Abstract
Humans typically show left-hemisphere dominance both for language and manual gestures. If this reflects a dependence of these behaviors on a common cerebral specialization, then healthy left-handers with atypical organization of language should show a similar pattern for gesture. Consistent with this hypothesis, we report fMRI data indicating that sinistrals (5/15) with bilateral, or right-lateralized, language representations in inferior frontal cortex exhibit a similar atypical pattern in inferior parietal representations of familiar gestures.
Keywords: praxis, language, lateralization, interrelations, asymmetries
Introduction
In the majority of humans, the left cerebral hemisphere plays a dominant role in the control of language and praxis (skilled gestural movements), including tool use (transitive) pantomimes and non-object-related (intransitive) gestures1 (Goodglass & Kaplan, 1963). Therefore, patients with extensive lesions of the left hemisphere often exhibit both aphasia – an impairment of productive and/or receptive language, and apraxia – a higher-level motor disorder that is most evident in tasks demanding pantomime, gesture, and/or imitation. Despite known cases of dissociation between aphasia and apraxia (e.g., Papagno, Della Sala, & Basso, 1993), it has long been hypothesized that the co-morbidity of these impairments reflects disruption of a left-lateralized cerebral specialization underlying both behaviors. One candidate specialization is the ability to construct symbolic representations, an essential component of productive language and praxis, as suggested by Finkelnburg’s concept of asymbolia (see Duffy & Liles, 19792). Alternatives include a left hemisphere advantage for representing motor sequences (Kimura & Archibald, 1974) or sequential hierarchies (Bradshaw & Nettleton, 1982; Greenfield, 1991).
To the extent that language and praxis rely on a common cerebral specialization, people with unusual lateralization of language should show a similar pattern in the organization of gesture representation. Indeed, epilepsy patients with atypically represented language tend to show similar lateralization of praxis, regardless of their handedness (Meador, et al., 1999). These results may, however, be influenced by disease-related functional reorganization. Though rare, at least some right-handed individuals with reversed lateralization of language provide evidence for a congruent praxis asymmetries, despite no history of brain injuries or seizures prior to abrupt onsets of their illnesses (Fischer, Alexander, Gabriel, Gould, & Milione, 1991; cf. Junque, Litvan, & Vendrell, 1986).
Here we used fMRI to investigate the cerebral organization of language and praxis in a sample known to show considerable natural variation in the cerebral organization of language, healthy left-handed adults (Knecht, et al., 2000). Because strongly left-handed individuals show considerably higher rates of right-hemisphere dominance for productive language (Knecht, et al., 2000), they provide a unique opportunity to evaluate the language-praxis relationship. The majority of our participants were expected to show the typical left-lateralized activation for productive language in inferior frontal cortex, in particular within Brodmann Areas (BAs) 44/45. These individuals should also show the usual pattern of left-lateralized increases in inferior parietal cortex (particularly in BA40) when representing familiar gestures. Left BA40 (supramarginal gyrus, SMG) lesions have long been associated with apraxia (Heilman, Rothi, & Valenstein, 1982; Haaland, Harrington, & Knight, 2000) and healthy adults show consistent, hand-independent increases in SMG activity during gesture planning and/or production (Kroliczak & Frey, 2009; Moll, et al., 2000; Rumiati, et al., 2004). Critically, roughly one-third of left-handers should show either bilateral, or right-lateralized activation in BA44/45 during productive language (Knecht, et al., 2000). If language and praxis rely on a common cerebral specialization, then these participants should show evidence for a congruent, atypical pattern of lateralization in BA40 activity when representing gestures for either hand.
Methods
From a pool of 51 self-identified left-handers, we selected only individuals in the upper quartile of left-handedness, as evaluated by the Edinburgh Handedness Inventory (Oldfield, 1971): N=15, 8 females, Mean Age = 24.8 years, SD = 8.4; Mean Edinburgh Laterality Index = −90.0; Range: −66 to −100; SD = 9.0. Ten of our subjects (67%) had a family history of left-handedness, and none had a history of neurological or psychiatric disorders.
