Abstract
Δ9 tetrahydrocannabinol (THC) and cannabidiol (CBD) are the principal psychoactive and non-psychoactive components of cannabis. While most THC-induced behavioral effects are thought to depend on endogenous cannabinoid 1 (CB1) receptors, the molecular targets for CBD remain unclear. Here, we report that CBD and THC inhibited the function of human 5-HT3A receptors (h5-HT3ARs) expressed in HEK 293 cells. The magnitude of THC and CBD inhibition was maximal 5 min after a continuous incubation with cannabinoids. The EC50 values for CBD and THC-induced inhibition were 110 nM and 322 nM respectively in HEK 293 cells expressing h5-HT3ARs. In these cells, CBD and THC did not stimulate specific [35S]-GTP-γs binding in membranes, suggesting that the inhibition by cannabinoids is unlikely mediated by a G-protein dependent mechanism. On the other hand, both CBD and THC accelerated receptor desensitization kinetics without significantly changing activation time. The extent of cannabinoid inhibition appeared to depend on receptor desensitization. Reducing receptor desensitization by nocodazole, 5-hydroxyindole and a point-mutation in the large cytoplasmic domain of the receptor significantly decreased CBD-induced inhibition. Similarly, the magnitude of THC and CBD-induced inhibition varied with the apparent desensitization rate of h5-HT3ARs expressed in Xenopus oocytes. For instance, with increasing amount of h5-HT3AR cRNA injected into the oocytes, the receptor desensitization rate at steady state decreased. THC and CBD-induced inhibition was correlated with the change in the receptor desensitization rate. Thus, CBD and THC inhibit h5-HT3A receptors through a mechanism that is dependent on receptor desensitization.
1. Introduction
While Δ9-tetrahydrocannabinol (THC) is a psychoactive substance, cannabidiol (CBD) has been known as the main nonpsychoactive component of marijuana (Pertwee, 2005, Pacher et al., 2006). CBD is the most abundant cannabinoid in marijuana next to THC. Like THC, CBD has been known for its therapeutic potential in the treatment of epilepsy, glaucoma, central and peripheral inflammatory disorders, anxiety, acute schizophrenia and cancer (Izzo et al., 2009, Scuderi et al., 2009). Both CBD and THC have been shown to be effective for the management of pain and emetic action induced by chemotherapy in humans (Pertwee, 2005). In contrast to THC, CBD has been found to inhibit psychotic effects induced by cannabis in humans (Leweke et al., 2000). Although CBD and THC share a high degree of similarity in their chemical structures, they act on the brain through distinct mechanisms (Pacher et al., 2006). While the majority of THC-induced behavioral effects are thought to involve activation of endogenous cannabinoid type 1 (CB1) receptors, the molecular targets for CBD-induced action remain unknown since CBD does not activate CB1 and CB2 receptors (Pertwee, 2005). Emerging evidence has also suggested that some of the THC-induced effects are not dependent on CB1 receptors. For instance, both THC and CBD can exert neuroprotective effect against NMDA receptor-induced neurotoxicity through a CB1 receptor-independent mechanism (Hampson et al., 1998). THC has also been found to directly inhibit the function of recombinant human 5-HT3A receptors (h5-HT3ARs) expressed in HEK 293 cells (Barann et al., 2002). A recent study has suggested that 5-HT3 receptors are involved in CB1/CB2 independent cannabinoid-induced analgesia in vivo (Racz et al., 2008).
5-HT3Rs are a member of a ligand-gated ion channel (LGIC) superfamily including γ-aminobutyric acid type A, glycine and neuronal nicotinic acetylcholine (nACh) receptors. 5-HT3Rs are involved in pain transmission, analgesia, mood disorders and drug abuse (Zhang and Lummis, 2006). Selective antagonists of 5-HT3R produce anxiolytic and antiemetic effects in animal models (Zhang and Lummis, 2006). Homopentameric receptors containing only the 5-HT3AR subunit are capable of forming functional ion channels in the central nervous system (CNS) (Maricq et al., 1991, Morales and Wang, 2002). 5-HT3ARs play a role in reward mechanism and actions of drug abuse (Grant, 1995).
One common feature shared by the members of LGICs is that these receptors desensitize rapidly in the prolonged presence of agonists. Although the molecular processes of receptor desensitization remain elusive, receptor desensitization has been shown to contribute significantly to acute ethanol interaction with the function of 5-HT3Rs and nAChRs (Zhou et al., 1998, Dopico and Lovinger, 2009). A number of factors can affect 5-HT3R desensitization. Desensitization kinetics can be affected by external calcium concentrations (Hu and Lovinger, 2005), posttranslational modification of receptor protein by activation of protein kinases (Yakel et al., 1991, Hubbard et al., 2000), cytoskeleton proteins (Emerit et al., 2005) and subunit composition (Hapfelmeier et al., 2003). Although the mechanisms of LGIC desensitization remain elusive, receptor desensitization is thought to contribute to controlling and shaping the efficacy of synaptic signaling (Jones and Westbrook, 1996, Giniatullin et al., 2005).
Like THC, CBD has been used for the treatment of anticancer drug-induced vomiting and nausea (Pertwee, 2005). The antiemetic action of cannabis has been proposed to occur partially via inhibition of 5-HT3 receptors (Townsend et al., 2002). However, whether or not CBD can inhibit h5-HT3ARs and the mechanism underlying cannabinoid inhibition have not been reported. To address these questions, we examined the effects of both CBD and THC on 5-HT-activated current in HEK 293 cells and Xenopus oocytes expressing h5-HT3ARs. Our results show that like THC, CBD potently inhibits h5-HT3AR function in a manner that is independent of agonist concentration. Both CBD and THC accelerate receptor desensitization. In addition, the extent of h5-HT3AR desensitization appears to influence the ability of cannabinoid-induced receptor inhibition. Some of this work has been presented previously in a preliminary form (Sun et al., 2009).
EXPERIMENTAL PROCEDURES
HEK 293 cell transfection and whole cell recording
HEK 293 cells were cultured as described previously (Xiong et al., 2008). The plasmid cDNA coding for the h5-HT3AR was transfected with the SuperFect Transfection kit (Qiagen, Valencia, CA). Currents were recorded 24–48 hr after transfection. Cells were lifted and continuously superfused with a solution containing 140 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 5 mM glucose, and 10 mM HEPES (pH 7.4 with NaOH; ~340 mosmol with sucrose). Membrane currents were recorded in the whole-cell configuration using an Axopatch 200B amplifier (Axon) at 20–22°C. Cells were held at −60 mV unless otherwise indicated. Data were acquired using pCLAMP 9 software (Molecular Devices, Sunnyvale, CA). Bath solutions were applied through 3 barrel square glass tubing (Warner Instrument, Hamden, CT) with tip diameter of ~200 µm. Drugs were applied using a Warner fast-step stepper-motor driven system. The 10–90% rise time of the junction potential during whole-cell recording was 4–12 ms.
Site-Directed mutagenesis
Point-mutations of a cloned h5-HT3A subunit were introduced using a QuikChange Site-Directed Mutagenesis Kit (Stratagene). The authenticity of the DNA sequence through the mutation sites was confirmed by double strand DNA sequencing using a CEQ 8000 Genetic Analysis System (Beckman Coulter, Inc).
[35S]-GTP-γs binding
HEK 293 cells were collected and homogenized in ice cold 50 mM Tris-HCl, pH 7.4. The homogenate was centrifuged at 30,000 g for 10 min at 4 °C. The membrane pellet was suspended in binding buffer used for [35S]-GTP-γs (PerkinElmer, Boston, MA) binding reaction, which contains 20 mM HEPES, 100 mM NaCl, 6 mM MgCl2, 0.1 mM DTT and 5 µM GDP. The membranes were incubated with 0.1 nM [35S]-GTP-γs for 2 hr at 30 °C. The filters were washed twice with 4 ml cold 10 mM Tri-HCl. Nonspecific binding was determined by 40 µM of GTP-γs. Bound and free [35S]-GTP-γs was separated by rapid vacuum filtration in a Brandell harvester through GF/B filters. The filters were punched into scintillation vials containing 5 ml liquid scintillation cocktail (LSC). The samples were counted in a scintillation counter at 50% efficiency. Assays were performed in triplicate and each experiment was repeated at least three times.
