SUMMARY
Normal cells require continuous exposure to growth factors, in order to cross a restriction point and commit to cell cycle progression. This can be replaced by two short, appropriately spaced pulses of growth factors, where the first pulse primes a process, which is completed by the second pulse, and enables restriction point crossing. Through integration of comprehensive proteomic and transcriptomic analyses of each pulse, we identified three processes that regulate restriction point crossing: (i) The first pulse induces essential metabolic enzymes and activates p53-dependent restraining processes. (ii) The second pulse eliminates, via the PI3K/AKT pathway, the suppressive action of p53, as well as (iii) sets an ERK-EGR1 threshold mechanism, which digitizes graded external signals into an all-or-none decision obligatory for S-phase entry. Together, our findings uncover two gating mechanisms, which ensure that cells ignore fortuitous growth factors, and undergo proliferation only in response to consistent mitogenic signals.
Keywords: AKT, cell cycle, EGR1, growth factor, p53, restriction point
INTRODUCTION
Growth factor (GF) signaling is continuously required during the G1 phase of the cell cycle, until cells cross a restriction (R) point, after which cell cycle progression becomes GF-independent (Pardee, 1974). Quiescent fibroblasts typically require 12 hours to progress through G1, until they enter the S-phase (Stiles et al., 1979). R-point crossing, however, occurs following prolonged (9 hour) exposure to GFs, and precedes initiation of DNA synthesis. Early studies proposed that this interval comprises two phases: in the first, GFs establish a competence state, which is complemented six hours later by the presence of nutrients and progression factors (Pledger et al., 1977; Stiles et al., 1979). A later report found that continuous exposure to the platelet-derived growth factor (PDGF) may be substituted by two pulses, separated by a fixed-length interval (Jones and Kazlauskas, 2001). Based on this scenario, it was proposed that the first pulse primes a process, which is completed by the second pulse and enables R-point transition (Kazlauskas, 2005).
Our study investigated the dual-step process in mammary epithelial cells, stimulated by the epidermal growth factor (EGF). Like in fibroblasts, GF signaling promotes epithelial proliferation by regulating cyclins, cyclin-dependent kinases (CDKs), as well as CDK inhibitors (Stull et al., 2004). CDK-mediated inactivation of pRb facilitates release and activation of a group of transcription factors (TFs), E2Fs, thus enabling progression from G1 to S-phase (Chen et al., 2009). E2Fs are regulated by a bistable, switch-like mechanism essential for R-point transition (Planas-Silva and Weinberg, 1997; Yao et al., 2008). Following extracellular cues, c-MYC acts as an additional critical regulator of progression through G1. Unlike transformed cells, which often harbour high expression of c-MYC, the abundance of this protein is tightly regulated in normal cells (Meyer and Penn, 2008). The expression and stabilization of c-MYC cooperate with the bistable activation mode of E2F by inducing the expression of cyclins, and by cooperating with E2F in a positive feedback loop (Leung et al., 2008).
To unravel the molecular events that precede R-point transition, we applied Kazlauskas’ two-pulse scenario to normal human mammary epithelial cells. Employing proteomic and transcriptomic analyses, we identified previously unknown mechanisms that refute mitogenic stimuli, unless they are consistent and appropriately timed. Specifically, along with forward-driving processes, the first pulse initiates also a restraining mechanism entailing p53 and a battery of anti-proliferative genes. The second pulse engages a phosphoinositide 3-kinase- (PI3K-) mediated mechanism that removes the p53-centered blockade. In addition, the second pulse enhances extracellular signal-regulated kinase (ERK) signaling, in what appears as a threshold-governed mechanism underlying the decision to cross the R-point.
RESULTS
Two pulses of EGF commit mammary epithelial cells to proliferation
To explore commitment to proliferation, we employed clone 184A1 of normal human mammary epithelial cells (Hammond et al., 1984). These cells were activated with EGF according to a protocol developed for fibroblasts (Jones and Kazlauskas, 2001): First, they were starved for GFs (16 hours), and then stimulated for one hour with EGF, washed and incubated in starvation medium for 7 hours. Subsequent exposure to a second 1-hour pulse initiated DNA synthesis three hours later (Figure 1A). This was confirmed by multiple repetitions of the experiment, which were averaged and presented in Supplementary Figure S1A without normalization. In contrast, cells treated with a single pulse, or with two pulses separated by a shorter interval, displayed no comparable DNA synthesis (Figure S1B). Importantly, the two-pulse protocol and the more conventional continuous exposure procedure similarly impacted the capacity of cells to enter S-phase (Figure 1B). A time-course analysis confirmed progressively higher BrdU incorporation signals and also indicated that the onset of DNA synthesis occurs 12 hours after stimulation (Figure S1C), in line with a previous study performed with these cells (Stampfer et al., 1993). To focus on events regulating S-phase entry, and avoid later effects, we adopted the 9–12 hour time window for measuring BrdU incorporation.
Figure 1.
Human mammary epithelial cells commit to proliferation upon two timed pulses of EGF. (A) 184A1 human mammary cells were GF-starved for 16 hours. They were then either pulsed with EGF (“1E”, red) for 1 hour, or mock pulsed (“1S”, green). Thereafter, cells were washed and incubated in starvation medium for 7 hours, as indicated, either followed by a second, one hour pulse of EGF, or not. Cells were then washed and incubated for 3 hours with BrdU in starvation medium. Thereafter, the cells were fixed, stained, and counted under a fluorescent microscope. BrdU incorporation into DNA was measured by determining the ratio of BrdU- to DAPI-stained nuclei, and normalized according to the starvation control (1S-7S-1S). Bars represent standard errors calculated from at least 15 non-overlapping photomicrograph fields (>500 nuclei). Significant p-values of two-tailed student’s T-test are indicated. The experiment was repeated thrice. (B) 184A1 cells were GF-starved as in A and then treated with two pulses of EGF, or continuously stimulated with EGF for 9 hours. Cells were then washed and incubated for 3 hours with BrdU, fixed and BrdU incorporation analysed as in A. Bars represent standard errors calculated from at least 15 non-overlapping photomicrograph fields (>500 nuclei). p-values of two-tailed student’s T-test are indicated. The experiment was repeated twice. (C) 184A1 cells were GF-starved and treated as in A. Following the second pulse, cells were left in starvation medium for 17 hours, fixed and stained with methyl violet. Cell-occupied area was then measured from four light photomicrographs of non-overlapping fields. Bars represent the standard errors calculated from triplicates. Significant p-values of two-tailed student’s T-test are indicated. Representative light photomicrographs are presented. The experiment was repeated twice. (D) Cells were GF-starved and treated as in A. At the indicated time points, cells were harvested, lysed, and cleared extracts electroblotted. Phosphorylation of Rb and abundance of c-MYC were determined (left panel), and quantified by densitometry. Signals were normalized to actin and fold phosphorylation or expression calculated (presented under each lane). The right panel presents the corresponding signals determined at the time of BrdU measurement (marked by Roman numbers). The experiment was repeated twice.
