
- 26.Garcia-German D, García JP, Sánchez AB, et al. Intramedullar telescopic nailing with Fassier–Duval rod in Osteogenesis Imperfecta. J Bone Joint Surg Br. 2009;91:58. doi: 10.2106/JBJS.I.00391. [DOI] [PubMed] [Google Scholar]
- 27.Daly K, Wisbeach A, Jr Sanpera I, et al. The prognosis for walking in osteogenesis imperfecta. J Bone Joint Surg Br. 1996;78:477–480. [PubMed] [Google Scholar]
- 28.Engelbert RH, Uiterwaal CS, Gulmans VA, et al. Osteogenesis imperfecta: profiles of motor development as assessed by a postal questionnaire. Eur J Pediatr. 2000;159:615–620. doi: 10.1007/s004310000505. [DOI] [PubMed] [Google Scholar]
- 29.Sułko J, Radło W. Operative management of long-bone of the upper limb in children with osteogenesis imperfecta. Chir Narzadow Ruchu Ortop Pol. 2005;70:195–199. [PubMed] [Google Scholar]
- 30.Novacheck TF, Stout JL, Tervo R. Reliability and validity of the Gillette Functional Assessment Questionnaire as an outcome measure in children with walking disabilities. J Pediatr Orthop. 2000;20:75–81. [PubMed] [Google Scholar]
- 31.Russell DJ, Rosenbaum PL, Avery LM, et al. The Gross Motor Function Measure (GMFM-66 and GMFM-88) user’s manual. London: MacKeith Press; 2002. [Google Scholar]
- 32.Wei S, Wang S-J, Liao Y-G, et al. Reliability and validity of the GMFM-66 in 0- to 3-year-old children with cerebral palsy. Am J Phys Med Rehabil. 2006;85:141–147. doi: 10.1097/01.phm.0000197585.68302.25. [DOI] [PubMed] [Google Scholar]
- 33.Ruck-Gibis J, Plotkin H, Hanley J, et al. Reliability of the Gross Motor Function Measure for children with osteogenesis imperfecta. Pediatr Phys Ther. 2001;13:10–17. doi: 10.1097/00001577-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Fraschini P (2009) Experience with OI at “La Nostra Famiglia” in Bosisio Parini and “Centro Vojta” in Rome. In: Proceedings of the OI in Motion Conference: On Rehabilitation and Physiotherapy in Osteogenesis Imperfecta, Rheinsberg, Germany, November 2009
- 35.Haley SM. The pediatric evaluation of disability inventory (PEDI) J Rehabil Outcomes Meas. 1997;1:61–69. [Google Scholar]
- 36.Engelbert RH, Uiterwaal CS, Gerver W-J, et al. Osteogenesis imperfecta in childhood: impairment and disability. A prospective study with 4-year follow-up. Arch Phys Med Rehabil. 2004;85:772–778. doi: 10.1016/j.apmr.2003.08.085. [DOI] [PubMed] [Google Scholar]
- 37.Russell DJ, Rosenbaum PL, Cadman DT, et al. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol. 1989;31:341–352. doi: 10.1111/j.1469-8749.1989.tb04003.x. [DOI] [PubMed] [Google Scholar]