Preparation of cRNAs and Expression of Receptors
The cDNA clone of the h5-HT3A subunit was purchased from OriGen, Inc (Rockville, MD). Complementary RNAs (cRNAs) were synthesized in vitro using a mMESSAGE mMACHINE RNA transcription kit (Ambion Inc., Austin, TX). The quality and sizes of synthesized cRNAs were confirmed by denatured RNA agarose gels. Mature female Xenopus laevis frogs were anesthetized by submersion in 0.2% 3-aminobenzoic acid ethyl ester (Sigma, St Louis MO). Oocytes were surgically excised and separated. The follicular cell layer of the oocytes was removed by treatment with type A collagenase (Sigma-Aldrich) for 10 min at room temperature. Although the amount of cRNA injected into oocytes varied from 1 to 100 ng, as indicated, the injection volume of diethylpyrocarbonate (DEPC)-treated water was kept at 20 nl for all injections. Oocytes were incubated at 19 °C in modified Barth’s solution (MBS): 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 2.0 mM CaCl2, 0.8 mM MgSO4, 10 mM HEPES, pH7.4.
Xenopus Oocyte Electrophysiological Recording
After incubation for 2–5 days, oocytes were studied at room temperature (20–22 °C) in a 90 µl chamber. The oocytes were superfused with MBS at a rate of 6 ml/min. Agonists and chemical agents were diluted in the bath solution and applied to the oocytes for a specified time, using a solenoid valve-controlled superfusion system. Membrane currents were recorded by two-electrode voltage-clamp at a holding potential of −70 mV, using a Gene Clamp 500 amplifier (Axon Instruments Inc., Burlingame, CA). The recording microelectrodes were filled with 3 M KCl and had electrical resistances of 0.5–3.0 MΩ. Data were acquired using pClamp 9.1 software (Axon). Average values are expressed as means ± S.E.
Data Analysis
Statistical analysis of concentration-response data was performed with the use of the nonlinear curve-fitting program Prism. Data were fit using the Hill equation
where I is the current amplitude activated by a given concentration of agonist ([Agonist]), Imax is the maximum response of the cell, nH is the Hill coefficient and EC50 is the concentration eliciting a half-maximal response. Data were statistically compared by the unpaired t-test, or analysis of variance (ANOVA), as noted. Average values are expressed as mean ± standard error (SE).
Kinetic Measurements
Activation rate of current induced by 30 µM 5-HT was estimated by measuring the slope of the initial inward component of current between 10 and 30% of the maximal current (10–30% rise time). Receptor desensitization was induced by prolonging incubation with 5-HT plus CBD or THC for 30 s. The time constants of deactivation and desensitization were determined by fitting with exponential functions using the Marquardt-Levenberg algorithm in Clampfit. The desensitization time constants for 5-HT3R-mediated currents were best fit using a bi-exponential function when expressed in HEK 293 cells. However, a single exponential function was sufficient to accurately fit the desensitization decays of current activated by 5-HT in the presence of CBD or THC. In comparing single to multiple exponential data, a weighted sum of the fast and slow components (AFAST*τFAST + ASLOW*τSLOW) was used to normalize the two components into a single component, where as τFAST and τSLOW were the fast and slow decay time constants, and AFAST and ASLOW were the relative proportions of the fast and slow components.
Chemicals
All chemicals used in preparing the solutions were from Sigma-Aldrich (St. Louis, MO). 5-HT was applied by gravity flow via a micropipette positioned 3 mm from the oocyte. Stock solutions of AEA, 5-hydroxyindole (5-HOID) and nocodazole (Noc) were prepared at a concentration of 10 mM in dimethylsulfoxide (DMSO). DMSO alone did not affect 5-HT receptors when added at concentrations up to 0.2 % (v/v), which was twice as high as the highest concentration of DMSO used in our experiments.
RESULTS
CBD and THC inhibit h5-HT3AR-Mediated Responses
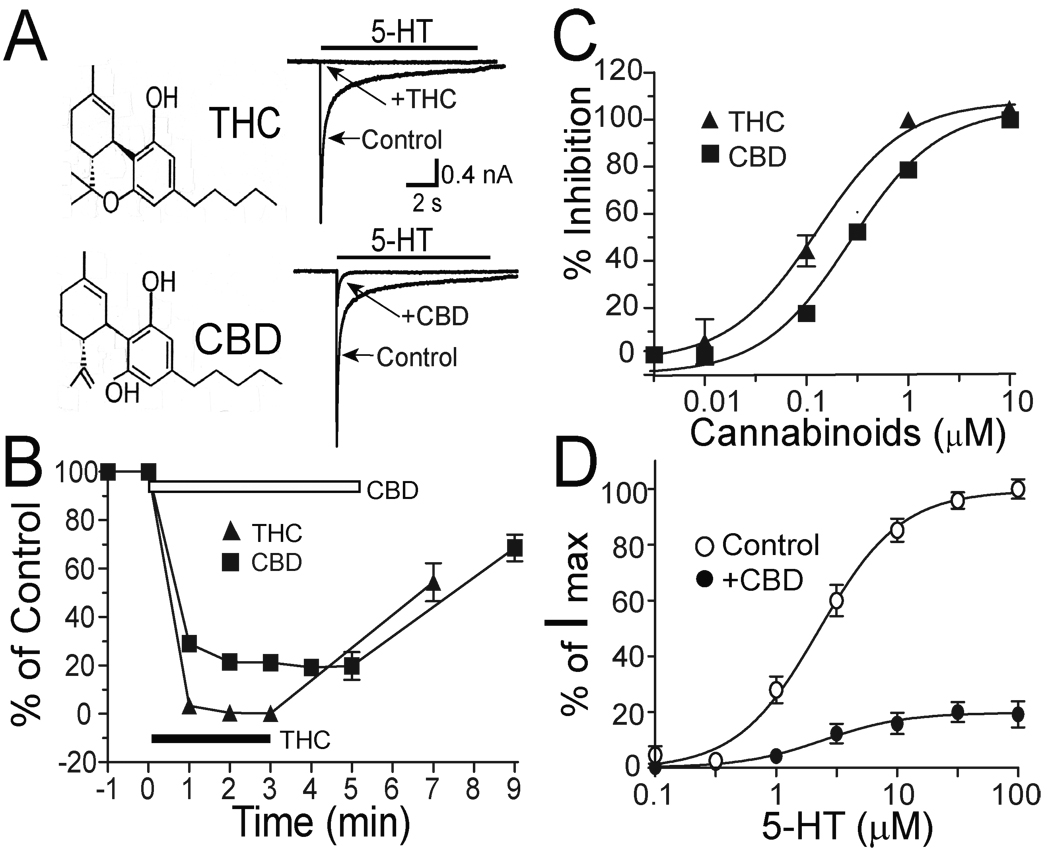
CBD and THC are structurally similar (Fig. 1A). THC at 1 µM reduced the amplitude of 5-HT (30 µM)-induced currents in HEK 293 cells expressing h5-HT3A subunits (Fig. 1A). This observation is consistent with a previous study (Barann et al., 2002). Similarly, CBD at 1 µM inhibited 5-HT3A receptor-mediated current (Fig. 1A). The inhibition by 1 µM CBD and THC was developed gradually after drug application, and reached a maximal level 3–5 min after continuous applications of the drugs (Fig. 1B). The magnitudes of the maximal inhibition by THC and CBD were 97 ± 5% and 82 ± 13%. These values were not significantly different (p = 0.1, unpaired t test, n = 5). The inhibition by CBD and THC were reversible after discontinuation of drug application (Fig. 1B). CBD and THC inhibited the amplitude of 5-HT-activated current in a concentration-dependent manner in HEK 293 cells expressing h5-HT3ARs (Fig. 1C). However, THC appeared to be more potent than CBD in inhibiting h5-HT3ARs. The EC50 values were 119 ±13 nM for THC and 329 ±19 nM for CBD. These values were significantly different (p < 0.001, unpaired t test, n = 4). The hill slope values were 1.59 ± 0.48 for THC and 1.34 ± 0.13 for CBD.
