Abstract
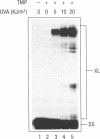
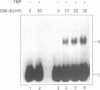
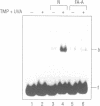
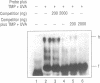
A DNA binding protein with specificity for DNA containing interstrand cross-links induced by 4,5',8-trimethylpsoralen (TMP) plus long wavelength ultraviolet (UVA) light has been identified in normal human chromatin. Protein binding to DNA was determined using a gel mobility shift assay and an oligonucleotide containing a hot spot for formation of psoralen interstrand cross-links. Specificity of the damage-recognition protein for cross-links was demonstrated both by a positive correlation between level of cross-link formation in DNA and extent of protein binding and by effective competition by treated but not undamaged DNA for the binding protein. Chromatin protein extracts from cells from individuals with the genetic disorder, Fanconi anemia, complementation group A (FA-A), which have decreased ability to repair damage produced by TMP plus UVA light, failed to show any protein binding to TMP plus UVA treated DNA. We have previously shown that these chromatin protein extracts contain a DNA endonuclease complex, pI 4.6, which specifically recognizes and incises DNA containing interstrand cross-links and which in FA-A cells is defective in its ability to incise this damaged DNA (Lambert et al. (1992) Mutation Res., 273, 57-71). Together, these findings suggest that the DNA binding protein identified is involved in recognition and repair of DNA interstrand cross-links.
Full text
PDF


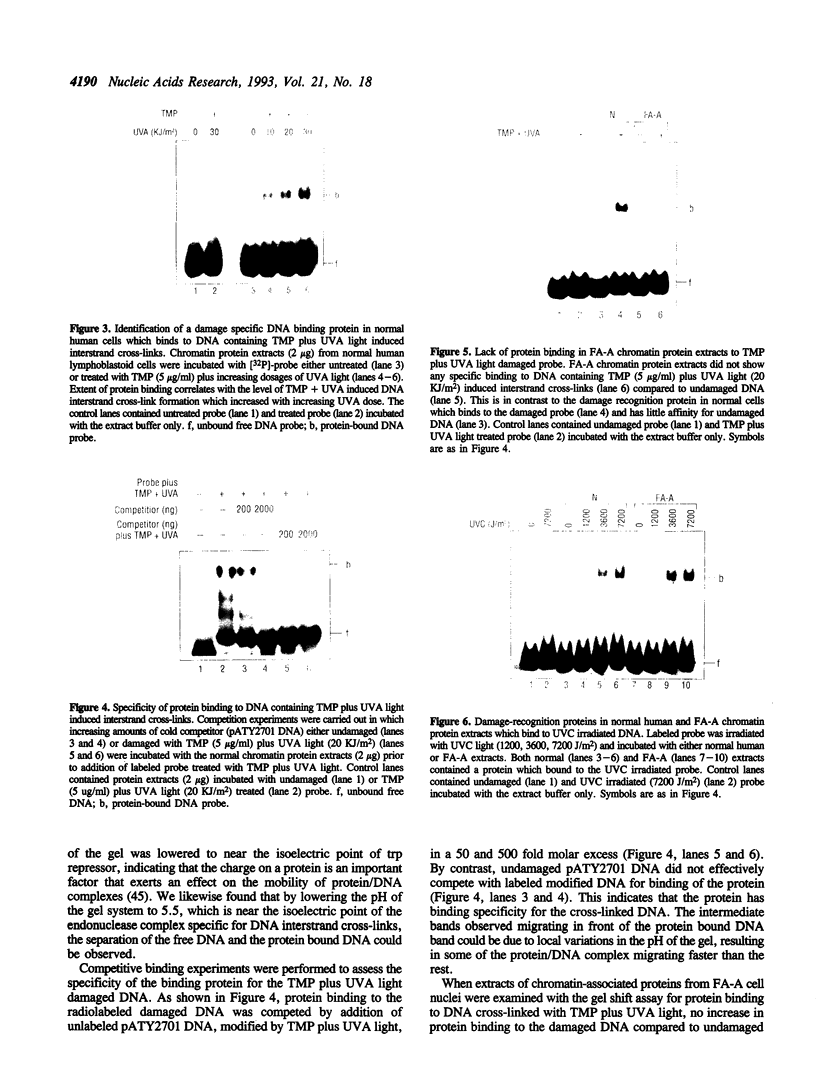


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A. D., Rogatko A., Schroeder-Kurth T. M. International Fanconi Anemia Registry: relation of clinical symptoms to diepoxybutane sensitivity. Blood. 1989 Feb;73(2):391–396. [PubMed] [Google Scholar]
- Averbeck D., Papadopoulo D., Moustacchi E. Repair of 4,5',8-trimethylpsoralen plus light-induced DNA damage in normal and Fanconi's anemia cell lines. Cancer Res. 1988 Apr 15;48(8):2015–2020. [PubMed] [Google Scholar]
- Baden H. P., Parrington J. M., Delhanty J. D., Pathak M. A. DNA synthesis in normal and xeroderma pigmentosum fibroblasts following treatment with 8-methoxypsoralen and long wave ultraviolet light. Biochim Biophys Acta. 1972 Mar 24;262(3):247–255. doi: 10.1016/0005-2787(72)90260-2. [DOI] [PubMed] [Google Scholar]
- Bredberg A., Lambert B., Söderhäll S. Induction and repair of psoralen cross-links in DNA of normal human and xeroderma pigmentosum fibroblasts. Mutat Res. 1982 Mar;93(1):221–234. doi: 10.1016/0027-5107(82)90137-3. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Chao C. C. Characterization of a UV-damage recognition factor in vitro that is associated with UV resistance in HeLa cells. Mutat Res. 1992 Feb;281(2):105–113. doi: 10.1016/0165-7992(92)90044-i. [DOI] [PubMed] [Google Scholar]