To verify cell cycle completion following the two-pulse scenario, we employed a methyl violet staining protocol, which is highly reproducible when applied to strongly adherent cells. This assay validated that two appropriately timed pulses promoted cell proliferation, whereas a single pulse was insufficient (Figure 1C). Parallel counting of DAPI-stained nuclei ensured that the differences measured by methyl violet staining where due to an increase in cell number (Figure S1D). c-MYC and E2F coordinate S-phase entry, and they are regulated at the level of protein abundance (c-MYC) and pRb phosphorylation (E2F) (Chen et al., 2009; Meyer and Penn, 2008). Consistently, both parameters were enhanced at the time of S-phase entry in cells treated with two pulses, relative to cells treated with a single pulse, or with two pulses separated by a shorter interval (Figure 1D and Figure S1E). In conclusion, the observed increase in BrdU incorporation, cell proliferation, c-MYC expression and pRb phosphorylation confirm that 184A1 cells enter S-phase upon two pulses of EGF, but not upon a single pulse.
Subtly different phosphorylation signals are induced by each pulse of EGF
Since the outcomes of the two pulses differ remarkably, we considered differences in the phosphorylation cascades outlined in Figure 2A. To characterize activation patterns, we analyzed cell extracts by reverse-phase protein array (RPPA) using antibodies to phosphorylated sites of key proteins (Figure 2B and Supplementary Table 1). To substantiate the RPPA results, we performed immunoblot analyses of ERK, AKT and ribosomal protein S6, which confirmed that phosphorylation of the three proteins was markedly increased by each pulse (Figure 2C and Figures S2A and S2B). Importantly, ERK phosphorylation was the only measured event that was more prolonged and enhanced upon the second pulse, compared to the first pulse. This difference in amplitude, as well as in the length of induction, was independently verified by immunostaining of active ERK in the nucleus (Figure S2C).
Figure 2.
Comparative analyses of phosphorylation cascades stimulated by each EGF pulse. (A) A scheme presenting phosphorylation cascades activated by EGFR. Light blue labelled proteins were analysed using RPPA. (B) 184A1 cells were GF-starved for 16 hours. Thereafter they were pulsed for 1 hour with EGF, washed, and incubated in starvation medium for 7 hours, followed by a second pulse with EGF (red) or no treatment (green). At the indicated time points, cells were harvested and equal amounts of protein were used for RPPA analysis using the indicated antibodies. The mean of phosphorylation signals normalized to the respective total expression level (in triplicates) was calculated. The heatmap presents the means in log2 scale, centered to the corresponding mean across all samples. The fold-change in phosphorylation between the second pulse peak and the first pulse peak is indicated (right column), if the difference between the peaks was significant. (C) 184A1 cells were GF-starved and treated as in B. Cells were harvested at the indicated time points, lysed, and analyzed by immunoblotting. Quantified and normalized signals are presented under each lane. The experiment was repeated thrice. (D) 184A1 cells were treated and BrdU incorporation measured as in Figure 1A. Cells were treated with U0126 at the indicated concentrations, 30 minutes prior to and throughout the second pulse (shaded area). Bars represent standard error values (>500 nuclei). ERK activation was calculated according to Figure S2D. The experiment was repeated thrice. (E) 184A1 cells were GF-starved, treated, and BrdU incorporation measured as in D. Cells were treated with LY294002 as indicated, 30 minutes prior to and throughout the second pulse (shaded). The experiment was repeated thrice.
To test whether enhanced ERK activation following the second pulse is essential for proliferation, we applied a MEK inhibitor, U0126. High concentration of U0126 (5 µM) strongly inhibited ERK activation, whereas lower concentrations resulted in proportionally reduced effects (Figure S2D). As shown in Figure 2D, when the second pulse of ERK was reduced by ~50% (0.1 µM U0126), entry into S-phase was essentially abrogated, despite the remaining 50% increase in ERK phosphorylation. In contrast, in cells treated with a lower concentration of U0126 (0.05 µM), where ERK activation reached 80% of its un-inhibited level, cells were able to enter S-phase (Figure 2D and Figure S2D). Taken together, our observations indicated that the relative amplitude and longer duration of ERK activation during the second pulse are critical for S-phase commitment. Interestingly, these results propose that ERK activation sets a threshold mechanism able to convert a graded input to an all-or-none output (digitization), required for S-phase entry decision (Pardee, 1989).
The increase in phosphorylation of AKT and S6 is compatible with activation of the PI3K cascade. To determine the importance of this pathway in inducing S-phase entry, we utilized the dual PI3K/mTOR inhibitor, LY294002. Figure 2E shows that inhibition of PI3K/mTOR, specifically at the second pulse, was sufficient for inhibition of S-phase entry, whereas inhibition of PI3K during the first pulse was ineffective (Figure S2E). This is in accordance with the conclusion that the second pulse may be substituted by direct activation of PI3K in cells expressing c-MYC and an active MEK (Jones and Kazlauskas, 2001).
Distinct programs of gene expression are induced by each pulse of EGF
Application of actinomycin D and cyclohexamide verified that de-novo transcription and translation are required during the late phase of EGF signaling, to achieve S-phase entry (Figure S3A). Hence, we employed Affymetrix GeneSet arrays at 17 time points to determine dynamic changes in the abundance of all mRNAs altered by EGF (Figure 3A). A set of logical rules was applied to categorize genes into groups sharing expression profiles (see Experimental Procedures). This identified ten profiles, including patterns representing genes transiently inducted by either pulse, profiles exhibiting persistent or interval-limited changes, and a group of genes downregulated by the second pulse (Supplementary Table 2 and Figures S3B-K).
Figure 3.
A short pulse of EGF is sufficient for the induction of metabolic enzymes essential for cell proliferation. (A) A scheme depicting the setup of the microarray experiment. mRNA samples were isolated at the indicated time points (blue triangles). Red and green segments indicate EGF pulses and intervals, respectively. (B) 184A1 cells were treated as in Figure 1A. mRNA abundance was measured at the indicated time points using Affymetrix GeneSet microarrays. Shown are centered and normalized expression patterns of genes associated with cholesterol biosynthetic processes included in the “persistently induced” profile (see Figure S3B). (C) 184A1 cells were grown and processed as in Figure 1A except that cells were treated with Metformin (0.1 mM), Mevastatin (1µM), or AICAR (0.5mM) during the interval (shaded). Bars represent standard errors calculated from at least 15 non-overlapping photomicrograph fields (>500 nuclei). A significant p-value of two-tailed student’s T-test is indicated. The experiment was repeated thrice.