Fig. 1.
CBD and THC inhibition of h5-HT3ARs expressed in HEK 293 cells. (A) The chemical structures of THC and CBD and traces showing currents activated by 30 µM 5-HT without and with preincubation of 1 µM THC or CBD for 3 min. The solid bar above each trace indicates the time of 5-HT application. (B) Time courses of THC and CBD inhibition of 5-HT3A receptors. Graphs represent average percent of control current amplitude after THC (triangles) and CBD (squares) application. The solid bar indicates the time of continuous application of THC. The open bar indicates the time of continuous application of CBD. Each data point represents mean ± S.E. from at least 9 cells. The error bars that are invisible are smaller than the size of symbols. (C) Concentration-response curves for CBD and THC inhibition of I5-HT. The curves are best fits using the Hill equation as described in ‘Methods’. Each data point represents mean ± S.E. from at least 4 cells. (D) Apparent non-competitive inhibition of h5-HT3AR-mediated response by 1 µM CBD. Graphs represent 5-HT concentration-response curves in the absence (open circles) and in the presence of 1 µM CBD (solid circles).
The CBD-induced inhibition of h5-HT3ARs was also examined in the presence of different concentrations of 5-HT (Fig. 1D). CBD inhibited h5-HT3ARs in a manner that was independent of agonist concentration. CBD at 1 µM reduced the amplitude of current activated by 1, 3, 10 and 30 µM 5-HT by 82 ± 10%, 78 ± 9%, 84 ± 11% and 82 ± 13%. These values were not significantly different (one-way ANOVA followed by Dunnett’s post hoc test, p > 0.2, n = 6). Moreover, CBD did not significantly alter the EC50 and slope values of the 5-HT-concentration response curve (2.2 ± 0.2 µM and 1.3 ± 0.2 in the absence of CBD, 2.5 ± 0.1 µM and 1.5 ± 1.0 in the presence of 1 µM CBD, p = 0.2, unpaired t test).
CBD and THC Do Not Directly Stimulate [35S]-GTP-γs Binding
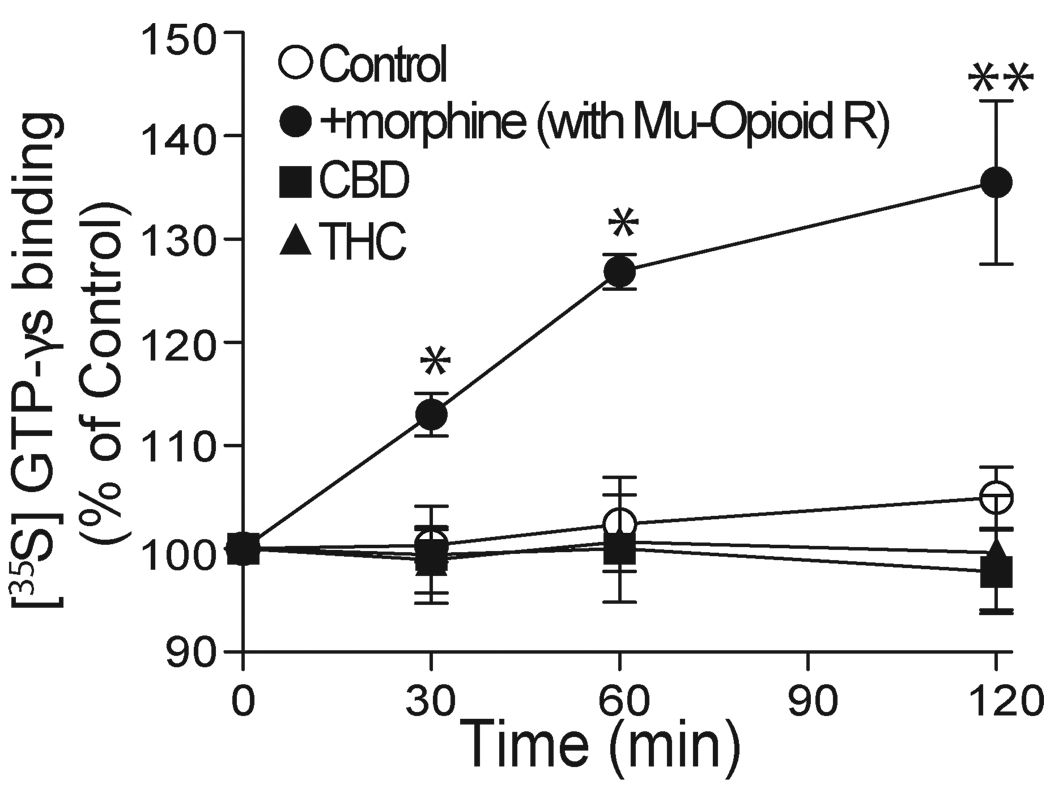
Although CBD is not the agonist of cloned CB1 and CB2 receptors, there is evidence to suggest that CBD may act on certain types of unknown CB-like receptors (Begg et al., 2003). To exclude the possibility that CBD may modulate 5-HT3A receptors by activating unknown G-protein-coupled receptors (GCRPs) endogenously expressed in HEK 293 cells, we directly measured the specific binding of [35S]-GTP-γs in HEK 293 cells. Morphine, a mu-opioid receptor agonist, at 1 µM, stimulated specific [35S]-GTP-γs binding by 13 ± 1%, 27 ± 2% and 36 ± 7% of control at increasing time points after agonist addition in HEK 293 cells expressing mu-opioid receptors (Fig. 2). In contrast, in cells without cotransfection of mu-opioid receptors, morphine did not significantly affect the basal level of specific [35S]-GTP-γs binding (0.3 ± 1%, 2.4 ± 4.5% and 4.9 ± 3%). The values of morphine-induced specific [35S]-GTP-γs binding at each point of incubation time significantly differed in cells with and without cotransfection of mu-opioid receptors (p < 0.004, unpaired t test, n = 5). This finding is in line with a previous observation (Xu et al., 2003). Next, we examined the effects of CBD and THC on specific [35S]-GTP-γs binding at various incubation times. CBD and THC at 1 µM did not significantly stimulate the specific [35S]-GTP-γs binding after 2 hr incubation (Fig. 2).
Fig. 2.
CBD and THC do not stimulate [35S]-GTP-γs binding. Graphs represent the time course of specific [35S]-GTP-γs binding in the presence of 1 µM morphine, CBD and THC in HEK 293 cells without (open circles, control) and with (solid circles) transient transfection of mu-opioid receptor cDNA. Each data point represents the average percent of control from at least 5 separate experiments. Asterisks indicate a significant difference as compared with control. *, P < 0.05; **, P<0.01.
