Abstract
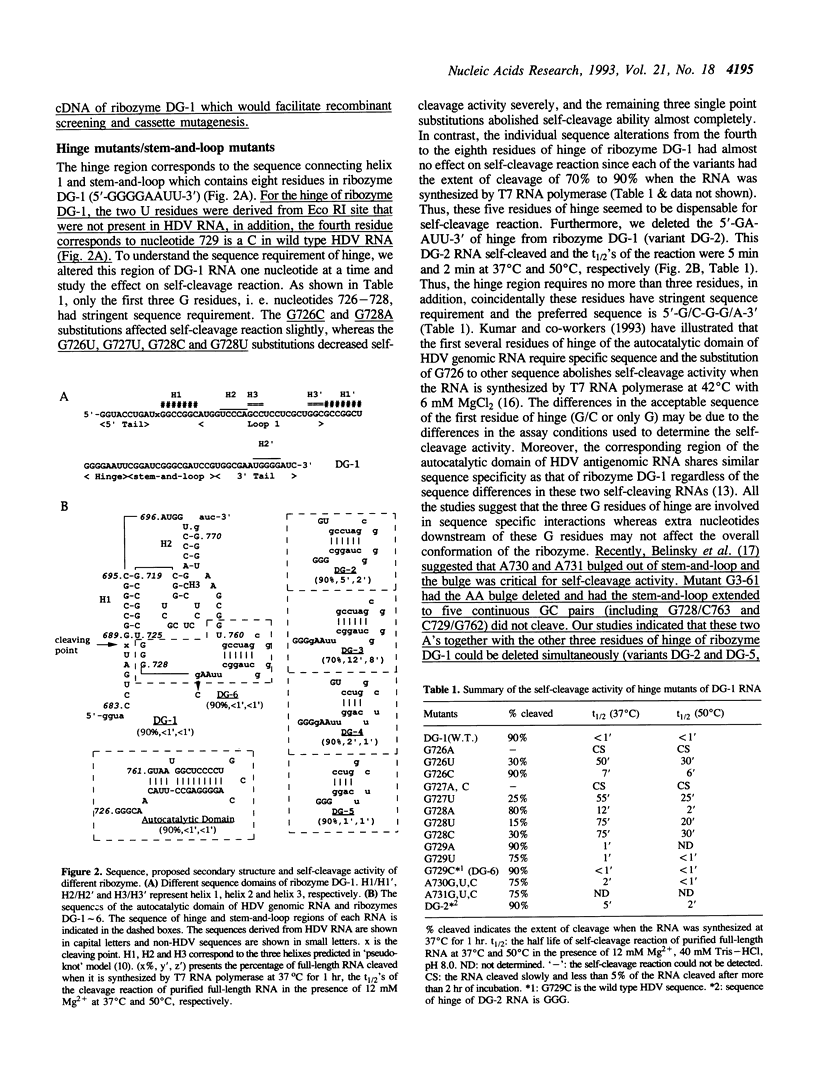
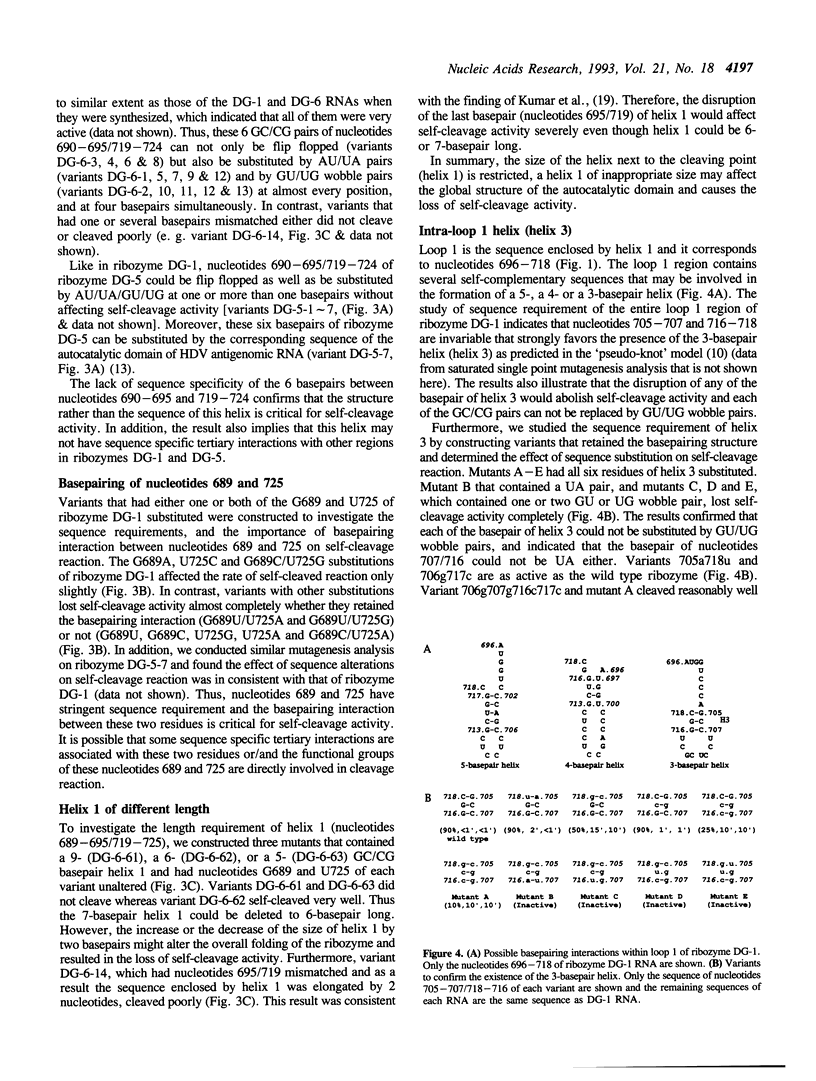
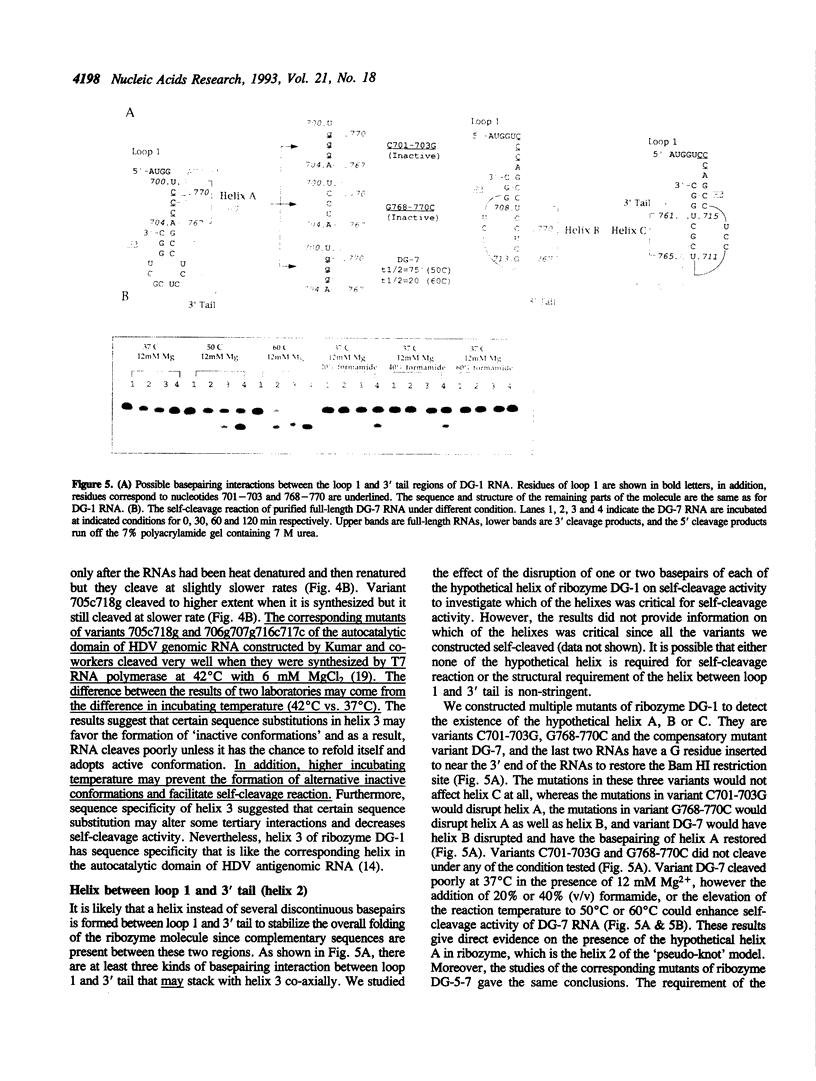
We conducted extensive mutagenesis analysis on a hepatitis delta virus (HDV) genomic ribozyme to study the sequence specificity of certain region and to derive the secondary structure associated with the catalytic core. The results confirmed that the autocatalytic domain of HDV genomic RNA contained four base-pairing regions as predicted in the 'pseudo-knot' model [Perrotta & Been (1990) Nature 350, 434-436]. The size and sequence of one of the base-pairing regions, i. e. stem-and-loop, could be flexible. Helix 3 and the first basepair of helix 1 required specific sequence to retain self-cleavage activity. The structural requirement of helix 2 was less stringent than the other base-pairing regions. Moreover, the size of helix 1 affected self-cleavage whereas the length of hinge could be variable even though the first three residues of hinge had stringent sequence requirement.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Been M. D., Perrotta A. T., Rosenstein S. P. Secondary structure of the self-cleaving RNA of hepatitis delta virus: applications to catalytic RNA design. Biochemistry. 1992 Dec 1;31(47):11843–11852. doi: 10.1021/bi00162a024. [DOI] [PubMed] [Google Scholar]
- Belinsky M. G., Britton E., Dinter-Gottlieb G. Modification interference analysis of a self-cleaving RNA from hepatitis delta virus. FASEB J. 1993 Jan;7(1):130–136. doi: 10.1096/fasebj.7.1.8422959. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D. Efficient trans cleavage and a common structural motif for the ribozymes of the human hepatitis delta agent. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10163–10167. doi: 10.1073/pnas.88.22.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong C., Varani G., Tinoco I., Jr Solution structure of an unusually stable RNA hairpin, 5'GGAC(UUCG)GUCC. Nature. 1990 Aug 16;346(6285):680–682. doi: 10.1038/346680a0. [DOI] [PubMed] [Google Scholar]
