Abstract
This paper is to study auditory event-related potential P300 in patients with anxiety and depressive disorders using dipole source analysis. Auditory P300 using 2-stimulus oddball paradigm was collected from 35 patients with anxiety disorder, 32 patients with depressive disorder, and 30 healthy controls. P300 dipole sources and peak amplitude of dipole activities were analyzed. The source analysis resulted in a 4-dipole configuration, where temporal dipoles displayed greater P300 amplitude than that of frontal dipoles. In addition, a right-greater-than-left hemispheric asymmetry of dipole magnitude was found in patients with anxiety disorder, whereas a left-greater-than-right hemispheric asymmetry of dipole magnitude was observed in depressed patients. Results indicated that the asymmetry was more prominent over the temporal dipole than that of frontal dipoles in patients. Patients with anxiety disorder may increase their efforts to enhance temporal dipole activity to compensate for a deficit in frontal cortex processing, while depressed patients show dominating reduction of right temporal activity. The opposite nature of results observed with hemispheric asymmetry in depressive and anxiety disorders could serve to be valuable information for psychiatric studies.
Keywords: Anxiety disorder, Depressive disorder, Auditory P300, Dipole source analysis
Introduction
P300 has attracted great interest of psychiatrists and psychologists because of its correlation with cognition and attention. Reduced magnitude of P300 component and reduction of coherences during oddball paradigm have been observed in patients with psychiatric disorders like schizophrenia (Hegerl et al. 1995), and Alzheimer’s disease patients (Ito et al. 1990; Guntekin and Basar 2010). A further study suggests P300 parameters and topography can be used to differentiate between forms of dementia, as well as between dementia and pseudodementia (Maurer and Dierks 1991).
When P300 parameters were used in patients with depressive disorder, there were conflicting reports as to whether patients displayed reduced P300 amplitude. In depressed patients, half of the studies resulted in reduced amplitude compared with healthy controls (Roth et al. 1986). In patients with anxiety disorder, several studies have suggested that P300 amplitude is reduced compared with healthy controls (Boudarene and Timsit-Berthier 1997; Bauer et al. 2001). Although P300 could have clinical value as a diagnostic tool for psychiatric disorders, it is widely accepted that the clinical value of scalp P300 has been impeded by unsatisfactory test–retest reliability and the existence of overlapping activities in scalp electroencephalography.
Dipole source analysis provides a tool to identify the generation of neuronal structures and to separate overlapping activity existent in scalp-recorded ERPs (Pockett et al. 2007). In addition, test–retest reliability of P300 could be improved with dipole source analysis, which provides higher test–retest reliability than single channel P300 analysis and principal components analysis (PCA) (Frodl et al. 2000). In this context, dipole source analysis might facilitate P300 applications in psychiatric research.
The present study aimed to develop dipole model of auditory P300 potentials for patients with anxiety disorder and depressive disorder. There are three relevant factors reflecting the interest of this ERPs study.
First, although previous behavioral and electroencephalographic studies have already found abnormal asymmetries of right-left brain function in patients with anxiety disorder and patients with depressive disorder (Bruder et al. 1997, 1999; Liotti et al. 1991), the present study aimed to find ERPs evidence to confirm the abnormal right-left hemispheric asymmetry and for interpretation of impaired neuronal process.
Second, P300 potential recorded at frontal scalp sites represents orienting response, while P300 recorded at parietal scalp sites represents the match process between the incoming stimulus and the voluntarily maintained attentional trace of the task relevant stimulus. However, little attempt has ever been made to compare P300 potential between frontal and tempo-parietal brain regions in psychiatric patients. In this context, we aimed to compare dipole P300 activity between these regions and determine whether depressive and anxiety disorder could be differentiated.
Third, although P300 parameters and topography have been used in previous ERPs studies of depression and anxiety, dipole source analysis has advantages in providing a useful mathematical processing for data reduction, as well as anatomical locations and source activities which may reflect functionally different physiological processes. Accordingly, comparison of dipole activities between depressive and anxiety disorder might result in finding of abnormal neuronal process associated with each disorder.