CBD Accelerates Receptor Desensitization
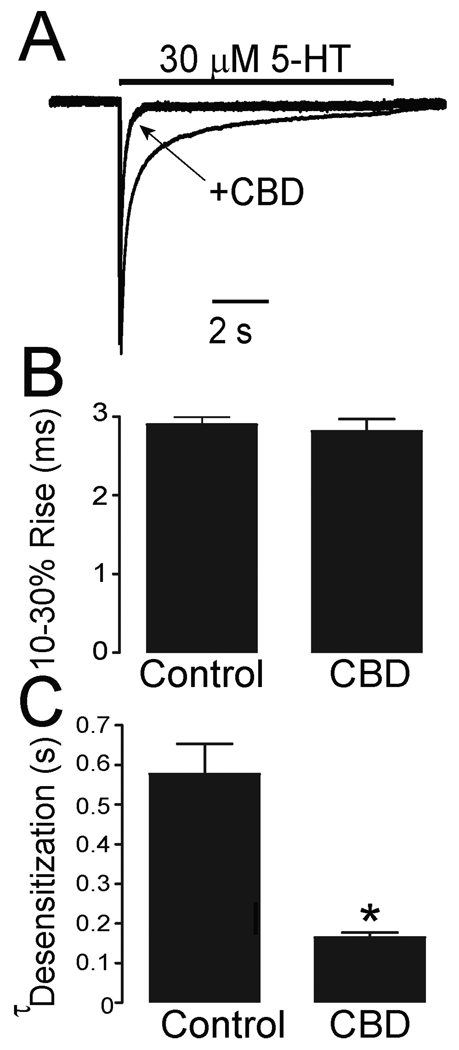
To further explore the mechanisms underlying CBD-induced inhibition, we examined the effects of CBD on the gating kinetics of h5-HT3ARs expressed in HEK 293 cells. In these cells, CBD was found to accelerate the decay of 5-HT-activated currents (Fig. 3A). The values of the 10–30% rise time of 5-HT-activated currents were 2.9 ± 0.1 ms and 2.8 ± 0.2 ms in the absence and presence of 0.1 µM CBD in HEK 293 cells expressing h5-HT3ARs (Fig. 3B). These values were not significantly different (unpaired t test, p = 0.2, n = 11). However, CBD appeared to accelerate the decay of 5-HT-activated current (desensitization) in the continuous presence of 5-HT (Fig. 3C). To properly evaluate the effect of CBD on desensitization kinetics, we normalized the fast and slow components using the weighted sum formula described in ‘Methods’. In HEK 293 cells, the values of the normalized desensitization time constant were 576 ± 81 ms and 181 ± 11 ms in the absence and presence of CBD, suggesting that CBD significantly accelerated the desensitization rate of h5-HT3ARs (unpaired t test, p < 0.001, n = 9).
Fig. 3.
CBD accelerates receptor desensitization kinetics. (A) Tracings of 5-HT-activated currents without and with preincubation of 1 µM CBD for 3 min recorded from a HEK 293 cell expressing h5-HT3ARs. The amplitude of current in the presence of CBD was normalized to that in the absence of CBD. (B) Bar graphs represent the average activation times of 5-HT-activated current with and without preincubation of 1 µM CBD for 3 min. Each data point represents mean ± S.E. from 7 cells. (C) Bar graphs represent the average weighted desensitization time constant of 5-HT-activated current with and without preincubation of 1 µM CBD for 3 min. Each data point represents mean ± S.E. from 7 cells. Asterisks indicate a significant difference as compared with control. *, P < 0.05.
CBD-Induced Inhibition Varies with the Different Receptor Desensitization Rates
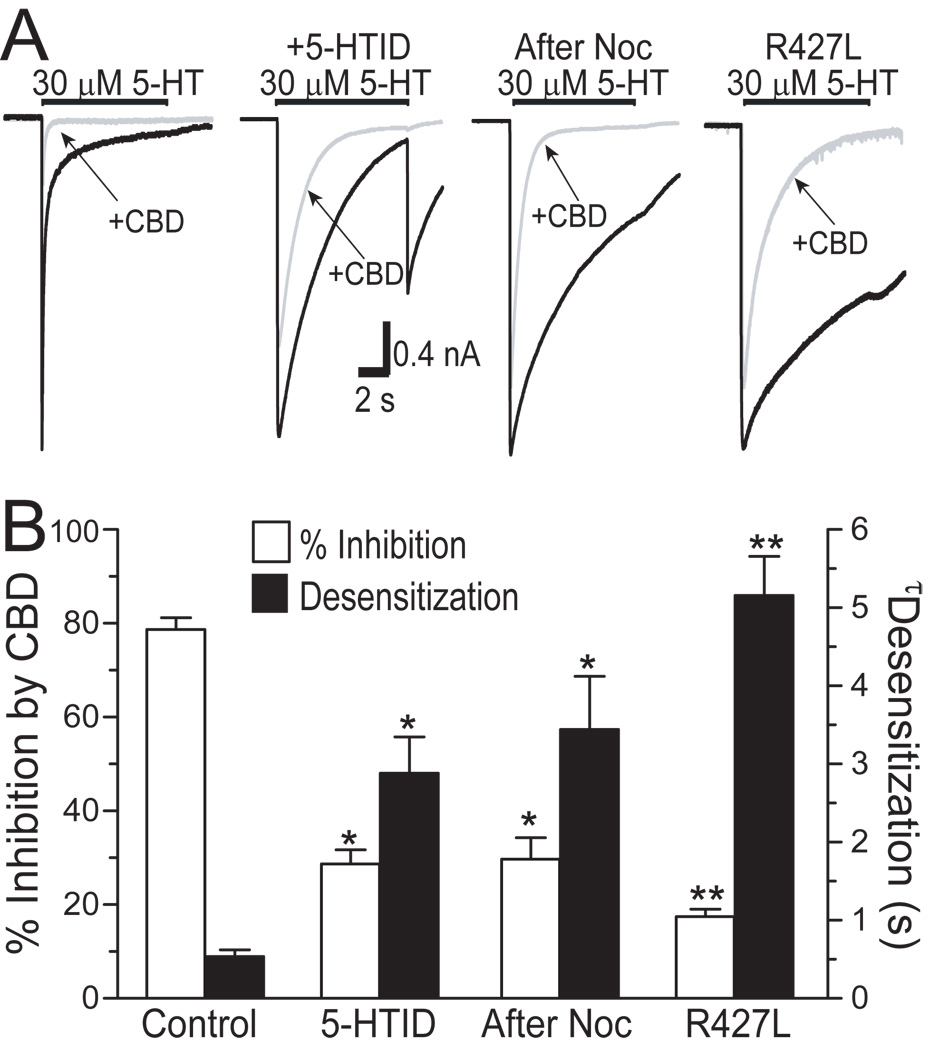
The observations noted above suggest that CBD can accelerate receptor desensitization. We next asked whether altering receptor desensitization will change CBD inhibition. We first treated cells with 5-hydroxyindole (5-HOID) or nocodazole (Noc) since these agents have been found to slow 5-HT3 receptor desensitization (van Hooft and Vijverberg, 1997, Hu et al., 2006, Sun et al., 2008). Moreover, we substituted a positively charged residue, arginine, with a hydrophobic residue, leucine, at position of 427 in the large cytoplasmic domain of the 5-HT3A subunit. This mutation has been reported to slow receptor desensitization (Hu et al., 2006). Consistent with previous studies, application of 5 µM 5-HOID or pretreatment with 25 µM Noc for 4 hrs slowed the decay of 5-HT-activated current (Fig. 4A). The average h5-HT3AR desensitization time was prolonged by ~5–6-fold in the presence of 5-HOID and after Noc treatment (Fig. 4B, solid bars). These pharmacological manipulations also significantly reduced the average percent inhibition by 1 µM CBD from 79 ± 3 % (control) to 28 ± 2% and 29 ± 6% (Fig. 4B; open bars). These values were significantly different (p < 0.01, one-way ANOVA followed by Dunnett’s post hoc test , n = 11). Consistent with our previous study (Hu et al, 2006), the decay of 5-HT-activated current slowed significantly in HEK 293 cells expressing the R427L mutant h5-HT3ARs. In these cells, the average percent inhibition of I5-HT by CBD was only 18 ± 2%, which was significantly less than CBD inhibition of the WT receptors (p < 0.01, one-way ANOVA followed by Dunnett’s post hoc test, n = 9).
Fig. 4.
5-HOID, Noc and the R427L mutation alter receptor desensitization and CBD-induced inhibition. (A) Tracings of 5-HT-activated currents without and with preincubationof 1 µM CBD for 3 min in HEK 293 cells expressing the wild type and the R427L mutant 5-HT3A receptors. 5 µM 5-HOID was applied simultaneously with 5-HT to cells. Cells were pretreated with 25 µM Noc for 4 hrs and washed thoroughly during the recording. (B) The open bars represent the average percent inhibition induced by 1 µM CBD. The solid bars represent average weighted desensitization time constant with and without treatment to alter desensitization. The average weighted time constant of receptor desensitization is the sum of fast and slow components of h5-HT3A receptor, as described in Methods. Each data point represents mean ± S.E. from at least 7 cells. Asterisks indicate a significant difference as compared with control (* p < 0.05; **, p < 0.01).