- Chao C. C., Huang S. L., Lee L. Y., Lin-Chao S. Identification of inducible damage-recognition proteins that are overexpressed in HeLa cells resistant to cis-diamminedichloroplatinum (II). Biochem J. 1991 Aug 1;277(Pt 3):875–878. doi: 10.1042/bj2770875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988 Oct 28;242(4878):564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Digweed M., Zakrzewski-Lüdcke S., Sperling K. Fanconi's anaemia: correlation of genetic complementation group with psoralen/UVA response. Hum Genet. 1988 Jan;78(1):51–54. doi: 10.1007/BF00291234. [DOI] [PubMed] [Google Scholar]
- Donahue B. A., Augot M., Bellon S. F., Treiber D. K., Toney J. H., Lippard S. J., Essigmann J. M. Characterization of a DNA damage-recognition protein from mammalian cells that binds specifically to intrastrand d(GpG) and d(ApG) DNA adducts of the anticancer drug cisplatin. Biochemistry. 1990 Jun 19;29(24):5872–5880. doi: 10.1021/bi00476a032. [DOI] [PubMed] [Google Scholar]
- Feldberg R. S. On the substrate specificity of a damage-specific DNA binding protein from human cells. Nucleic Acids Res. 1980 Mar 11;8(5):1133–1143. doi: 10.1093/nar/8.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenert D. C., Cleaver J. E. Repair of psoralen-induced cross-links and monoadducts in normal and repair-deficient human fibroblasts. Cancer Res. 1985 Nov;45(11 Pt 1):5399–5404. [PubMed] [Google Scholar]
- Gruenert D. C., Cleaver J. E. Repair of psoralen-induced cross-links and monoadducts in normal and repair-deficient human fibroblasts. Cancer Res. 1985 Nov;45(11 Pt 1):5399–5404. [PubMed] [Google Scholar]
- Hasegawa S. L., Doetsch P. W., Hamilton K. K., Martin A. M., Okenquist S. A., Lenz J., Boss J. M. DNA binding properties of YB-1 and dbpA: binding to double-stranded, single-stranded, and abasic site containing DNAs. Nucleic Acids Res. 1991 Sep 25;19(18):4915–4920. doi: 10.1093/nar/19.18.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld S., Levine A. S., Ozato K., Protić M. A constitutive damage-specific DNA-binding protein is synthesized at higher levels in UV-irradiated primate cells. Mol Cell Biol. 1990 May;10(5):2041–2048. doi: 10.1128/mcb.10.5.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E. N., Engelsberg B. N., Billings P. C. Purification of nuclear proteins that bind to cisplatin-damaged DNA. Identity with high mobility group proteins 1 and 2. J Biol Chem. 1992 Jul 5;267(19):13520–13527. [PubMed] [Google Scholar]
- Jiricny J., Hughes M., Corman N., Rudkin B. B. A human 200-kDa protein binds selectively to DNA fragments containing G.T mismatches. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8860–8864. doi: 10.1073/pnas.85.23.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. K., Yeung A. T. DNA base composition determines the specificity of UvrABC endonuclease incision of a psoralen cross-link. J Biol Chem. 1990 Feb 25;265(6):3489–3496. [PubMed] [Google Scholar]
- Kaye J., Smith C. A., Hanawalt P. C. DNA repair in human cells containing photoadducts of 8-methoxypsoralen or angelicin. Cancer Res. 1980 Mar;40(3):696–702. [PubMed] [Google Scholar]
- Klocker H., Burtscher H. J., Auer B., Hirsch-Kauffmann M., Schweiger M. Fibroblasts from patients with Fanconi's anemia are not deficient in excision of thymine dimer. Eur J Cell Biol. 1985 May;37:240–242. [PubMed] [Google Scholar]