The first EGF pulse is sufficient for the induction of metabolic enzymes essential for S-phase entry
Interestingly, a profile of 35 persistently induced genes, whose ascending abundance was not affected by a second pulse, displayed enrichment (q-value<0.001) for enzymes associated with steroid, cholesterol and lipid metabolism (Figure 3B, Figure S3B and Supplementary Table 3). Elevated expression of two of these genes, isopentenyl-diphosphate delta isomerase 1 (IDI1), and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), was validated by real-time quantitative PCR (RT-qPCR; Figure S3L). Notably, the early induction of S6 phosphorylation, a crucial determinant of protein synthesis (Figure 2B), was consistent with an increase in lipid biosynthetic processes upon the initial pulse. Hence, we speculated that early induction of metabolic processes enables the increases in cell size and membrane mass required for cell division. To test this, we pharmacologically targeted two pathways: the AMPK pathway was activated using Metformin and AICAR, while Mevastatin was employed to inhibit HMG-CoA reductase. Of relevance, metabolism inhibitors were previously shown to inhibit cell growth downstream to EGFR, primarily through inhibition of cholesterol and fatty acid synthesis (Guo et al., 2009). In the same vein, lipid metabolism genes are often up-regulated in cancer models (Hirsch et al., 2010). Consistently, incubating cells with either inhibitor during the interval completely abolished EGF-induced proliferation (Figure 3C), supporting the hypothesis that increased lipid and cholesterol metabolism is essential for proliferation, despite being independent of the second pulse.
Differential transient induction of TFs by the early and late pulses
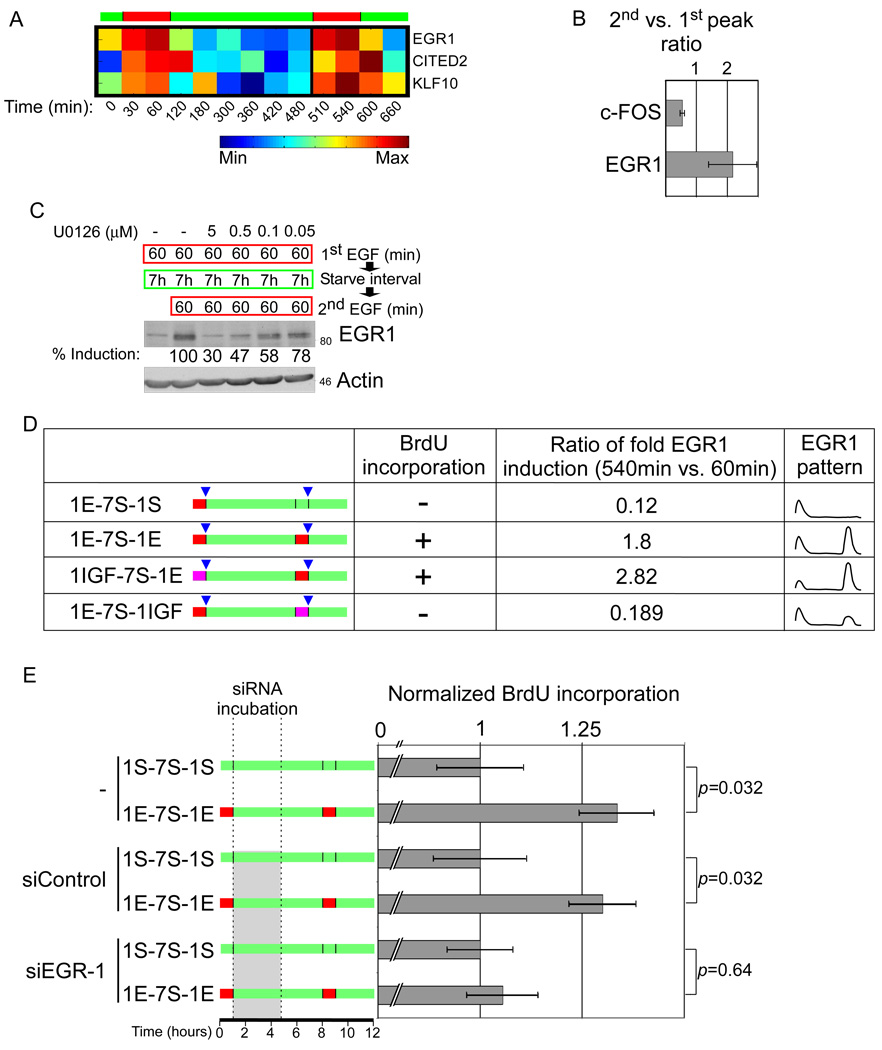
To focus on early transcriptional programs, we studied genes that exhibited transient induction by both pulses, and assigned them to three profiles, according to the ratio of induction between the two pulses (Figures S3C-S3E). The immediate-early TFs included in the profile denoted 1st<2nd (greater induction upon the second pulse; e.g., EGR1) and the profile 1st>2nd (e.g., c-FOS) are shown in Figures 4A and in Figure S4A, respectively, along with verification of differential induction (Figs. S4B, S4C and 4B). Interestingly, both induction of EGR1, an ERK-induced TF essential for mitogenic responses, and the profile of ERK activation (Figure 2B), were enhanced at the second pulse. Hence, we hypothesized that the enhanced ERK signals are coupled to increased transcription of genes of the 1st<2nd profile, and together they license R-point transition. To examine this model, we related EGR1 to the aforementioned ability of U0126 to block R-point transition (see Figure 2D). As expected, complete ERK inhibition (U0126 at 5 µM, see Figure S2D) essentially abolished EGR1 induction (Figure 4C), and prevented cell proliferation (Fig. 2D). In contrast, lower concentrations (0.05 µM), which reduced EGR1 expression by only 22%, did not interfere with proliferation (Figure 2D). Application of an intermediate concentration (0.1 µM) inhibited ERK activation by 54% and EGR1 transcription by 42%, thereby quenching extra activation of the pathway. Importantly, this was sufficient to prevent R-point transition. In conclusion, EGR1 and possibly other TFs that exhibit the 1st<2nd expression pattern, might participate in the digitization of ERK signals.
Figure 4.