Methods
Participants
Three groups of participants were recruited for the present study: (1) 30 healthy controls (16 males and 14 females, aged 22–38 years); (2) 32 patients with depressive disorder (15 males and 17 females, aged 25–40 years); and (3) 35 patients with anxiety disorder (16 males and 19 females, aged 26–43 years). The three groups were comparable in gender and age. Healthy controls were recruited from the university hospital staff, and were screened by psychiatrists and Hamilton anxiety and depression ratings scale to exclude any psychopathology. Patients were recruited from the Depression and Anxiety Disorders Clinic in Shenzhen Kangning Hospital, and the diagnoses were completed by psychiatrists in this hospital. The patients in Group 2 met the China Criteria of Mental Diseases III (CCMD-3) for anxiety disorders, but not for a depressive disorder, and were diagnosed with panic disorder (n = 9), social phobia (n = 13), or generalized anxiety (n = 13), and their scores of Hamilton Anxiety Scale were 24.25 ± 5.12 (mean ± SD). The patients in Group 3 met the criteria for CCMD-3 for major depressive disorder (n = 18) and dysthymia (n = 14), but not for any anxiety disorder, and their scores of Hamilton Depression Scale were 27.35 ± 5.06 (mean ± SD). Prior to oddball testing, the patients should have never taken drugs or at least drug-free period of 14 days.
Experimental procedure
The auditory 2-stimulus oddball paradigm was employed in the following tests, and the stimuli were defined as ‘standard’ and ‘target’ and were presented with a probability of 0.85 and 0.15, respectively. A 500-Hz tone was defined for the standard stimulus, and a 1-kHz tone was defined for the target stimulus. The tones were presented binaurally through earphones at 70 dB SPL once every 2 s, and each stimulus lasted for 100 ms. Subjects were instructed to press a button as quickly and accurately as possible following identification of a target stimulus, and the reaction time and error rates were recorded. The experiments were performed in a shielded room free from noise or interruption.
Electroencephalographic (EEG) signals were recorded during 4 blocks, each of which comprised 100 stimulus presentations. Subjects responded with their right hand in half of their blocks and with their left hand in the other half, the order of hand use was counterbalanced across participants.
EEG signal acquisition and processing
The electrodes were placed according to the 10-20 System (Fp1, Fpz, Fp2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P7, P3, Pz, P4, P8, O1, Oz, and O2). Additional intermediate sites were FC5, FC1, FC2, FC6, CP5, CP1, CP2, CP6, PO3, POz, PO4). All channels were referenced to linked mastoids and had an impedance of ≤10 kΩ, the ground electrode was located at the nasion. An additional channel was used for electrooculography (EOG) recording. The EEG data recorded at each electrode was amplified with a band pass filter of 0.5–30 Hz.
The analyzed EEG time epochs lasted for 1,200 ms (300 ms pre-stimulus and 900 ms post-stimulus). Waveforms were averaged off-line for ERPs calculation. To avoid eye movement in the processed EEG, all epochs with excessive eye movements (>120 μV) were eliminated from the average calculation. On average, more EEG epochs in depressive patients were eliminated than the other groups after the artifact rejection (36.7% of all epochs in depressive patients were eliminated vs. 18.4% in healthy group and 27.5% in anxiety group).
The individual ERPs waveforms were averaged over subjects to obtain grand average ERPs for each group, and the grand average ERPs were computed separately for standard and target stimuli. The P300 component was defined as the largest positive deflection occurring within the time window of 280–500 ms, while the peak latency was defined as the time from stimulus onset to the peak of P300. Topographical maps of scalp potential were calculated for response to target and standard stimuli.
Dipole source analysis
Estimation of the dipole sources of scalp-recorded P300 data was performed with signal source analysis software ASA 3.0 (Advanced Source Analysis; ANT Software BV, www.ant-neuro.com/products/asa/). ASA delivered the MRI of the MNI305 brain which was already transformed to the Talairach space. The software also delivered standard electrodes and head model files that matched the MNI305 MRI. Therefore, the estimated coordinates from the source localization were in the Talairach space.