- Lambert M. W., Fenkart D., Clarke M. Two DNA endonuclease activities from normal human and xeroderma pigmentosum chromatin active on psoralen plus ultraviolet light treated DNA. Mutat Res. 1988 Jan;193(1):65–73. doi: 10.1016/0167-8817(88)90008-9. [DOI] [PubMed] [Google Scholar]
- Lambert M. W., Lee D. E., Okorodudu A. O., Lambert W. C. Nuclear deoxyribonuclease activities in human lymphoblastoid and mouse melanoma cells. A comparative study. Biochim Biophys Acta. 1982 Dec 31;699(3):192–202. doi: 10.1016/0167-4781(82)90107-5. [DOI] [PubMed] [Google Scholar]
- Lambert M. W., Tsongalis G. J., Lambert W. C., Hang B., Parrish D. D. Defective DNA endonuclease activities in Fanconi's anemia cells, complementation groups A and B. Mutat Res. 1992 Jan;273(1):57–71. doi: 10.1016/0921-8777(92)90050-d. [DOI] [PubMed] [Google Scholar]
- Lambert W. C., Lambert M. W. Co-recessive inheritance: a model for DNA repair and other surveillance genes in higher eukaryotes. Mutat Res. 1992 Mar;273(2):179–192. doi: 10.1016/0921-8777(92)90079-i. [DOI] [PubMed] [Google Scholar]
- Lambert W. C., Lambert M. W. Co-recessive inheritance: a model for DNA repair, genetic disease and carcinogenesis. Mutat Res. 1985 May;145(3):227–234. doi: 10.1016/0167-8817(85)90031-8. [DOI] [PubMed] [Google Scholar]
- Lenz J., Okenquist S. A., LoSardo J. E., Hamilton K. K., Doetsch P. W. Identification of a mammalian nuclear factor and human cDNA-encoded proteins that recognize DNA containing apurinic sites. Proc Natl Acad Sci U S A. 1990 May;87(9):3396–3400. doi: 10.1073/pnas.87.9.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moranelli F., Lieberman M. W. Recognition of chemical carcinogen-modified DNA by a DNA-binding protein. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3201–3205. doi: 10.1073/pnas.77.6.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustacchi E., Papadopoulo D., Diatloff-Zito C., Buchwald M. Two complementation groups of Fanconi's anemia differ in their phenotypic response to a DNA-crosslinking treatment. Hum Genet. 1987 Jan;75(1):45–47. doi: 10.1007/BF00273837. [DOI] [PubMed] [Google Scholar]
- Okorodudu A. O., Lambert W. C., Lambert M. W. Nuclear deoxyribonuclease activities in normal and xeroderma pigmentosum lymphoblastoid cells. Biochem Biophys Res Commun. 1982 Sep 30;108(2):576–584. doi: 10.1016/0006-291x(82)90867-1. [DOI] [PubMed] [Google Scholar]
- Parrish D. D., Lambert M. W. Chromatin-associated DNA endonucleases from xeroderma pigmentosum cells are defective in interaction with damaged nucleosomal DNA. Mutat Res. 1990 Mar;235(2):65–80. doi: 10.1016/0921-8777(90)90059-e. [DOI] [PubMed] [Google Scholar]
- Parrish D. D., Lambert W. C., Lambert M. W. Xeroderma pigmentosum endonuclease complexes show reduced activity on and affinity for psoralen cross-linked nucleosomal DNA. Mutat Res. 1992 Mar;273(2):157–170. doi: 10.1016/0921-8777(92)90077-g. [DOI] [PubMed] [Google Scholar]
- Pil P. M., Lippard S. J. Specific binding of chromosomal protein HMG1 to DNA damaged by the anticancer drug cisplatin. Science. 1992 Apr 10;256(5054):234–237. doi: 10.1126/science.1566071. [DOI] [PubMed] [Google Scholar]