Differential induction of immediate-early transcription factors by the two pulses. (A) 184A1 cells were treated with two pulses of EGF, as in Figure 3B. Shown are centered and normalized expression levels of the indicated immediate-early induced TFs from the profile denoted 1st<2nd (Figure S3D). (B) 184A1 cells were treated with two pulses of EGF. At the end of each pulse, mRNA was isolated, followed by cDNA synthesis and RT-qPCR with primers for either c-FOS or EGR1. Presented are the average ratios of expression, calculated from four biological repeats. (C) GF-starved 184A1 cells were pulsed for 1 hour with EGF, washed, and incubated for 7 hours in starvation medium, followed by a second EGF pulse. Cells were treated with U0126 at the indicated concentrations, 30 minutes prior to and throughout the second pulse. Immunoblotting was used to quantify EGR1 levels of induction relative to the level at 1E-7S-1E, normalized to actin. (D) GF-starved 184A1 cells were pulsed for 1 hour with EGF (“1E”, red), IGF-1 (“1IGF”, purple), or left untreated (“1S”, green). Following GF removal, cells were incubated in starvation medium for 7 hours, followed by a second pulse of EGF or IGF-1, as indicated. For BrdU incorporation analysis see Figure S4D. To determine EGR1 fold induction, mRNA was isolated at the end of each pulse (blue triangles), and cDNA analysed by RT-qPCR. The right column schematically presents time profiles of EGR1’s patterns of expression. (E) GF-starved 184A1 cells were treated and analyzed as in Figure 1A. The shaded rectangle indicates transfection with control or EGR1-specific siRNA oligonucleotides.
To further address a model of mild EGR1 overshoot, we switched pulses between EGF and the insulin-like growth factor 1 (IGF-1). Although IGF-1 could replace EGF as a first-pulse inducer, the reciprocal order was ineffective (Figure S4D). Correspondingly, we observed enhanced EGR1 induction when cells were treated with IGF-1 and then with EGF, but no EGR1 enhancement (or ERK activation) was observed with the inverse combination (Figures 4D and S4E). This observation further supported the model, and motivated manipulation of the second peak of EGR1. Cells were transfected with EGR1-specific siRNAs immediately after the first pulse, which resulted in partial knockdown during the second pulse (Figure S4F). Importantly, the observed reduction in EGR1 expression during the second pulse abrogated S-phase entry (Figure 4E). In conclusion, the enhancement of ERK activation instructs enhanced EGR1 induction at the second pulse, and this is required for S-phase entry, in agreement with a threshold-setting mechanism that licenses crossing of the R-point (Figure S4G).
The second pulse downregulates p53-controlled anti-proliferative genes that are up-regulated by the first pulse
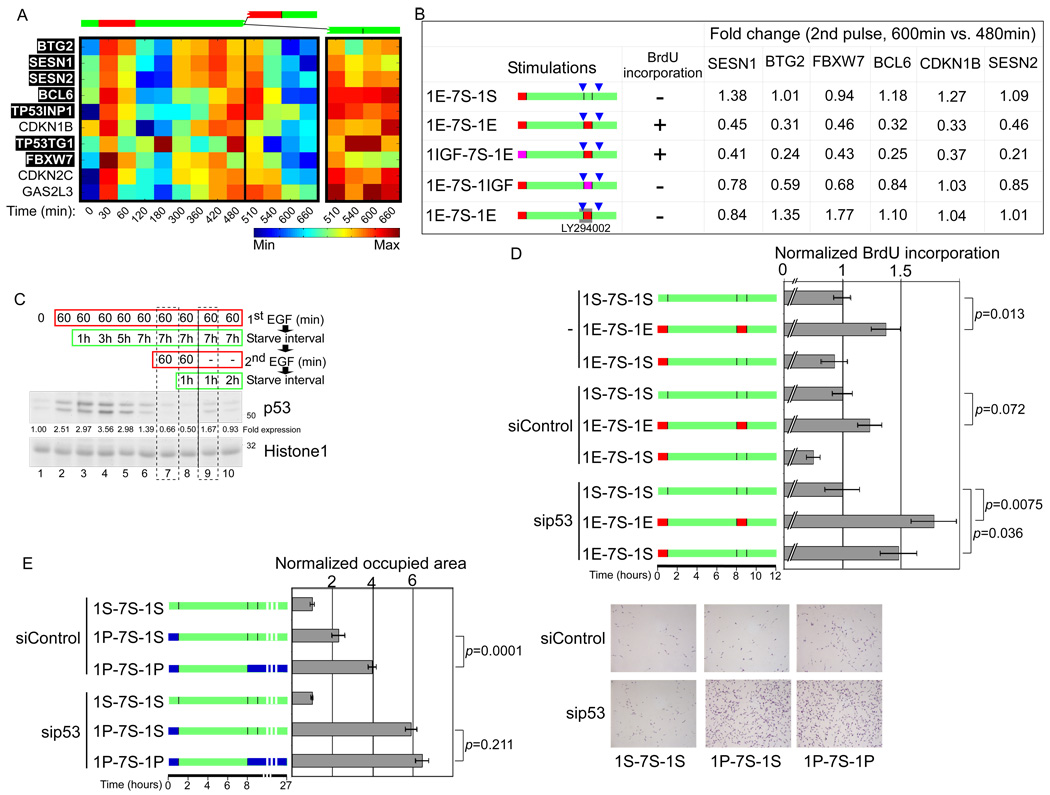
The expression profiles of one large group of EGF-induced genes, denoted ‘down-regulated by a second pulse’, implied a restraining mechanism. Validation of the patterns of five genes of this group by using RT-qPCR, confirmed the unique kinetics, in line with immunoblotting of one gene product, p27 (CDKN1B; Figures S5A and S5B). Interestingly, we noted that this profile contains well-characterized anti-proliferative genes, seven of which are known transcriptional targets of the p53 tumor suppressor (Figure 5A). Hence, we focused on the possibility that p53 might be involved in a restraining mechanism, which is set up by the first pulse of EGF, but is removed by the second pulse. To test this model we examined down-regulation of six of the anti-proliferative genes following treatment with the previously utilized combinations of EGF and IGF-1. As shown in Figure 5B, when cells were treated with two pulses of EGF, or with a first pulse of IGF-1 followed by a second pulse of EGF, all six anti-proliferative genes we examined underwent more than 2-fold down-regulation following the second pulse. In contrast, treatments that do not promote proliferation, such as a single EGF pulse or a combination of EGF followed by IGF-1, elicited very modest down-regulation of the six genes, consistent with restraining roles.
Figure 5.