Levels of Functional Receptor Expression Alter Desensitization Kinetics
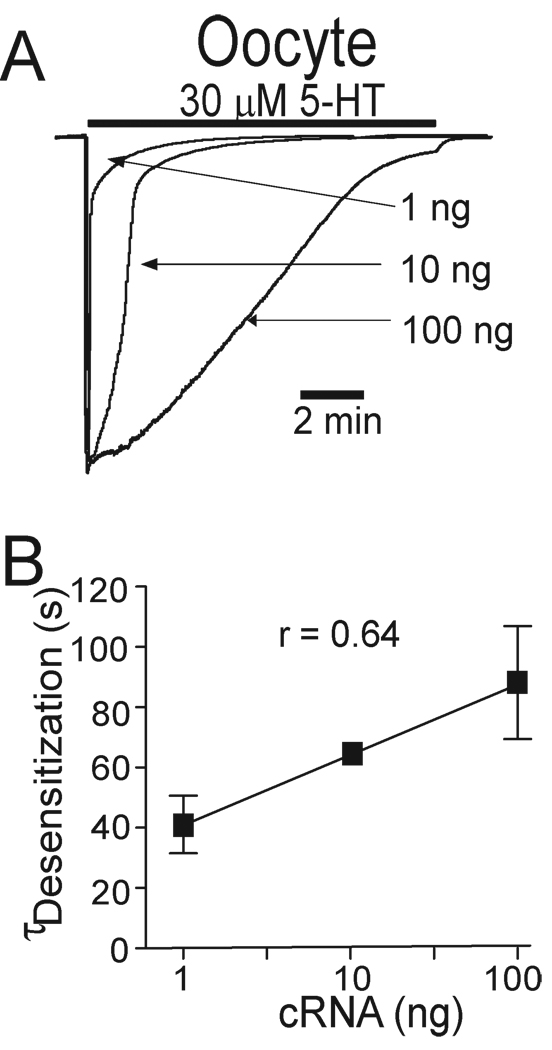
Accumulating evidence has revealed that the desensitization rate of GABAA, glycine and 5-HT3 receptors varies with the steady state receptor density (Chen et al., 2000, Legendre et al., 2002, Boileau et al., 2005, Xiong et al., 2008). Xenopus ooyctes have been widely used as expression system in the studies of 5-HT3 receptor functionality. Moreover, one advantage of using Xenopus oocytes is that the expression level of 5-HT3 receptor protein can be relatively precisely manipulated. Our previous study has shown the relationship between amount of cRNA injected and receptor expression in oocytes (Xiong et al., 2008). So to explore the interrelationship between functional h5-HT3AR expression level, desensitization kinetics and CBD/THC-induced inhibiting effect on I5-HT, we injected different concentrations (1, 10, and 100 ng) of h5-HT3A subunit cRNA into Xenopus oocytes. In these cells, the decays of currents activated by prolonged application of 5-HT appeared to be different (Fig. 5A). With increasing the concentrations of h5-HT3AR cRNAs injected into oocytes, the receptor desensitization time increased (Fig. 5B; r = 0.64, linear regression, p < 0.01). Thus, h5-HT3AR desensitization varied with functional expression of the receptors expressed in Xenopus oocytes.
Fig. 5.
Receptor desensitization kinetics are correlated with functional receptor expression. (A) Tracings of 5-HT-activated currents in Xenopus oocytes injected with different amount of h5-HT3A receptor cRNA. (B) Correlation between the levels of receptor cRNA injection and receptor desensitization time constant (Linear regression, p < 0.01).
CBD and THC-Induced Inhibition Is Correlated with Receptor Expression
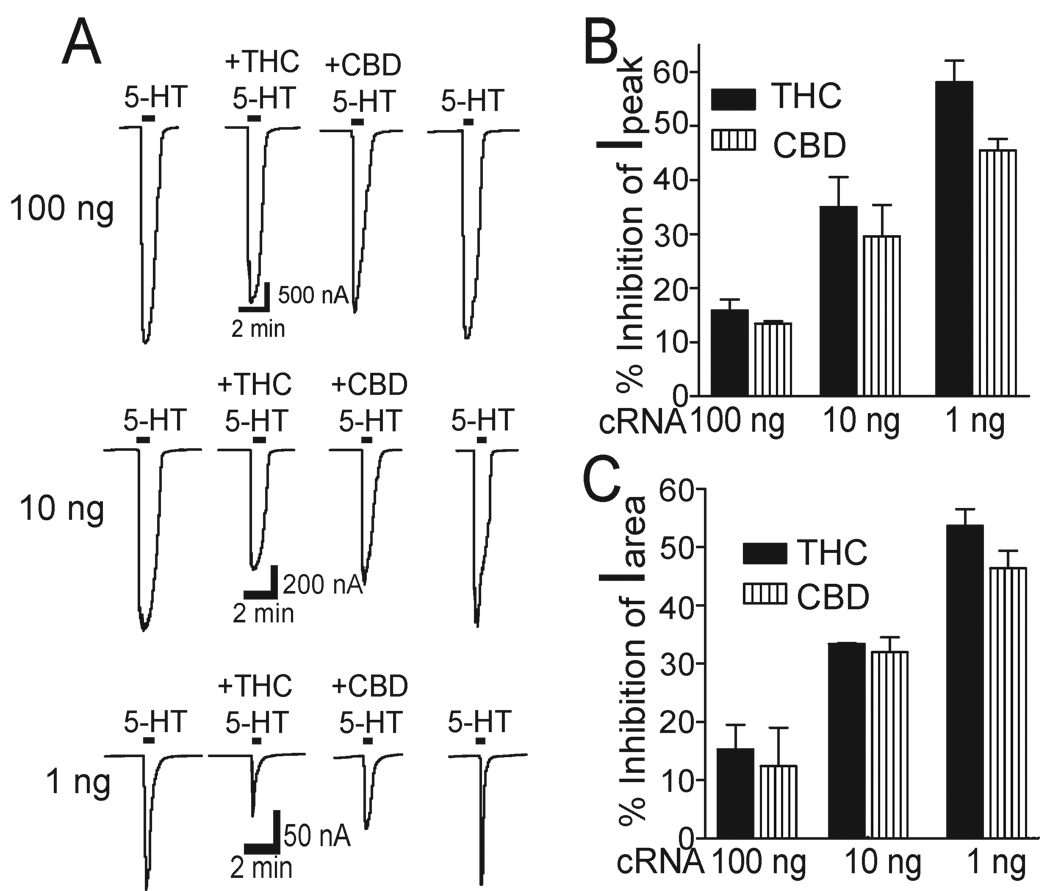
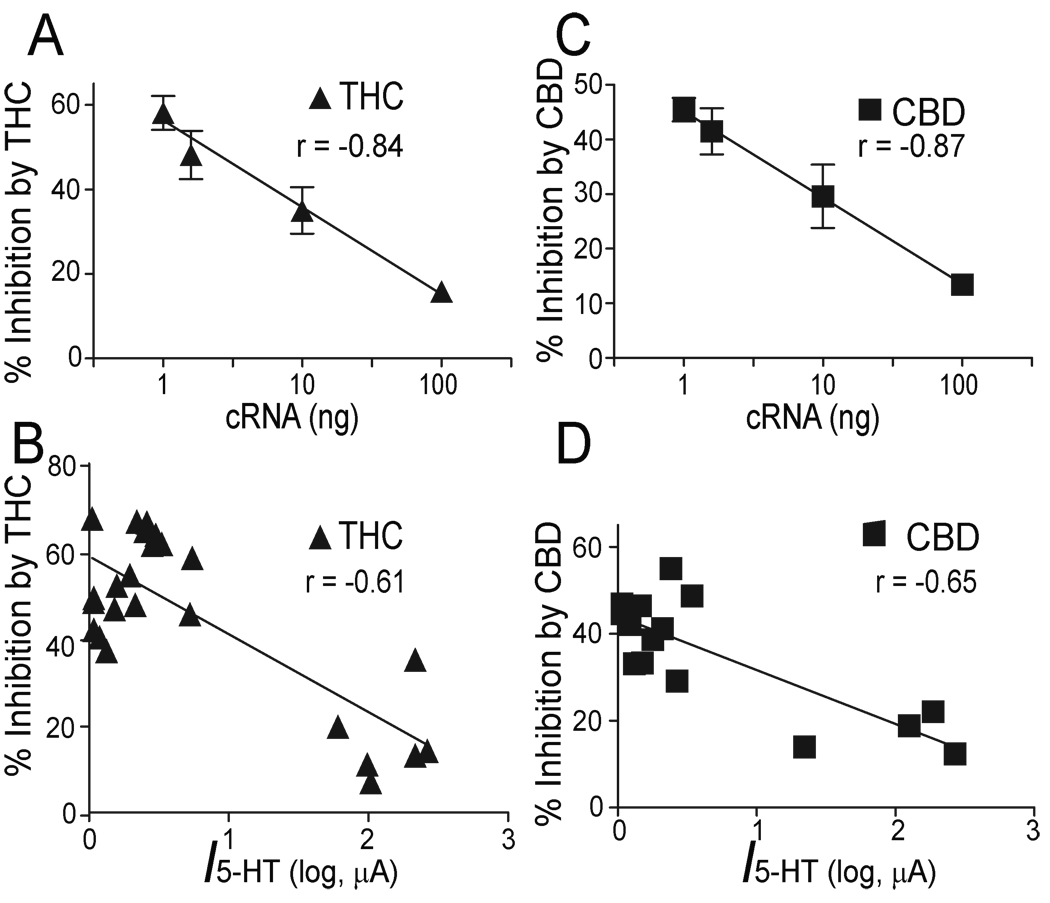
Next we investigated if alteration of receptor density can change CBD inhibition. We observed that THC and CBD inhibited h5-HT3AR-mediated current in a manner that depended on the amount of h5-HT3AR-cRNA injected into the oocytes (Fig. 6A). To investigate the nature of CBD and THC inhibition, we first calculated the average percentage inhibition of 5-HT current peak. The inhibition by CBD and THC was the strongest at the lowest level of receptor expression (Fig. 6B). For instance, the magnitudes of average inhibition by 10 µM CBD and THC were 57 ± 4% and 47 ± 3 % of control in oocytes injected with 1 ng of h5-HT3AR-cRNA, whereas the magnitudes of the inhibition were only 16 ± 2 % and 13 ± 1 % of control in oocytes injected with 100 ng of h5-HT3AR-cRNA. These values were significantly different (one-way ANOVA followed by Dunnett’s post hoc test against, p < 0.001, n = 6). Next, we calculated the average percentage inhibition of 5-HT integral current by measuring current area. The result was found to be similar to that of CBD and THC inhibition of 5-HT current peak (Fig. 6C). Correlation analysis showed that the magnitude of current peak inhibition produced by 10 µM THC was strongly correlated with the amount of the cRNA injected into the oocytes (Fig. 7A linear regression, p < 0.001) and the current activated by 30 µM 5-HT (Fig. 7B; linear regression, p = 0.01). The magnitude of current peak inhibition produced by 10 µM CBD was also strongly correlated with the amount of the cRNA injected into the oocytes (Fig. 7C, linear regression, p < 0.001) and the current activated by 30 µM 5-HT (Fig. 7D; p < 0.01).