- Hampel A., Tritz R., Hicks M., Cruz P. 'Hairpin' catalytic RNA model: evidence for helices and sequence requirement for substrate RNA. Nucleic Acids Res. 1990 Jan 25;18(2):299–304. doi: 10.1093/nar/18.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos A., Dijkema R., Arnberg A. C., van der Meide P. H., Schellekens H. The hepatitis delta (delta) virus possesses a circular RNA. Nature. 1986 Oct 9;323(6088):558–560. doi: 10.1038/323558a0. [DOI] [PubMed] [Google Scholar]
- Kumar P. K., Suh Y. A., Miyashiro H., Nishikawa F., Kawakami J., Taira K., Nishikawa S. Random mutations to evaluate the role of bases at two important single-stranded regions of genomic HDV ribozyme. Nucleic Acids Res. 1992 Aug 11;20(15):3919–3924. doi: 10.1093/nar/20.15.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P. K., Suh Y. A., Taira K., Nishikawa S. Point and compensation mutations to evaluate essential stem structures of genomic HDV ribozyme. FASEB J. 1993 Jan;7(1):124–129. doi: 10.1096/fasebj.7.1.8422958. [DOI] [PubMed] [Google Scholar]
- Kuo M. Y., Sharmeen L., Dinter-Gottlieb G., Taylor J. Characterization of self-cleaving RNA sequences on the genome and antigenome of human hepatitis delta virus. J Virol. 1988 Dec;62(12):4439–4444. doi: 10.1128/jvi.62.12.4439-4444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Chang M. F., Shieh C. K., Kamahora T., Vannier D. M., Govindarajan S., Lai M. M. Molecular cloning and sequencing of a human hepatitis delta (delta) virus RNA. Nature. 1987 Sep 24;329(6137):343–346. doi: 10.1038/329343a0. [DOI] [PubMed] [Google Scholar]