The second pulse down-regulates anti-proliferative genes induced by the first pulse. (A) 184A1 cells were treated with two pulses of EGF. Shown are centered and normalized levels of anti-proliferation genes (marked in black are known p53 targets) from the profile “down-regulated by a second pulse” (see Figure S3F). (B) 184A1 cells were treated as in Figure 4D. Where indicated, cells were treated for 30 minutes with LY294002. BrdU incorporation results are presented in Figure 2E and Figure S4D. To determine fold change, mRNA was isolated before the second pulse and 60 minutes after completion of the pulse (blue triangles), and cDNA analysed by RT-qPCR. Listed are the ratios of expression levels after and before the second pulse. (C) GF-starved cells were pulsed for 60 minutes with EGF, washed, and incubated in starvation medium for 7 hours, followed by a second pulse. At the indicated time points, cells were harvested for a chromatin association assay and DNA-bound proteins isolated and analysed by immunoblotting. (D) Cells were transfected with control or p53-specific siRNAs. Twenty-four hours later, the cells were re-plated on cover-slips, and 24 hours later, they were GF-starved for 16 hours, treated with EGF, and BrdU incorporation measured. Bars represent standard errors calculated from 15 non-overlapping photomicrograph fields. P-values of two-tailed student’s T-test are indicated. (E) NIH-3T3 cells were transfected with control or p53-specific siRNAs. Twenty-four hours later, the cells were re-plated, and 24 hours later they were GF-starved for 24 hours, treated for 1 hour without (“1S”, green) or with PDGF (“1P”, blue), washed, and incubated in starvation medium for 7 hours, followed by a second PDGF pulse. Cells were left in starvation medium or PDGF-containing medium for additional 18 hours, and then fixed and stained with methyl violet. Representative photomicrographs are shown (right part). Cell-occupied area was measured from five photomicrographs (left part). Bars represent standard errors of triplicates. The experiment was repeated twice.
To identify signaling pathways mediating down-regulation of the group of anti-proliferative genes, we used specific MEK (U0126) and PI3K (LY294002) drugs. This revealed that activation of PI3K is necessary for down-regulation of the set of six anti-proliferative genes (Figure 5B), but U0126 did not affect the capacity of the second pulse to down-regulate their expression (Figure S5C). In line with PI3K involvement, it has been reported that a subset of apoptosis-promoting E2F1 target genes is specifically repressed by PI3K signaling (Hallstrom et al., 2008). Moreover, PDGF induces immediate, as well as delayed waves of PI3K activity, only the latter is obligatory for DNA synthesis (Jones et al., 1999; Kumar et al., 2006). In conclusion, our results indicate that a late, PI3K-mediated process that down-regulates a set of anti-proliferation genes is necessary for S-phase entry.
Because the anti-proliferative genes we identified undergo up-regulation at the first pulse, and a fraction of the set is regulated by p53, we predicted that the early pulse of EGF activates p53. Of relevance, cell cycle-related pulses of p53 have recently been detected (Loewer et al., 2010). To test this prediction, we isolated chromatin following EGF stimulation, and measured the fraction of DNA-bound p53. This uncovered early EGF-induced activation of p53’s transcriptional function, which persisted for 6–8 hours and decreased towards the end of the interval (Figure 5C). Importantly, upon a second pulse, p53 activation almost disappeared (compare lanes 7 and 9 in Figure 5C). Thus, the pattern of p53 activation correlated with the kinetics of expression of its targets. Notably, the abundance of p53 in the soluble fraction exhibited only moderate changes on EGF treatment (Figure S5D).
In an effort to firmly establish involvement of p53, we knocked-down its expression using specific siRNAs. This treatment reduced p53 levels by 85%, and resulted in 50–70% lower expression of the set of p53 target genes (Figures S5E and S5F). We therefore examined whether a reduction in p53 would bypass the need for a second pulse. Remarkably, p53 knockdown enabled cells to enter S-phase upon a single pulse of EGF, in contrast to control cells that required two pulses (Figs. 5D and S5G). Interestingly, comparison of p53-knocked-down with control cells detected increased expression of EGR1, as long as seven hours after the first EGF pulse (Figure S5H), suggesting a mechanism that sensitizes cells to proliferation upon a single EGF pulse, once the p53-dependent restraining mechanism is abrogated. Next we tested the prediction that PI3K activity is not needed during the second pulse, if p53 activity is compromised. Indeed, inhibition of PI3K using LY294002 completely blocked S-phase entry by control cells, but no effect of LY294002 was observed upon p53 knockdown (Figure S5I). This result underscores the role of PI3K as a late G1 suppressor of p53-regulated anti-proliferative processes.
Since forced p53 reduction in epithelial cells pre-empted the requirement for a second pulse, we asked whether the underlying mechanism is generalizable, and can be extended to the original fibroblast cell system, in which the two-pulse scenario was first established. Congruent with the original report (Jones and Kazlauskas, 2001), stimulating NIH-3T3 cells, which express wild type p53 (Huang et al., 1996), with two pulses of PDGF significantly increased their proliferation (Figure 5E). Although knockdown of p53 was less efficient than in 184A1 cells, it enhanced growth of starved fibroblasts (Figures S5E and S5J). Importantly, forced reduction of p53 expression completely eliminated the necessity for a second pulse of PDGF to promote proliferation (Figure 5E), similar to the results obtained with 184A1 cells.
Taken together, our results indicate that a single pulse of EGF initiates a priming process, which includes lipid metabolism and drives cells through early G1, but at the same time it also induces a restraining process that prevents commitment to proliferation. The latter involves activation of p53 and a set of anti-proliferative genes. When cells are exposed to a second EGF pulse, signaling through PI3K/AKT releases the constraint. If enhanced activation of ERK and consequent induction of EGR1 exceed a threshold, and in parallel the anti-proliferation genes are down-regulated, cells will cross the R-point and commit to proliferation (see model in Figure 6).
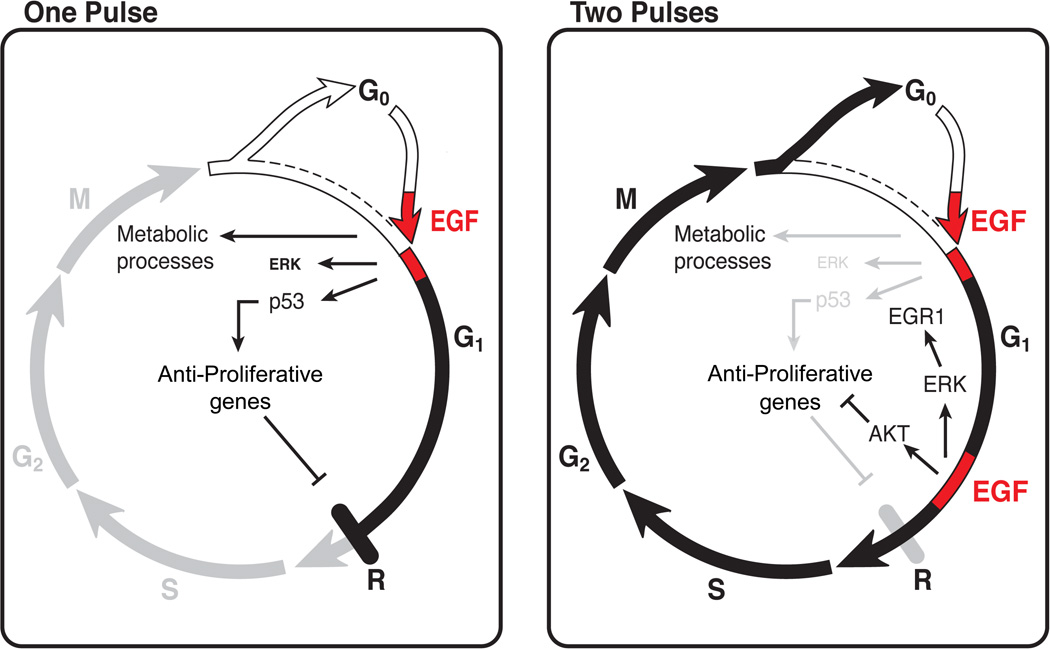
Figure 6.