The strength of the dipoles in the present source space was altered over time, but the orientation and location remained stable. A realistic three-shell head model (scalp, skull, and brain) was applied to calculate dipole activities. The conductivity values were 0.33 S/m, 0.0042 S/m, and 0.33 S/m for the scalp, skull, and brain compartments, respectively, and the compartments were assumed to be isotropic in ASA source reconstruction.
A grand average dipole model over each group of subjects was calculated for the target P300 of the grand average data, and was performed according to the following steps:
Referring to the study by Hegerl and Frodl-Bauch (1997), two dipole pairs, one initially placed at bilateral posterior brain regions and the other initially placed at bilateral anterior brain regions, were used to calculate the centers of activity in both hemispheres. The interhemispheric symmetry constraint for coordinates of the dipole pair was used for dipole estimation; locations of dipoles were fitted for the P300 time interval.
Because the first two pairs of dipoles already explained the majority of the scalp data (> 93%), the third pair of bilateral dipoles was discarded.
The location coordinates of the grand average dipole model were then transferred to MNI305 MRI to identify the corresponding anatomical regions. The healthy control group, anxiety disorder patients, and depressive disorder patients were analyzed using the above-described procedures to obtain the respective grand average dipole model.
The individual data were subsequently analyzed. Dipole source analysis was performed for individual data using the grand average dipole configuration without individually fitting this dipole configuration. The dipole orientation and strength were individually adjusted within the P300 time intervals.
Once the time-varying individual dipole waveform was obtained, the large positive deflection that occurred within the time window of 280–500 ms was defined as the dipole peak. Individual dipole activities were averaged across subjects to obtain grand average dipole activities.
Statistical analysis
P300 amplitudes in dipole activities of target stimuli were submitted to repeated measures ANOVA with ‘group’ (depressive disorder, anxiety disorder, or healthy controls) serving as the between-subjects factor and the dipole site (frontal or temporal), hemisphere (right or left) serving as the within-subjects factors, respectively.
Results
The correct responses to the target stimuli was very high for all three groups (Control [%]: 98.3 ± 3.2, Anxiety [%]: 96.6 ± 2.7, Depression [%]: 97.0 ± 1.9). The reaction time for target tones did not differ significantly across groups. (Control [ms]: 496 ± 87, Anxiety [ms]: 519 ± 92, Depression [ms]: 527 ± 105).