Fig. 6.
CBD and THC-induced inhibition varies with the functional receptor expression. (A) Tracings showing preincubation (10 min) of CBD and THC induced inhibition of 5-HT-activated currents in Xenopus oocytes previously injected with 100 ng (Upper), 10 ng (Middle) and 1 ng (Lower) of h5-HT3A receptor cRNA. (B) Bar graphs represent average percent inhibition of peak current by CBD and THC. Each data point represents mean ± S.E. from at least 5 oocytes. (C) Bar graphs represent average percent inhibition of I5-HT area by CBD and THC. Each data point represents mean ± S.E. from at least 5 oocytes.
Fig. 7.
CBD and THC inhibition correlates with the levels of functional receptor expression. (A) Correlation between the magnitude of inhibition induced by 10 µM THC and the concentration of h5-HT3A receptor cRNA injected into oocytes (linear regression, p < 0.001, n = 4). Each data point represents mean ± S.E. from at least 7 oocytes. (B) Correlation between the extent of THC-induced inhibition of 5-HT3A receptors and current amplitude activated by a maximally-effective concentration of 5-HT (100 µM) in oocytes previously injected with different amounts of h5-HT3AR-cRNA (Linear regression, p < 0.01, n = 24). (C) Correlation between the magnitude of inhibition induced by 10 µM CBD and the concentration of h5-HT3AR-cRNA injected into oocytes (linear regression, p < 0.001, n = 4). Each data point represents mean ± S.E. from at least 7 oocytes. (D) Correlation between the extent of CBD-induced inhibition of h5-HT3ARs and current amplitude activated by a maximally-effective concentration of 5-HT (100 µM) in oocytes previously injected with a different amount of h5-HT3AR-cRNA (Linear regression, p < 0.01, n = 15).
CBD and THC-Induced Inhibition Is Correlated with Receptor Desensitization
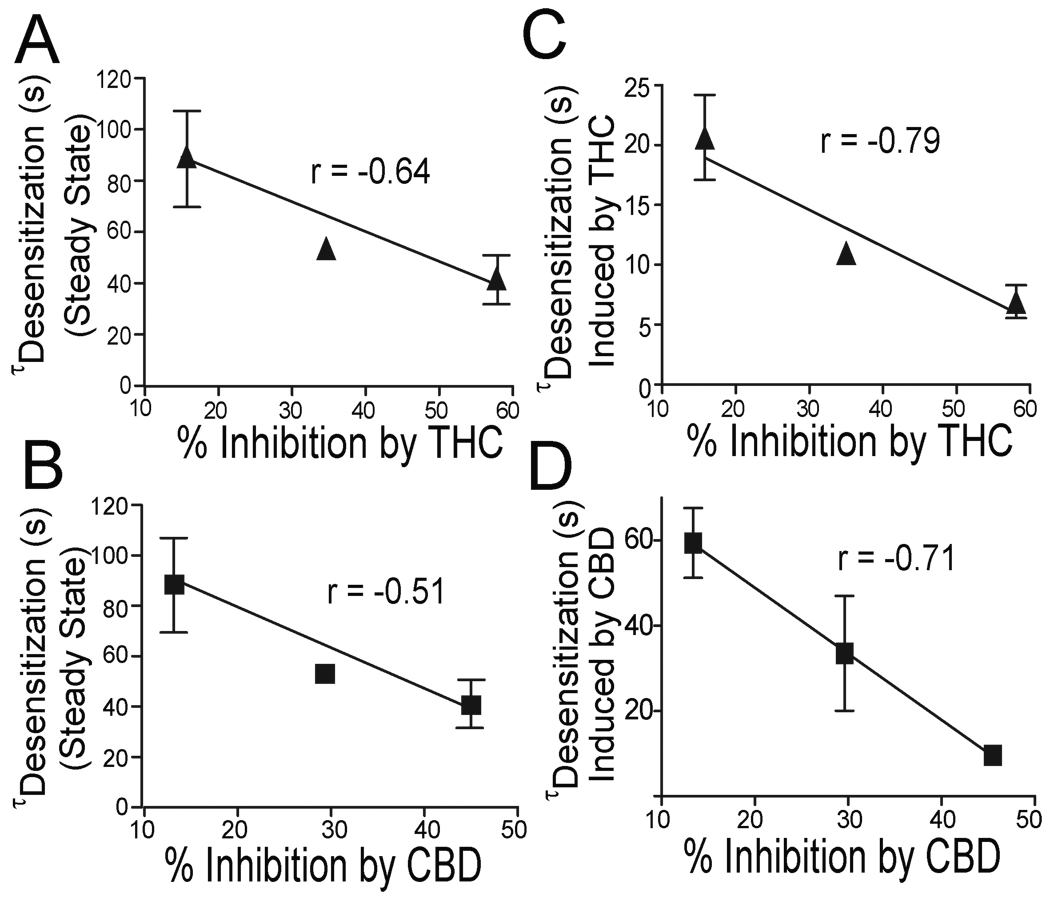
The above observations suggest that the levels of receptor expression alter receptor desensitization and the magnitude of inhibition of h5-HT3ARs induced by THC and CBD. In view of these findings, we predicted that CBD and THC-induced inhibition should be correlated with receptor desensitization in cells injected with different concentrations of h5-HT3AR-cRNAs. Indeed, a correlation was observed between the magnitude of THC (Fig. 8A) or CBD (Fig. 8B)-induced inhibition and receptor desensitization time (linear regression, p < 0.01). Moreover, the receptor desensitization rate was significantly increased after application of THC/CBD respectively. Interestingly, we found a correlation between the THC (Fig.8C) or CBD (Fig.8D)-induced inhibition and the receptor desensitization even after THC/CBD application (linear regression, p < 0.001). In contrast, no significant correlations were found between receptor activation time and THC or CBD-induced inhibition (data not shown).