Verbal Fluency Test
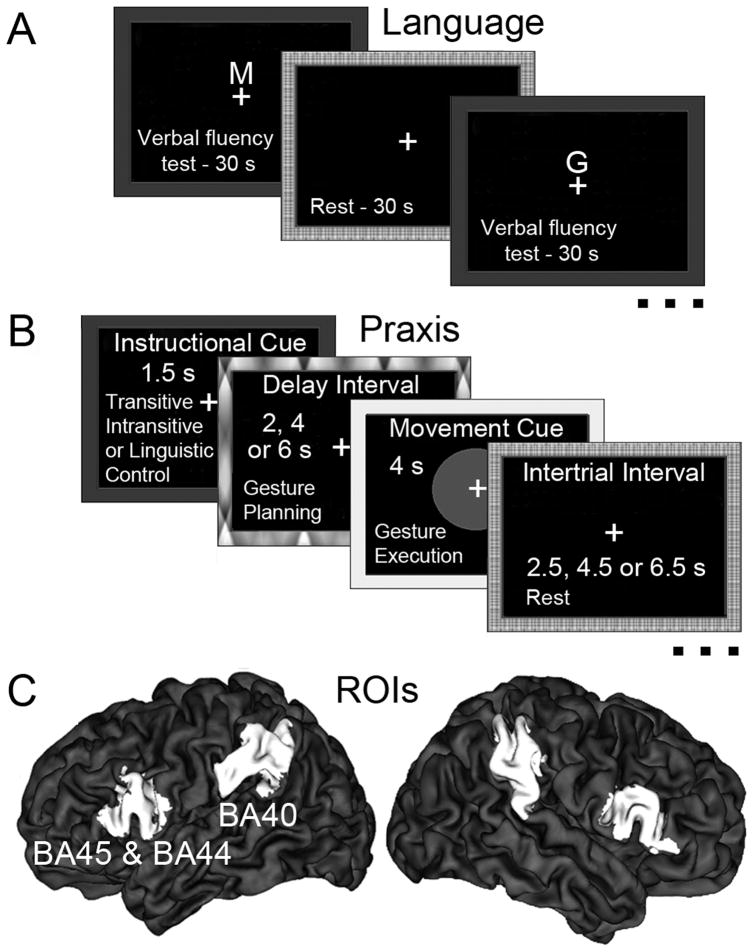
Results from previous fMRI studies employing this task have shown strong increases in the inferior frontal gyrus, including BA44/45, and laterality indices (LIs) derived from this activity show high concordance with results of the intracarotid barbiturate (Wada) test (Chlebus, et al., 2007), as well as strong test-retest reliability (Adcock, Wise, Oxbury, Oxbury, & Matthews, 2003). Participants silently generated as many words as possible that began with a visually presented letter (see Fig. 1A). These and remaining stimuli were back-projected on screen behind the scanner using Presentation software (http://www.neurobs.com) and were viewed via a mirror. An AB block design with a 30s verbal fluency task alternating with a 30s rest was used.
Figure 1.
(A) Trial timing in the productive language test. Instructional cue indicated that participants should silently generate as many words as possible starting with the presented letter (M, G, E, T, H, or L). (B) Trial structure and timing in the praxis test. The 1.5s instructional cue was followed by a variable delay (2, 4, or 6s) interval during which gestures were planned, and a 4s movement cue signaling gesture execution. Inter-trial intervals were 2.5, 4.5, or 6.5 s. (C) Regions of interest (ROIs). The probabilistic maps of Broca’s area and its counterpart in the right hemisphere (defined as Brodmann areas [BAs]44/45), as well as the left and right BA40 (defined as divisions PF and PFm of the inferior parietal lobule) were obtained from the Juelish histological atlas implemented in FSL. To avoid overlap with neighboring cytoarchitectonic areas, these maps were first thresholded at the 50th percentile of their maximum probability. These ROIs are shown on a three-dimensional, partially inflated surface rendering of an individual brain implemented in the CARET imaging software.
Gesture Planning Test
To assess the lateralization of praxis skills, participants were asked to plan familiar manual gestures in response to randomly presented visual cue words (see Fig. 1B). Thirty-three percent of the trials involved transitive gesture cues (e.g., “cutting”), and another 33% intransitive gesture cues (e.g., “scolding”). The remaining trials involved cues for non-physical actions (e.g., “believing”), and served as controls for activation of perceptual and linguistic processes such as reading and lexical access. Each volunteer completed two imaging sessions, one with the left hand and the other with the right hand, in counterbalanced order. As in our earlier work with right-handers (Kroliczak & Frey, 2009) we used an event-related design. Both sessions consisted of six (6), six-minute functional runs. For trial structure and timing see Fig. 1B and its caption.
The analyses focused on the premovement gesture-planning phase, with linguistic processing accounted for, i.e., the contrasts used were transitive and intransitive gesture planning vs. linguistic control. Planning-related activity in each condition was modeled as the 3.5s period starting with the onset of the instructional cue (1.5 s) and lasting through the offset of the shortest (2s) delay interval. Our previous work with right-handers (Kroliczak & Frey, 2009) has shown that this task is associated with strongly left-lateralized activity in posterior parietal cortex, including BA40, regardless of the hand (left, right) tested or gesture type (transitive, intransitive).