Schematic representations of the proposed biochemical events elicited by a single and a dual EGF pulse (shown in red). The first pulse of EGF induces expression of lipid biosynthesis-associated genes, along with activation of p53. The latter propels expression of anti-proliferation genes, such as BTG2 and SESN1. When cells are treated with a second pulse of EGF, enhanced activation of ERK and subsequent induction of EGR1 exceed a critical threshold. In parallel, signaling through PI3K, and the resulting suppressed expression of anti-proliferative genes, permit cells to cross the restriction point (R) and enter the S-phase.
DISCUSSION
To irreversibly cross the restriction point (R) of the cell cycle, fibroblasts have been shown to require either continuous exposure to GFs, or two short pulses confined to a time window of approximately 9 hours (Jones and Kazlauskas, 2001). In this report, we applied the discontinuous stimulation protocol to human mammary epithelial cells and unravelled three transcriptional mechanisms that orchestrate entry into the S-phase: (i) a persistent increase in expression of genes associated with lipid biosynthesis (initiated by the first EGF pulse), (ii) a threshold mechanism that requires enhanced activation of ERK and induction of EGR1 by the second pulse, and (iii) activation (by the second pulse) of PI3K, which suppresses a set of anti-proliferative genes. Several of these anti-proliferative genes are transcriptional targets of p53, which is activated by the first pulse, safeguarding the system against proliferative responses to inconsistent, fortuitous GFs signaling.
Early induction of metabolic enzymes
In order to divide, cells require relatively large quantities of lipids, proteins and nucleotides, which are used to increase cell size and replicate DNA. Accordingly, it was shown that cellular growth rates are tightly coordinated with the length of the cell cycle, suggesting the existence of a size sensor at G1, which maintains a roughly constant cell mass over many cycles (Dolznig et al., 2004). A recent study of the relationships between cell size and the cell cycle demonstrated an accelerative, size-dependent growth rate during G1, implying an intrinsic size-regulating machinery (Tzur et al., 2009). Consistent with these studies, three aspects of our experimental evidence indicated an increase in metabolic processes following a single pulse of EGF: elevated S6 phosphorylation, which translates to accelerated protein synthesis (Figure 2), induction of genes associated with lipid and sterol biosynthetic pathways (Figure 3B), and inhibition of the two-pulse commitment to cell cycle engagement induced by metabolic inhibitors (Figure 3C). It is important to note, that although they are obligatory, the early metabolic events are not sufficient for cell proliferation, since a single pulse of EGF gave rise to their induction, whereas S-phase entry required an additional pulse.
An ERK/EGR1 gating mechanism
Because metabolic processes could not explain the gating mechanism involved in restriction point crossing, we focused on events that differ between the first and the second pulses of EGF. Two activation indicators of the ERK pathway, namely ERK phosphorylation and EGR1 abundance, displayed enhanced signals following the second EGF pulse (Figures 2 and 4). We further found that inhibition of the excessive activation of ERK or of the increased induction of EGR1 abolished cell proliferation, which implied a threshold mechanism. Accordingly, to cross the R-point, the activation of ERK and downstream induction of EGR1 must reach the set threshold, thus ensuring that weak or inconsistent signals would not trigger cell proliferation. Compatible with a late ERK-centered gating mechanism, it has previously been reported that the pattern of ERK activation upon continuous exposure of fibroblasts to GFs is biphasic (Meloche et al., 1992). Further, the late phase of ERK activation, which occurs at mid-G1, is essential for S-phase entry, and its inhibition prevents cell proliferation (Sah et al., 2002). Our study leaves open mechanisms underlying the second pulse enhancement of the ERK-EGR1 signals. One potential mechanism involves ERK-specific dual-specificity phosphatases, which are newly synthesized following EGF stimulation to feedback control downstream signals (Amit et al., 2007).
A p53-mediated mechanism dampens mitogenic noise
The transcriptional response to EGF is very complex, yet a portion of the response could be grouped into 10 temporal patterns (Figure S3). One group, which includes multiple anti-proliferative genes, was strongly suppressed by the second pulse of EGF (Figure 5). Suppression of anti-proliferative genes during G1 has previously been shown in quiescent fibroblasts, 6–12 hours after serum stimulation (Iyer et al., 1999), or upon sustained activation of ERK (Yamamoto et al., 2006). Interestingly, several genes included in this group have previously been characterized as targets of p53. Congruent with p53 involvement, the first pulse of EGF increased the tight association of p53 with chromatin, which persisted for 6–8 hours (Figure 5C). It is worth noting that excessive mitogenic signals involving RAS and c-MYC similarly engaged the anti-proliferative activity of p53 (Zindy et al., 1998). To confirm a role for p53, we knocked-down its expression. As expected, removal of p53 enabled both epithelial and fibroblastic cells to enter S-phase upon a single pulse of EGF, in contrast to the two-pulse requirement for normal cells. These observations indicate that p53 protects cells from excessive or untimely responses by imposing a constraining mechanism, which is relieved by a second GF stimulation. In line with this, it has previously been reported that lung carcinomas that harbour EGFR mutations also carry p53 mutations (Mounawar et al., 2007), which is likely to unleash full transformation. In addition, the efficacy of treating hepatocellular carcinoma with an EGFR-blocking antibody, Cetuximab, displayed dependence on wild-type p53 (Huether et al., 2005).
The PI3K/AKT pathway contributes to cell survival and suppresses the expression of anti-proliferative genes, such as CDKN1B/p27, thus shifting the balance toward proliferation following activation of E2F (Hallstrom et al., 2008; Sa and Stacey, 2004). Furthermore, activation of AKT reduced hypoxia-induced transcriptional activation of p53 (Yamaguchi et al., 2001), and delayed the onset of p53-mediated apoptosis (Sabbatini and McCormick, 1999). Accordingly, we demonstrate here that inhibition of PI3K during the second pulse of EGF abrogated suppression of the anti-proliferative group of genes, and prevented proliferation.