Scalp data measurement
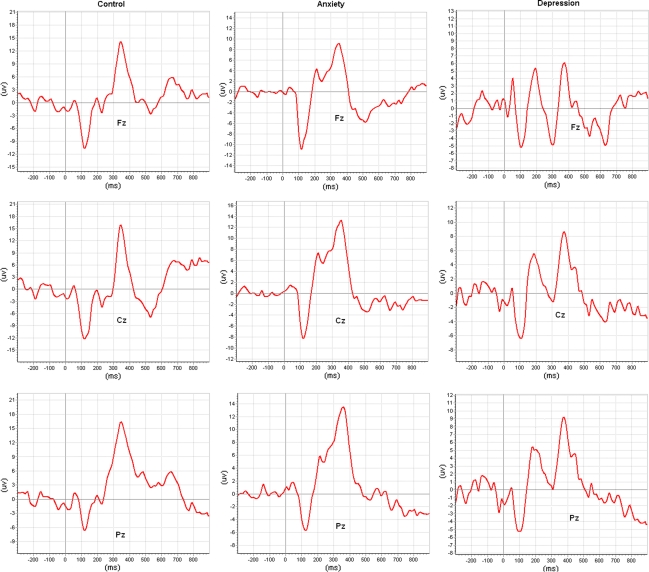
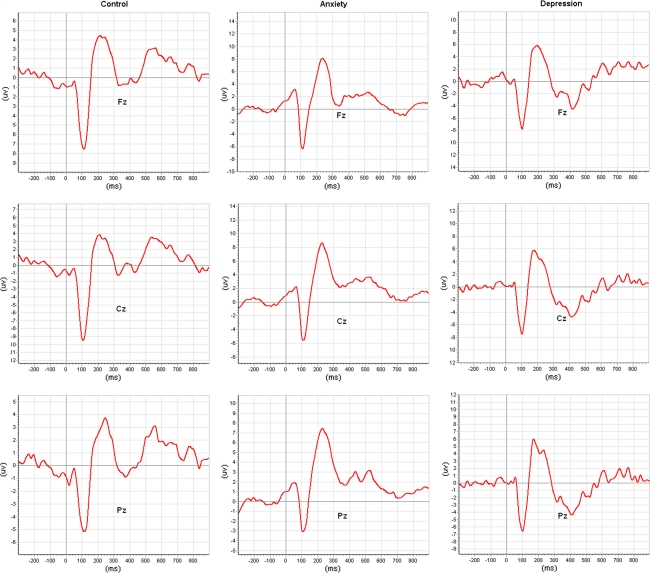
The grand average ERP waveforms at Fz, Cz, and Pz electrode sites in response to target stimuli are shown in Fig. 1 for healthy controls, as well as patients with anxiety disorder and patients with depressive disorder, and also the corresponding waveforms to standard stimuli are showed in Fig. 2. Results of P300 parameters at Pz scalp site are presented in Table 1. Specially, the small peaks around P300 observed in depressive patients were probably due to high proportion of eliminated EEG epochs from average calculation.
Fig. 1.
Grand average ERPs of the healthy controls, patients having anxiety disorders and patients having depressive disorders in response to target stimuli, where ERPs are respectively from the Fz,Cz and Pz electrode sites
Fig. 2.
Grand average ERPs of the healthy controls, patients with anxiety disorders and patients with depressive disorders in response to standard stimuli, where ERPs are respectively from the Fz,Cz and Pz electrode sites
Table 1.
Amplitudes and latencies of P300 component of healthy controls and patients at Pz electrode (mean ± SD)
| P300 parameter | Healthy controls (n = 30) | Anxiety disorder (n = 35) | Depressive disorder (n = 32) |
|---|---|---|---|
| Latency (ms) | 356.3 ± 25.1 | 359.7 ± 30.9 | 374.3 ± 35.2 |
| Amplitude (μv) | 15.8 ± 4.5 | 13.6 ± 4.8 | 9.5 ± 3.7 |
ERPs topographies to target stimuli at peak latency are shown in Fig. 3. The auditory P300 was maximally positive over parietal scalp regions when observed at peak latency. The target stimulus effect displayed a midline focus of activity at centro-parietal area in the healthy control group, and the focus shifted to left hemisphere in depressed patients. In anxiety disorder patients, the foci were displayed in the right posterior scalp area. ERPs topographies to standard stimuli at the same time points are shown in Fig. 3 where all topographies showed obvious foci close to midline at posterior scalp area.
Fig. 3.
ERPs potential maps to target stimuli and standard stimuli of the healthy control and patient groups at P300 peak latency. The upper row presents maps to target stimuli and the low row presents maps to standard stimuli
Dipole source analysis
In the target P300 dipole model of grand average data, two dipoles per hemisphere were located respectively. Most of the variance of scalp-recorded data could be explained. Individual Goodness of Fit (GOF) was situated between 95.3 and 99.5% for healthy controls, 93.3–98.2% for anxiety disorder, and 93.0–97.6% for depressive disorder.