Fig. 8.
Receptor desensitization correlates with THC and CBD inhibition. (A–B) Correlation between receptor desensitization time constant and average percentage of inhibition by THC/CBD ( Linear regression, p < 0.01). (C–D) Correlation between receptor desensitization after THC/CBD application and average percentage of inhibition by THC/CBD (Linear regression, p < 0.01).
DISCUSSION
The data presented in this study have shown that a non-psychotropic cannabinoid, CBD, inhibited h5-HT3ARs in a concentration-dependent manner. The EC50 for CBD inhibition was 310 nM, which is within the concentration range (0.1–1 µM) of CBD to produce neuroprotection, anticonvulsant, anxiolytic and antipsychotic effects (Pertwee, 2005). The inhibition by CBD was independent of agonist concentration, suggesting that CBD is an apparent non-competitive inhibitor of 5-HT3ARs. This is consistent with previous findings on THC inhibition of 5-HT3A receptors (Barann et al., 2002). Although CBD shares a high degree of structural similarity with THC, there is strong evidence that CBD does not activate CB1 and CB2 receptors (Pertwee, 2005). It is also unlikely that CBD-induced inhibition of h5-HT3ARs is mediated via endogenous cannabinoid and capsaicin receptors since CBD did not stimulate [35S]-GTP-γs binding.
The precise mechanism underlying CBD and THC inhibition of 5-HT3Rs is unclear. Our study has suggested that receptor desensitization critically contributes to the magnitude of cannabinoid inhibition. Both THC and CBD accelerated receptor desensitization. The sensitivity of h5-HT3ARs to CBD and THC-induced inhibition varied with different receptor desensitization rates. For example, Noc and 5-HOID treatment slowed 5-HT3A receptor desensitization and significantly reduced CBD and THC-induced inhibition. Similarly, a point-mutation of R427 in the large cytoplasmic domain of the 5-HT3A subunit was also found to slow receptor desensitization and attenuate CBD inhibition. Conversely, reducing receptor expression at the cell surface accelerated receptor desensitization kinetics in Xenopus oocytes expressing h5-HT3ARs. In these cells, CBD-induced inhibition of I5-HT increased. Overall, the magnitude of CBD and THC inhibition was correlated with the extent of receptor desensitization.
Desensitization plays a critical role in the regulation of neuronal excitability and the efficacy of synaptic transmission (Jones and Westbrook, 1996, Giniatullin et al., 2005). CBD and THC altered 5-HT3A receptor desensitization kinetics without changing activation kinetics. This observation is consistent with a previous study showing that THC did not alter the specific binding of the 5-HT3 receptor antagonist [3H]-GR65630 in HEK 293 cells expressing h5-HT3ARs (Barann et al., 2002). It is plausible to predict that because CBD and THC inhibit 5-HT3ARs, at least in part, by accelerating the rate of receptor desensitization, the energy barrier that governs the channels from the open state to the desensitization state is critical for the potency of CBD and THC.
Our study has shown that the ability of CBD and THC to inhibit 5-HT3ARs varies with receptor density at the cell membrane surface. This observation is potentially important since there are multiple factors regulating 5-HT3AR density at synaptic membrane surfaces under physiological and pathological processes. For instance, the expression levels of surface 5-HT3ARs were altered by activation of protein kinases (Sun et al., 2003), cytoskeleton proteins (Emerit et al., 2005, Niesler et al., 2007) and naturally occurring genetic variants (Niesler et al., 2007). The expression level of 5-HT3Rs was elevated in serotonin transporter deficient mice, suggesting that the extracellular concentration of 5-HT also influenced receptor density (Mossner et al., 2004). Collectively, these factors could determine the sensitivity of 5-HT3Rs to cannabinoid-induced inhibiting effect.
How receptor density change alters the cannabinoid inhibition is unclear. One possible explanation is that low density receptors tend to enter the desensitization state with less energy barrier. This idea is consistent by a recent study showing that overexpression of the light chain of the microtubule-associated protein 1B reduced steady state receptor density at the cell surface and accelerated receptor desensitization kinetics (Sun et al., 2008). Similar observations were reported in the studies of GABAARs in which receptor desensitization was modulated by different levels of the steady state density of GABAA receptors at the cell surfaces (Chen et al., 2000, Petrini et al., 2003, Boileau et al., 2005). There is also evidence showing that desensitization of glycine receptors varies with receptor density (Legendre et al., 2002).
One can ask if CBD and THC are open channel blockers (OCB) of 5-HT3 receptors because the inhibition induced by both compounds developed and reversed slowly. However, our experimental data do not favor this idea. An OCB should maximize its blocking effect when channels open. With an OCB the inhibiton of peak current should be greater at higher agonist concentrations, as the availability of the binding site increase with increasing concentration. In addition, blocking a channel at the open state by a given OCB usually slows receptor desensitization. Neither of these scenarios occurred in CBD-induced inhibition as CBD inhibition was independent on agonist concentrations and was associated with an acceleration of receptor desensitization. Moreover, the blocking effect induced by CBD appeared to develop before 5-HT was present, which does not fit to the use-dependence, a typical character of an OCB.
CONCLUSION
We conclude that CBD inhibits h5-HT3ARs and accelerates receptor desensitization. The ability of inhibition by CBD and THC is highly dependent on receptor desensitization rate. Such a mechanism could be applicable to the effects of endo/exocannabinoid-induced modulation of other members of the LGIC superfamily since anandamide has been shown to accelerate 5-HT3A, glycine and nAChR desensitization (Barann et al., 2002, Lozovaya et al., 2005, Spivak et al., 2007). Both CBD and THC exert strong antinociceptive and antiemetic effects (Pacher et al., 2006). Inhibition of h5-HT3ARs by CBD and THC may contribute to the therapeutic mechanisms of cannabinoids for control of pain, emesis and other neurological disorders.
Acknowledgements
We thank Dr. David M. Lovinger for help with critical comments. This work was supported by funds from the intramural program of National Institute on Alcohol Abuse and Alcoholism.
ABBREVIATIONS
- THC
Δ9 tetrahydrocannabinol
- CBD
cannabidiol
- AEA
anandamide
- ANOVA
analysis of variance
- DA
dopamine
- 5-HT
5-hydroxytryptophan
- GABA
γ-aminobutyric acid
- TM
transmembrane domain
- VTA
ventral tegmental area
- WT
wild type
- Noc
nocodazole
- 5-HOID
5-hydroxyindole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest.