The three transcription-based regulatory mechanisms of S-phase entry we unveiled open interesting questions for future research. For example, p53 and PI3K are assembled into an incoherent feed-forward process, which has the characteristics of a checkpoint allowing transition to the next step only when sufficient GF signals are provided (Figure S6). On the other hand, p53 is often deleted and PI3K is frequently mutated in human cancers, raising the intriguing possibility that the aberrant p53/PI3K checkpoint sensitizes many tumors to sporadically available GFs. Mutations of EGFR, which induce basally weak activation, are similarly frequent in cancer, reinforcing potential clinical implications of the feed-forward process. The gating mechanism involving late enhancement of the ERK-EGR1 module raises additional questions. While it is likely that this module digitizes graded GF signals, thereby blocking induction of S-phase entry by inconsistent pulses of GFs, molecular mechanisms that generate enhance late signals and compute fold activation are matters for future investigation.
EXPERIMENTAL PROCEDURES
Cell lines and siRNA transfection
Human mammary 184A1 cells were maintained in DFCI medium (Band and Sager, 1989). NIH-3T3 cells were grown in DME medium supplemented with 1mM sodium pyruvate. For further details, see Supplementary Experimental Procedures. siRNA transfections were carried out using HiPerFect (184A1 cells; Qiagen, Germany), or Dharmafect1 (NIH-3T3; Lafayette, CO).
BrdU incorporation assay
Incubation with the BrdU labelling reagent was carried out for 3 hours, followed by fixation and staining using a BrdU detection kit (Roche Diagnostics GmbH, Germany). Cells were visualized using a Nikon Eclipse 90i microscope, and photos captured using the Image-Pro software. BrdU- and DAPI-stained nuclei were counted from at least 15 fields of each treatment.
Methyl violet staining
Cells were washed with ice-cold saline, fixed in methanol and incubated with 0.3% methyl violet for 5 minutes at −20°C, and then washed. Pictures from four non-overlapping fields were collected using a Leica binocular. Cell-occupied area was determined using Cell Profiler (www.cellprofiler.org) (Carpenter et al., 2006).
Reverse-phase protein array analysis
Cell pellets were lysed in RPPA buffer [1% Triton X-100, 50mM HEPES (pH 7.4), 150mM NaCl, 1.5mM MgCl2, 1mM EGTA, 100mM NaF, 10mM Na-pyrophosphate, 1mM Na3VO4, 10% glycerol, and protease and phosphatase inhibitors (Roche Diagnostics GmbH, Germany)], for 20 minutes on ice. Following centrifugation, protein concentration was assayed using the BCA reagent (Pierce, Rockford, IL), and then 4XPSB [40% glycerol, 8% SDS, 0.25M Tris-HCl (pH 6.8), and 10% 2-mercaptoehtanol] was added to the cleared lysates followed by boiling. Samples were serially diluted, spotted onto nitrocellulose-coated slides, probed with antibodies (Supplementary Experimental Procedures), and signals quantified as previously described (Amit et al., 2007).
Immunofluorescence staining
Cells were grown on cover-slips and treated as indicated. Cells were then fixed in paraformaldehyde (3%) for 15 minutes, and stained with anti-pERK antibody followed by Cy2-conjugated anti-mouse secondary antibody. Nuclei were stained with DAPI during the last washing step. See Supplementary Experimental Procedures for details regarding visualization and intensity measurement.
Chromatin association assay
Cells were washed, incubated for 5 minutes at 4°C with buffer A [100mM NaCl, 300mM sucrose, 3mM MgCl2, 10mM Pipes (pH6.8), 1mM EGTA, 0.2% Triton X-100, protease inhibitor cocktail, and 1mM Na3VO4]. After collecting the Triton-soluble fraction, plates were washed once, and adherent material harvested. The triton-insoluble pellets were re-suspended in buffer B [50mM NaCl, 300mM sucrose, 3mM MgCl2, 10mM Pipes (pH6.8), 1mM EGTA] supplemented with DNAseI (60U). Samples were incubated at 37°C for 45 minutes. Protein content of both fractions was measured, and samples resolved by electrophoresis.
RNA purification, RT-qPCR, and microarray analyses
Cellular RNA was purified using a kit (PerfectPure; 5 Prime GmbH, Germany). cDNA was synthesised using the SuperScriptII first-strand synthesis kit (Invitrogen, Carlsbad, CA). Real-time-qPCR analysis was performed using Power-SYBR Green (ABI, Carlsbad, CA). All experiments were carried out in triplicates, and results normalized to β2-microglobulin RNA levels. RT-qPCR primers (Supplementary Experimental Procedures) were designed using ProbeLibrary. RNA (600 ng) was labelled, fragmented and hybridized to an Affymetrix Human Gene 1.0 ST oligonucleotide array. The data was summarized using an iterative module (http://media.affymetrix.com/support/technical/whitepapers/exon_gene_signal_estimate_whitepaper.pdf), and normalized with Parallel LOWESS (Ballman et al., 2004). To reliably assess noise, we selected two time points, where we expected minimal change. The noise σ as a function of intensity was estimated over all probe sets of the array using a robust method that ignores outliers (Zeisel et al., 2010). Intensity values of 3 or less (Log2 scale) were considered below detection and replaced by 3. Probe sets that varied less than 0.03 were considered unchanged, leaving 20,810 probe sets. Probe sets were categorized into profiles of interest using logical classifying rules with precision up the noise estimator (see Supplementary Experimental Procedures). Each profile was tested for enrichment of GO biological process annotations using DAVID (Dennis et al., 2003; Huang da et al., 2009). The data presented in this report have been deposited in NCBI’s Gene Expression Omnibus, and are accessible through GEO series using accession number GSE27629 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE27629).