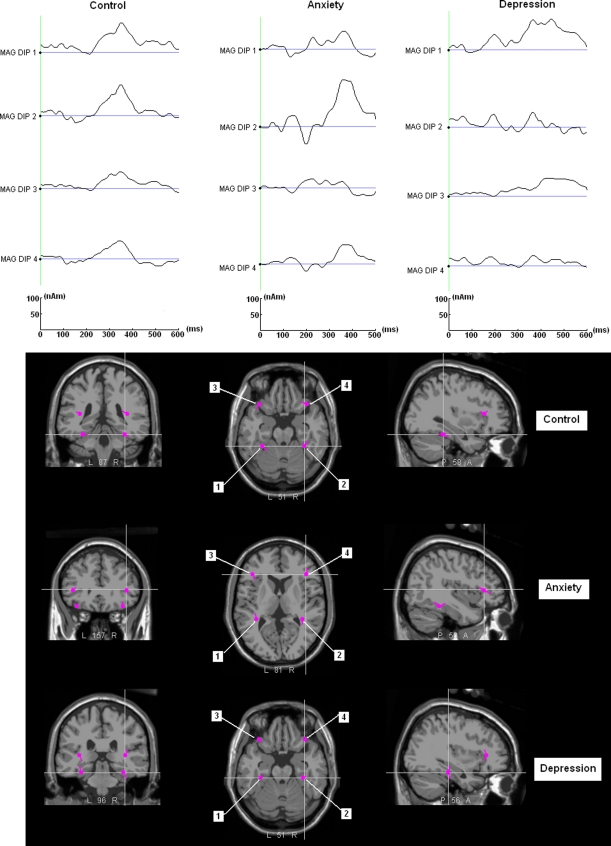
Table 2 presents coordinates of the dipole locations in the nasion-ear frame of the realistic head model. Figure 4 shows dipole locations, as well as orientations of grand average target ERPs superimposed on an MNI305 anatomical template brain with grand average time-varying dipole waveforms for healthy controls, anxiety disorder patients, and depressive disorder patients. A pair of posterior dipoles was located at bilateral temporo-basal regions and the other pair was located at bilateral frontal regions. While EEG data were fit to three models with slightly different dipole locations for each group, distances between corresponding dipoles were around 1 cm.
Table 2.
Coordinates of the dipole locations in the nasion-ear frame of the realistic head model
| Group | Dipole No. | X (mm) | Y (mm) | Z (mm) |
|---|---|---|---|---|
| Healthy control | 1 | −39.4 | 34.3 | −23.6 |
| 2 | −39.4 | −34.3 | −23.6 | |
| 3 | 20.7 | 35.2 | 12.4 | |
| 4 | 20.7 | −35.2 | 12.4 | |
| Anxiety disorder | 1 | −42.8 | 34.0 | −20.9 |
| 2 | −42.8 | −34.0 | −20.9 | |
| 3 | 28.5 | 37.4 | 10.5 | |
| 4 | 28.5 | −37.4 | 10.5 | |
| Depressive disorder | 1 | −36.2 | 35.2 | −22.8 |
| 2 | −36.2 | −35.2 | −22.8 | |
| 3 | 27.8 | 36.5 | 1.9 | |
| 4 | 27.8 | −36.5 | 1.9 |
Fig. 4.
The upper part presents grand average dipole source waveforms of the healthy control and patient groups, where two kinds of P300 activities are observed in each group, monophasic P300 peak and small P300 peak followed by a slow negative wave. The low part illustrates dipole locations and orientations superimposed on MNI305 anatomical template brain
In the healthy group, the waveforms from the bilateral temporal dipoles and right frontal dipole presented with a monophasic P300 peak, while the waveforms from the frontal dipoles in the right hemisphere resulted in a small P300 peak followed by a negative slow wave. In the anxiety disorder group, the two dipoles in the left hemisphere presented with a small P300 peak followed by a negative slow wave, whereas the two dipoles in the right hemisphere displayed a large monophasic P300 peak. In the depressive disorder group, the right-hemispheric temporal dipole presented with a small P300 peak followed by a negative slow wave, and the left-hemispheric dipoles displayed a large monophasic P300 peak.
Statistical analysis
Since deviation of corresponding dipole location in three dipole models is around 1 cm which will not much affect dipole waveform, these models were regarded as same mathematical processing for further group comparisons.
The results indicated that ‘group’ had a main effect on dipole P300 amplitude (F(2,94) = 3.36; P = 0.042). More specifically, the depressive disorder group displayed lower dipole amplitudes when compared with anxiety disorder patients (F(1,94) = 7.86, P < 0.01) and the healthy control group (F(1,94) = 8.83, P < 0.01).