- Perrotta A. T., Been M. D. A pseudoknot-like structure required for efficient self-cleavage of hepatitis delta virus RNA. Nature. 1991 Apr 4;350(6317):434–436. doi: 10.1038/350434a0. [DOI] [PubMed] [Google Scholar]
- Perrotta A. T., Been M. D. Cleavage of oligoribonucleotides by a ribozyme derived from the hepatitis delta virus RNA sequence. Biochemistry. 1992 Jan 14;31(1):16–21. doi: 10.1021/bi00116a004. [DOI] [PubMed] [Google Scholar]
- Rosenstein S. P., Been M. D. Evidence that genomic and antigenomic RNA self-cleaving elements from hepatitis delta virus have similar secondary structures. Nucleic Acids Res. 1991 Oct 11;19(19):5409–5416. doi: 10.1093/nar/19.19.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Wang K. S., Choo Q. L., Weiner A. J., Ou J. H., Najarian R. C., Thayer R. M., Mullenbach G. T., Denniston K. J., Gerin J. L., Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986 Oct 9;323(6088):508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]
- Wu H. N., Huang Z. S. Mutagenesis analysis of the self-cleavage domain of hepatitis delta virus antigenomic RNA. Nucleic Acids Res. 1992 Nov 25;20(22):5937–5941. doi: 10.1093/nar/20.22.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. N., Lin Y. J., Lin F. P., Makino S., Chang M. F., Lai M. M. Human hepatitis delta virus RNA subfragments contain an autocleavage activity. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1831–1835. doi: 10.1073/pnas.86.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. N., Wang Y. J., Hung C. F., Lee H. J., Lai M. M. Sequence and structure of the catalytic RNA of hepatitis delta virus genomic RNA. J Mol Biol. 1992 Jan 5;223(1):233–245. doi: 10.1016/0022-2836(92)90728-3. [DOI] [PubMed] [Google Scholar]