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Sara Lavi, Doron Ginsberg, Noa Bossel, Ido Amit, and Ami Citri for their help, and Eylon Shahar for technical support. Our research is supported by grants from the National Cancer Institute (including 4R37CA072981, CCSG and grant P30 CA16672), the European Commission, the German-Israeli Project Cooperation, the Israel Cancer Research Fund, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Kekst Family Institute for Medical Genetics, and the M.D. Moross Institute for Cancer Research. Y.Y. is the incumbent of the Harold and Zelda Goldenberg Professorial Chair and E.D. of the Henry J. Leir Professorial Chair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amit I, Citri A, Shay T, Lu Y, Katz M, Zhang F, Tarcic G, Siwak D, Lahad J, Jacob-Hirsch J, et al. A module of negative feedback regulators defines growth factor signaling. Nat Genet. 2007;39:503–512. doi: 10.1038/ng1987. [DOI] [PubMed] [Google Scholar]
- Ballman KV, Grill DE, Oberg AL, Therneau TM. Faster cyclic loess: normalizing RNA arrays via linear models. Bioinformatics. 2004;20:2778–2786. doi: 10.1093/bioinformatics/bth327. [DOI] [PubMed] [Google Scholar]
- Band V, Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci U S A. 1989;86:1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9:785–797. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- Dolznig H, Grebien F, Sauer T, Beug H, Mullner EW. Evidence for a size-sensing mechanism in animal cells. Nat Cell Biol. 2004;6:899–905. doi: 10.1038/ncb1166. [DOI] [PubMed] [Google Scholar]
- Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, Czernin J, Shyy JY, Watson AD, Phelps M, et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci U S A. 2009;106:12932–12937. doi: 10.1073/pnas.0906606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrom TC, Mori S, Nevins JR. An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell. 2008;13:11–22. doi: 10.1016/j.ccr.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SL, Ham RG, Stampfer MR. Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci U S A. 1984;81:5435–5439. doi: 10.1073/pnas.81.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch HA, Iliopoulos D, Joshi A, Zhang Y, Jaeger SA, Bulyk M, Tsichlis PN, Shirley Liu X, Struhl K. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell. 2010;17:348–361. doi: 10.1016/j.ccr.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TS, Kuo ML, Shew JY, Chou YW, Yang WK. Distinct p53- mediated G1/S checkpoint responses in two NIH3T3 subclone cells following treatment with DNA-damaging agents. Oncogene. 1996;13:625–632. [PubMed] [Google Scholar]
- Huether A, Hopfner M, Baradari V, Schuppan D, Scherubl H. EGFR blockade by cetuximab alone or as combination therapy for growth control of hepatocellular cancer. Biochem Pharmacol. 2005;70:1568–1578. doi: 10.1016/j.bcp.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J, Jr, Boguski MS, et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- Jones SM, Kazlauskas A. Growth-factor-dependent mitogenesis requires two distinct phases of signalling. Nat Cell Biol. 2001;3:165–172. doi: 10.1038/35055073. [DOI] [PubMed] [Google Scholar]
- Jones SM, Klinghoffer R, Prestwich GD, Toker A, Kazlauskas A. PDGF induces an early and a late wave of PI 3-kinase activity, and only the late wave is required for progression through G1. Curr Biol. 1999;9:512–521. doi: 10.1016/s0960-9822(99)80235-8. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A. The priming/completion paradigm to explain growth factor-dependent cell cycle progression. Growth Factors. 2005;23:203–210. doi: 10.1080/08977190500096020. [DOI] [PubMed] [Google Scholar]
- Kumar A, Marques M, Carrera AC. Phosphoinositide 3-kinase activation in late G1 is required for c-Myc stabilization and S phase entry. Mol Cell Biol. 2006;26:9116–9125. doi: 10.1128/MCB.00783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Ehmann GL, Giangrande PH, Nevins JR. A role for Myc in facilitating transcription activation by E2F1. Oncogene. 2008;27:4172–4179. doi: 10.1038/onc.2008.55. [DOI] [PubMed] [Google Scholar]
- Loewer A, Batchelor E, Gaglia G, Lahav G. Basal dynamics of p53 reveal transcriptionally attenuated pulses in cycling cells. Cell. 2010;142:89–100. doi: 10.1016/j.cell.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche S, Seuwen K, Pages G, Pouyssegur J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol Endocrinol. 1992;6:845–854. doi: 10.1210/mend.6.5.1603090. [DOI] [PubMed] [Google Scholar]
- Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- Mounawar M, Mukeria A, Le Calvez F, Hung RJ, Renard H, Cortot A, Bollart C, Zaridze D, Brennan P, Boffetta P, et al. Patterns of EGFR, HER2, TP53, and KRAS mutations of p14arf expression in non-small cell lung cancers in relation to smoking history. Cancer Res. 2007;67:5667–5672. doi: 10.1158/0008-5472.CAN-06-4229. [DOI] [PubMed] [Google Scholar]
- Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974;71:1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Planas-Silva MD, Weinberg RA. The restriction point and control of cell proliferation. Curr Opin Cell Biol. 1997;9:768–772. doi: 10.1016/s0955-0674(97)80076-2. [DOI] [PubMed] [Google Scholar]
- Pledger WJ, Stiles CD, Antoniades HN, Scher CD. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977;74:4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa G, Stacey DW. P27 expression is regulated by separate signaling pathways, downstream of Ras, in each cell cycle phase. Exp Cell Res. 2004;300:427–439. doi: 10.1016/j.yexcr.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Sabbatini P, McCormick F. Phosphoinositide 3-OH kinase (PI3K) and PKB/Akt delay the onset of p53-mediated, transcriptionally dependent apoptosis. J Biol Chem. 1999;274:24263–24269. doi: 10.1074/jbc.274.34.24263. [DOI] [PubMed] [Google Scholar]
- Sah JF, Eckert RL, Chandraratna RA, Rorke EA. Retinoids suppress epidermal growth factor-associated cell proliferation by inhibiting epidermal growth factor receptor-dependent ERK1/2 activation. J Biol Chem. 2002;277:9728–9735. doi: 10.1074/jbc.M110897200. [DOI] [PubMed] [Google Scholar]
- Stampfer MR, Pan CH, Hosoda J, Bartholomew J, Mendelsohn J, Yaswen P. Blockage of EGF receptor signal transduction causes reversible arrest of normal and immortal human mammary epithelial cells with synchronous reentry into the cell cycle. Exp Cell Res. 1993;208:175–188. doi: 10.1006/excr.1993.1236. [DOI] [PubMed] [Google Scholar]
- Stiles CD, Isberg RR, Pledger WJ, Antoniades HN, Scher CD. Control of the Balb/c-3T3 cell cycle by nutrients and serum factors: analysis using platelet-derived growth factor and platelet-poor plasma. J Cell Physiol. 1979;99:395–405. doi: 10.1002/jcp.1040990314. [DOI] [PubMed] [Google Scholar]
- Stull MA, Rowzee AM, Loladze AV, Wood TL. Growth factor regulation of cell cycle progression in mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2004;9:15–26. doi: 10.1023/B:JOMG.0000023585.95430.f4. [DOI] [PubMed] [Google Scholar]
- Tzur A, Kafri R, LeBleu VS, Lahav G, Kirschner MW. Cell growth and size homeostasis in proliferating animal cells. Science. 2009;325:167–171. doi: 10.1126/science.1174294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Tamatani M, Matsuzaki H, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J Biol Chem. 2001;276:5256–5264. doi: 10.1074/jbc.M008552200. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ebisuya M, Ashida F, Okamoto K, Yonehara S, Nishida E. Continuous ERK activation downregulates antiproliferative genes throughout G1 phase to allow cell-cycle progression. Curr Biol. 2006;16:1171–1182. doi: 10.1016/j.cub.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Yao G, Lee TJ, Mori S, Nevins JR, You L. A bistable Rb-E2F switch underlies the restriction point. Nat Cell Biol. 2008;10:476–482. doi: 10.1038/ncb1711. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Amir A, Kostler WJ, Domany E. Intensity dependent estimation of noise in microarrays improves detection of differentially expressed genes. BMC Bioinformatics. 2010;11:400. doi: 10.1186/1471-2105-11-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, Roussel MF. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.