Following ANOVA analysis, results revealed a main effect of dipole site on dipole P300 amplitude (F(1,94) = 21.15, P < 0.001). Namely, the temporal dipoles resulted in greater P300 amplitude than the frontal dipoles, and the effect was strongest in patients with anxiety disorder (F(1,94) = 32.15, P < 0.001) and weakest in patients with depressive disorder (F(1,94) = 7.85, P < 0.01). The effect was intermediate for the healthy control group (F(1,94) = 18.15, P < 0.001).
ANOVA analysis also indicated a main effect of hemisphere on dipole P300 amplitude (F(1,94) = 9.57, P < 0.01). The dipole P300 amplitude was greater in the right compared with the left hemisphere in patients with anxiety disorders, indicating that the hemispheric effect was significantly right-greater-than-left asymmetry (F(1,94) = 17.70, P < 0.001). In contrast, the dipole P300 amplitude was greater in the left compared with the right hemisphere in depressive disorder patients, resulting in an hemispheric effect on dipole amplitude that had significant left-greater-than-right asymmetry (F(1,94) = 21.15, P < 0.001). In the healthy control group, the hemispheric effect was not significant (F(1,94) = 1.52, P = 0.257).
ANOVA also revealed a dipole site by hemisphere interaction in patients with anxiety disorder, which confirmed that hemispheric asymmetry is dependent on the dipole site (F(1,94) = 24.35, P < 0.001). Specifically, the right-greater-than-left asymmetry of the P300 amplitude was more prominent in the temporal dipole compared with the frontal dipole. A dipole site by hemisphere interaction in depressive disorder patients confirmed that the left-greater-than-right asymmetry of the P300 amplitude was more prominent in the temporal dipole compared with the frontal dipole (F(1,94) = 19.15, P < 0.001). This interaction effect was not significant in the healthy control group (F(1,94) = 0.591, P = 0.430).
Discussion
The present P300 source reconstruction study used two dipoles per hemisphere in the final source space. The 4-dipole configuration was in basic agreement with previously published dipole studies (Frodl et al. 2000; Hegerl and Frodl-Bauch 1997), except for interhemispheric orientation symmetry of the dipole pairs. In fMRI studies of auditory oddball paradigm (Linden et al. 1999; Mulert et al. 2004), predominating activations of bilateral supramarginal gyri or temporoparietal regions and insula were always found, fMRI activations and EEG–LORETA current source density activations of auditory oddball also showed good correspondence in the bilateral temporoparietal region and insula (Mulert et al. 2004). Although anatomical locations of the 4-dipole model are not accurately consistent with fMRI activations, there is an approximate correspondence. Since dipole source waveforms are not much affected by errors in the center location of up to 1–2 cm (Scherg and Picton 1991; Bledowski et al. 2004), it is reasonable they reflect the brain electric activities in around bilateral temporoparietal and insula regions.
The present results revealed two kinds of P300 activities in the dipole waveforms: a large, monophasic, P300 peak, which reflected the P300 data recorded at the parietal electrode sites, and a small, P300 peak followed by a negative slow wave, which reflected P300 data recorded at the frontal electrode sites. In the healthy control group, only the right frontal dipole resulted in a small, P300 peak, which may reflect an orienting response in stimuli-driven attention processes, and the result supported the target discrimination process underlying P300 response originates with right frontal activation. In the anxiety disorder group, a small, P300 peak followed by a negative wave was visible in the left-hemispheric frontal dipole and left-hemispheric temporal dipole, which reflects abnormal orienting response originating with left hemispheric activation. In depressive disorder patients, a small, P300 dipole peak followed by a negative, slow wave was obviously displayed in the right-hemispheric temporal dipoles, which suggests abnormal orienting response pronounced over the right-hemispheric temporal site.
Although linked mastoid as reference scheme in this experiment might attenuate effects of laterality, hemispheric asymmetries of dipole P300 amplitude were obvious during auditory P300 paradigm testing in patients with anxiety and depressive disorders. It is not implemented in this paper but deduced that significant asymmetry also existed in these patients when with average mastoid reference scheme. The optimal reference scheme for cognitive ERPs recording in depressive and anxiety disorders still demands a further verification.