REFERENCES
- Barann M, Molderings G, Bruss M, Bonisch H, Urban BW, Gothert M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br J Pharmacol. 2002;137:589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertaler L, Batkai S, Pacher P, Razdan RK, Lovinger DM, Kunos G. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J Biol Chem. 2003;278:46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Pearce RA, Czajkowski C. Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein. J Neurosci. 2005;25:11219–11230. doi: 10.1523/JNEUROSCI.3751-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang H, Vicini S, Olsen RW. The gamma-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc Natl Acad Sci U S A. 2000;97:11557–11562. doi: 10.1073/pnas.190133497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico AM, Lovinger DM. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev. 2009;61:98–114. doi: 10.1124/pr.108.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerit MB, Sun H, Hu X-Q, Schoenebeck JC, Peoples RW, Miko A, Williams CK, L Z. 5-HT3 receptor linkage to cytoskeleton by MAP1B determines receptor desensitization kinetics. Abst Soc Neurosci. 2005;844:817. [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Grant KA. The role of 5-HT3 receptors in drug dependence. Drug Alcohol Depend. 1995;38:155–171. doi: 10.1016/0376-8716(95)01120-n. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−) Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier G, Tredt C, Haseneder R, Zieglgansberger W, Eisensamer B, Rupprecht R, Rammes G. Co-expression of the 5-HT3B serotonin receptor subunit alters the biophysics of the 5-HT3 receptor. Biophys J. 2003;84:1720–1733. doi: 10.1016/S0006-3495(03)74980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XQ, Lovinger DM. Role of aspartate 298 in mouse 5-HT3A receptor gating and modulation by extracellular Ca2+ J Physiol. 2005 doi: 10.1113/jphysiol.2005.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XQ, Peoples RW. Arginine 246 of the pretransmembrane domain 1 region alters 2,2,2-trichloroethanol action in the 5-hydroxytryptamine3A receptor. J Pharmacol Exp Ther. 2008;324:1011–1018. doi: 10.1124/jpet.107.131011. [DOI] [PubMed] [Google Scholar]
- Hu XQ, Sun H, Peoples RW, Hong R, Zhang L. An interaction involving an arginine residue in the cytoplasmic domain of the 5-HT3A receptor contributes to receptor desensitization mechanism. J Biol Chem. 2006;281:21781–21788. doi: 10.1074/jbc.M600676200. [DOI] [PubMed] [Google Scholar]
- Hubbard PC, Thompson AJ, Lummis SC. Functional differences between splice variants of the murine 5-HT(3A) receptor: possible role for phosphorylation. Brain Res Mol Brain Res. 2000;81:101–108. doi: 10.1016/s0169-328x(00)00138-8. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- Legendre P, Muller E, Badiu CI, Meier J, Vannier C, Triller A. Desensitization of homomeric alpha1 glycine receptor increases with receptor density. Mol Pharmacol. 2002;62:817–827. doi: 10.1124/mol.62.4.817. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Schneider U, Radwan M, Schmidt E, Emrich HM. Different effects of nabilone and cannabidiol on binocular depth inversion in Man. Pharmacol Biochem Behav. 2000;66:175–181. doi: 10.1016/s0091-3057(00)00201-x. [DOI] [PubMed] [Google Scholar]
- Lozovaya N, Yatsenko N, Beketov A, Tsintsadze T, Burnashev N. Glycine receptors in CNS neurons as a target for nonretrograde action of cannabinoids. J Neurosci. 2005;25:7499–7506. doi: 10.1523/JNEUROSCI.0977-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Morales M, Wang SD. Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci. 2002;22:6732–6741. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossner R, Schmitt A, Hennig T, Benninghoff J, Gerlach M, Riederer P, Deckert J, Lesch KP. Quantitation of 5HT3 receptors in forebrain of serotonin transporter deficient mice. J Neural Transm. 2004;111:27–35. doi: 10.1007/s00702-003-0074-y. [DOI] [PubMed] [Google Scholar]
- Niesler B, Walstab J, Combrink S, Moeller D, Kapeller J, Rietdorf J, Boenisch H, Goethert M, Rappold G, Bruess M. Characterization of the Novel Human Serotonin Receptor Subunits 5-HT3C, 5- HT3D and 5-HT3E. Mol Pharmacol. 2007 doi: 10.1124/mol.106.032144. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The pharmamcology and therapeutic potential of cannabidiol: Landes Bioscience. 2005 [Google Scholar]
- Petrini EM, Zacchi P, Barberis A, Mozrzymas JW, Cherubini E. Declusterization of GABAA receptors affects the kinetic properties of GABAergic currents in cultured hippocampal neurons. J Biol Chem. 2003;278:16271–16279. doi: 10.1074/jbc.M213081200. [DOI] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Markert A, Stamer F, Gothert M, Zimmer A. Anandamide effects on 5-HT(3) receptors in vivo. Eur J Pharmacol. 2008;596:98–101. doi: 10.1016/j.ejphar.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G. Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytother Res. 2009;23:597–602. doi: 10.1002/ptr.2625. [DOI] [PubMed] [Google Scholar]
- Spivak CE, Lupica CR, Oz M. The Endocannabinoid Anandamide Inhibits the Function of {alpha}4{beta}2 Nicotinic Acetylcholine Receptors. Mol Pharmacol. 2007 doi: 10.1124/mol.107.036939. [DOI] [PubMed] [Google Scholar]
- Sun H, Hu XQ, Emerit MB, Schoenebeck JC, Kimmel CE, Peoples RW, Miko A, Zhang L. Modulation of 5-HT3 receptor desensitization by the light chain of microtubule-associated protein 1B expressed in HEK 293 cells. J Physiol. 2008;586:751–762. doi: 10.1113/jphysiol.2007.136440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Hu XQ, Moradel EM, Weight FF, Zhang L. Modulation of 5-HT3 receptor-mediated response and trafficking by activation of protein kinase C. J Biol Chem. 2003;278:34150–34157. doi: 10.1074/jbc.M303584200. [DOI] [PubMed] [Google Scholar]
- Sun H, Xiong W, Koo BDL, Zhang L. Psychotropic and Nonpsychotropic Cannabis Derivatives Inhibit Human 5-HT3A receptors via a Receptor Desensitization-Dependent Mechanism. Society for Neuroscience annunal meeting. 2009 doi: 10.1016/j.neuroscience.2011.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DD, Thayer SASA, Brown DRDR. Cannabinoids throw up a conundrum. British journal of pharmacology. 2002;137:575. doi: 10.1038/sj.bjp.0704913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooft JA, van der Haar E, Vijverberg HP. Allosteric potentiation of the 5-HT3 receptor-mediated ion current in N1E-115 neuroblastoma cells by 5-hydroxyindole and analogues. Neuropharmacology. 1997;36:649–653. doi: 10.1016/s0028-3908(97)00045-2. [DOI] [PubMed] [Google Scholar]
- van Hooft JA, Vijverberg HP. Full and partial agonists induce distinct desensitized states of the 5-HT3 receptor. J Recept Signal Transduct Res. 1997;17:267–277. doi: 10.3109/10799899709036608. [DOI] [PubMed] [Google Scholar]
- Xiong W, Hosoi M, Koo BN, Zhang L. Anandamide inhibition of 5-HT3A receptors varies with receptor density and desensitization. Mol Pharmacol. 2008;73:314–322. doi: 10.1124/mol.107.039149. [DOI] [PubMed] [Google Scholar]
- Xu H, Lu YF, Rothman RB. Opioid peptide receptor studies. 16. Chronic morphine alters G-protein function in cells expressing the cloned mu opioid receptor. Synapse. 2003;47:1–9. doi: 10.1002/syn.10144. [DOI] [PubMed] [Google Scholar]
- Yakel JL, Shao XM, Jackson MB. Activation and desensitization of the 5-HT3 receptor in a rat glioma x mouse neuroblastoma hybrid cell. J Physiol. 1991;436:293–308. doi: 10.1113/jphysiol.1991.sp018551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lummis SC. 5-HT3 receptors. Marcell Dekker, Inc.; 2006. [Google Scholar]
- Zhou Q, Verdoorn TA, Lovinger DM. Alcohols potentiate the function of 5-HT3 receptor-channels on NCB-20 neuroblastoma cells by favouring and stabilizing the open channel state. J Physiol. 1998;507(Pt 2):335–352. doi: 10.1111/j.1469-7793.1998.335bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]