- Plooy A. C., van Dijk M., Berends F., Lohman P. H. Formation and repair of DNA interstrand cross-links in relation to cytotoxicity and unscheduled DNA synthesis induced in control and mutant human cells treated with cis-diamminedichloroplatinum(II). Cancer Res. 1985 Sep;45(9):4178–4184. [PubMed] [Google Scholar]
- Rydberg B., Dosanjh M. K., Singer B. Human cells contain protein specifically binding to a single 1,N6-ethenoadenine in a DNA fragment. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6839–6842. doi: 10.1073/pnas.88.15.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Franklin K. A., Sancar G. B. Escherichia coli DNA photolyase stimulates uvrABC excision nuclease in vitro. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7397–7401. doi: 10.1073/pnas.81.23.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale B., Singh S., Khanna K. K., Findik D., Lavin M. F. Purification and characterization of a DNA-binding protein activated by ionizing radiation. J Biol Chem. 1992 May 25;267(15):10295–10301. [PubMed] [Google Scholar]
- Treiber D. K., Chen Z., Essigmann J. M. An ultraviolet light-damaged DNA recognition protein absent in xeroderma pigmentosum group E cells binds selectively to pyrimidine (6-4) pyrimidone photoproducts. Nucleic Acids Res. 1992 Nov 11;20(21):5805–5810. doi: 10.1093/nar/20.21.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang S. S., Kuhnlein U. DNA-binding protein from HeLa cells that binds preferentially to supercoiled DNA damaged by ultraviolet light or N-acetoxy-N-acetyl-2-aminofluorene. Biochim Biophys Acta. 1982 May 31;697(2):202–212. doi: 10.1016/0167-4781(82)90078-1. [DOI] [PubMed] [Google Scholar]
- Tsongalis G. J., Lambert W. C., Lambert M. W. Electroporation of normal human DNA endonucleases into xeroderma pigmentosum cells corrects their DNA repair defect. Carcinogenesis. 1990 Mar;11(3):499–503. doi: 10.1093/carcin/11.3.499. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Sancar A., Hearst J. E. DNase I footprint of ABC excinuclease. J Biol Chem. 1987 Sep 25;262(27):13180–13187. [PubMed] [Google Scholar]
- Vigny P., Gaboriau F., Voituriez L., Cadet J. Chemical structure of psoralen-nucleic acid photoadducts. Biochimie. 1985 Mar-Apr;67(3-4):317–325. doi: 10.1016/s0300-9084(85)80074-2. [DOI] [PubMed] [Google Scholar]
- Wunder E., Fleischer-Reischmann B. Response of lymphocytes from Fanconi's anemia patients and their heterozygous relatives to 8-methoxy-psoralene in a cloning survival test system. Hum Genet. 1983;64(2):167–172. doi: 10.1007/BF00327118. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Miller C. G. A general method of optimizing automated DNA synthesis to decrease chemical consumption to less than half. Anal Biochem. 1990 May 15;187(1):66–75. doi: 10.1016/0003-2697(90)90418-9. [DOI] [PubMed] [Google Scholar]
- Zytkovicz T. H. Identification and characterization of a high-affinity saturable binding protein for the carcinogen benzo(a)pyrene. Cancer Res. 1982 Nov;42(11):4387–4393. [PubMed] [Google Scholar]