Left-greater-than-right asymmetry occurred in the depressive disorder patients, whereas right-greater-than-left asymmetry occurred in patients with anxiety disorders. These patient-dependent hemispheric asymmetries of dipole magnitude reflected foci locations in the scalp P300 topography at peak latency. Dipole activities in depressed patients revealed a left hemisphere advantage (in particular over the temporal site) when asked to perceive and respond to target tones, which was consistent with dichotic listening studies (Bruder et al. 1999; Liotti et al. 1991). Depressive disorder patients appeared to activate rather left than right-hemisphere regions during auditory tasks. The anxiety disorder group showed P300 evidence that a right hemisphere advantage existed in the frontal and temporal dipoles, particularly in the temporal dipoles. These results are consistent with previous PET studies suggesting decreased metabolism and cerebral blood flow in the left temporoparietal and inferior frontal cortex in panic disorder patients (Meyer et al. 2000; Nordahl et al. 1998; Lee et al. 2006; Sakai et al. 2006).
In another P300 principal components analysis (PCA) study (Bruder et al. 2002), tonal and phonetic oddball tasks have been used to study difference of scalp topography between the two tasks or to determine whether anxiety and depression disorders could be differentiated with tonal-minus-phonetic difference, similar hemispheric asymmetry of PCA factor scores in the patients was reported in this previous study.
The present findings demonstrated that temporal dipole activities possess stronger P300 activity compared with frontal dipoles in all groups, which is consistent with the target scalp P300 trait of parietal advantage. The primary interest of the present study was to determine whether the advantage of temporal dipole over frontal dipole could be used to differentiate between anxiety disorder and depressive disorder patients. Because the anxiety disorder patients displayed the strongest temporal advantage, enhanced activity in temporal dipole was compared with the frontal dipole, which suggested that these patients might attempt to enhance posterior dipole activity during target discrimination processing to compensate for deficits in frontal cortex processing.
Patients with depressive disorder displayed the weakest temporal advantage among all three groups. Dominating reduction of temporal activity pronounced in right hemisphere suggested a right-hemispheric temporal cortex hypofunction, as well as a right-hemispheric inferior prefrontal cortex hypofunction, which may represent impaired arousal and vigilance mechanisms in depression.
It has been reported depression engage the right hemisphere’s mechanisms and influence arousal and vigilance mechanisms lateralized to the right hemisphere (Liotti et al. 1991), According to results of the present study, the severer interference of functioning existed in the right-hemispheric temporal cortex.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No:30970779); Natural Science Foundation of Guangdong Province (Grant No.06028566, No. 9151806001000009).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bauer LO, Costa L, Hesselbrock VM. Effects of alcoholism, anxiety and depression on P300 in women: a pilot study. J Stud Alcohol. 2001;62:571–579. doi: 10.15288/jsa.2001.62.571. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Hoechstetter K, et al. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. J Neurosci. 2004;24(42):9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudarene M, Timsit-Berthier M. Stress, anxiety and event related potentials. Encephale. 1997;23:237–250. [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, McGrath PJ, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biol Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Wexler BE, Stewart JW, Price LH, Quitkin FM. Perceptual asymmetry differences between major depression with or without a comorbid anxiety disorder: a dichotic listening study. J Abnorm Psychol. 1999;108:233–239. doi: 10.1037/0021-843X.108.2.233. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke J, Leite P, Schneier FR, Stewart JW, Quitkin FM. Cognitive ERPs in depressive and anxiety disorders during tonal and phonetic oddball tasks. Clin Electroencephalogr. 2002;33(3):119–124. doi: 10.1177/155005940203300308. [DOI] [PubMed] [Google Scholar]
- Frodl T, Juckel G, Gallinat J, Bottlender R, Riedel M, Preuss U, Moller H, Hegerl U. Dipole localization of P300 and normal aging. Brain Topogr. 2000;13(1):3–9. doi: 10.1023/A:1007831017318. [DOI] [PubMed] [Google Scholar]
- Guntekin B, Basar E. A new interpretation of P300 responses upon analysis of coherences. Cogn Neurodyn. 2010;4:107–118. doi: 10.1007/s11571-010-9106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerl U, Frodl-Bauch T. Dipole source analysis of P300 component of the auditory evoked potential: a methodological advance? Psychiatry Research: Neuroimaging. 1997;74:109–118. doi: 10.1016/S0925-4927(97)03129-6. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Juckel G, Muller-Schubert A, Pietzcker A, Gaebel W. Schizophrenics with small P300: a subgroup with a neurodevelopmental disturbance and a high risk for tardive dyskinesia? Acta Psychiatr Scand. 1995;91:120–125. doi: 10.1111/j.1600-0447.1995.tb09751.x. [DOI] [PubMed] [Google Scholar]
- Ito J, Yamao S, Fukuda H, Mimori Y, Nakamura S. The P300 event-related potentials in dementia of the Alzheimer type. Correlations between P300 and monoamine metabolites. Electroencephalogr Clin Neurophysiol. 1990;77:174–178. doi: 10.1016/0168-5597(90)90035-C. [DOI] [PubMed] [Google Scholar]
- Lee YS, Hwang J, Kim SJ, Sung YH, Kim J, Sim ME, Bae SC, Kim MJ, Lyoo IK. Decreased blood flow of temporal regions of the brain in subjects with panic disorder. J Psychiatr Res. 2006;40(6):528–534. doi: 10.1016/j.jpsychires.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Linden DEJ, Prvulovic D, Formisano E, et al. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex. 1999;9(8):815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Liotti M, Sava D, Rizzolatti G, Caffarra P. Differential hemispheric asymmetries in depression and anxiety: a reaction-time study. Biol Psychiatry. 1991;29:887–899. doi: 10.1016/0006-3223(91)90055-Q. [DOI] [PubMed] [Google Scholar]
- Maurer K, Dierks T. Atlas of brain mapping-topographic mapping of EEG and evoked potentials. In: Maurer K, Dierks T, editors. Atlas of brain mapping. Berlin-Heidelberg: Springer; 1991. [Google Scholar]
- Meyer JH, Swinson R, Kennedy SH, Houle S, Brown GM. Increased left posterior parietal-temporal cortex activation after D-fenfluramine in women with panic disorder. Psychiatry Res: Neuroimaging. 2000;98:133–143. doi: 10.1016/S0925-4927(00)00048-2. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jager L, Schmitt R, et al. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22(1):83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Stein MB, Benkelfat C, Semple WE, Andreason P, Zametkin A, Uhde TW, Cohen RM. Regional cerebral metabolic asymmetries replicated in an independent group of patients with panic disorders. Biol Psychiatry. 1998;44:998–1006. doi: 10.1016/S0006-3223(98)00026-2. [DOI] [PubMed] [Google Scholar]
- Pockett S, Whalen S, McPhail AVH, Freeman WJ. Topography, independent component analysis and dipole source analysis of movement related potentials. Cogn Neurodyn. 2007;1:327–340. doi: 10.1007/s11571-007-9024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth WT, Duncan CC, Pfefferbaum A, Timsit-Berthier M. Applications of cognitive ERPs in psychiatric patients. In: McCallum WC, Zapppoli R, Denoth F, editors. Cerebral Psychophysiology: Studies in Event-Related Potentials (EEG Suppl 38) Amsterdam: Elsevier; 1986. pp. 419–438. [PubMed] [Google Scholar]
- Sakai Y, Kumano H, Nishikawa M, Sakano Y, Kaiya H, Imabayashi E, Ohnishi T, Matsuda H, Yasuda A, Sato A, Diksic M, Kuboki T. Changes in cerebral glucose utilization in patients with panic disorder treated with cognitive-behavioral therapy. Neuroimage. 2006;33(1):218–226. doi: 10.1016/j.neuroimage.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Scherg M, Picton TW. Separation and identification of event-related potential components by brain electric source analysis. Electroencephalogr Clin Neurophysiol. 1991;42:24–37. [PubMed] [Google Scholar]