All scans were performed on a Siemens 3T Allegra MRI scanner at the Lewis Center for NeuroImaging and data were analyzed with FSL 4.1.4 (http://www.fmrib.ox.ac.uk/fsl/). All imaging parameters and preprocessing steps were identical to those described in our previous work with right-handers (Kroliczak & Frey, 2009). Upon averaging across multiple functional runs in each participant using a fixed effects model (recommended for a single-subject analysis in the FSL software), every subject’s activation was registered to his/her high-resolution anatomical brain. Subsequently, each individual’s activation maps were warped to the standard space of the MNI template brain using FMRIB’s Linear Image Registration Tool (FLIRT) (Jenkinson & Smith, 2001).
Using z-statistic images obtained from the two experimental tasks (i.e., silent word generation vs. rest, and transitive/intransitive gesture planning vs. linguistic control), in each individual we performed region-of-interest (ROI) analyses in probabilistically defined areas (Eickhoff, et al., 2007). ROIs were delineated with the Juelich Histological Atlas implemented in FSL 4.1.4. Because this atlas provides probabilistic cytoarchitectonic maps, to avoid an overlap with neighboring areas, these maps were first thresholded at the 50th percentile probability value (Eickhoff, et al., 2007). This way we achieved more precise assignments of functional activations to anatomically defined regions.
Language and Praxis Laterality Assessments
We assessed the laterality of language in BA44/45 (Amunts, et al., 2004). Known as Broca’s area in the left hemisphere, this region has long been implicated in language production (Dronkers, Plaisant, Iba-Zizen, & Cabanis, 2007). Gesture laterality was evaluated in BA40 (Caspers, et al., 2006). In the left hemisphere, this inferior parietal region has been consistently linked to praxis representation based on data from brain-injured patients (Haaland, et al., 2000; Heilman, et al., 1982) and from functional neuroimaging studies of healthy individuals (Kroliczak & Frey, 2009; Moll, et al., 2000; Rumiati, et al., 2004). Both ROIs are illustrated in Figure 1C on a partially inflated brain (Van Essen, 2005). The majority of subjects (73%) failed to show significant activation clusters in Broca’s area for gesture planning when linguistic processing was controlled. We therefore did not calculate LIs for praxis in BA44/45.
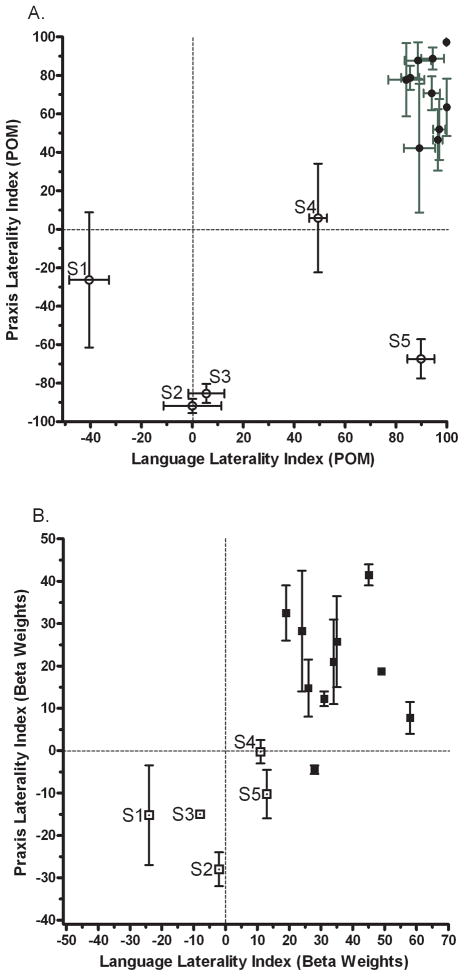
To establish task-related cerebral asymmetries in each participant, we first calculated language and gesture LIs using the spatial extent of activation in the left and right hemisphere ROIs. To this end, we counted the number of voxels whose activity exceeded several pre-specified percentage of maximum (POM) activation thresholds (Chlebus, et al., 2007), here 40%–90% of maximum z-value, in increments of 10 (see also Fig. 2A caption). This was done to guard against the possibility that results were biased by use of a particular threshold; a possibility given that the number of voxels in an activation cluster is influenced by threshold choice. For this reason, POM-derived LIs (POMLIs) yield more stable results when all activated voxels (with positive z-values) within anatomical ROIs are considered before applying the POM cut-offs (see Chlebus, et al., 2007). Moreover, to avoid basing conclusions only on voxel counting, which does not differentiate between activity levels of suprathreshold voxels, we also computed LIs using the estimated signal amplitude, i.e., beta weights. Only voxels with positive z-values within the selected anatomical ROIs contributed to this calculation (Jansen, et al., 2006).
Figure 2.
(A) Laterality indices of language and praxis based on activation extent. Voxels in the left and right ROIs that survived a specified percentage of maximum (POM) threshold of the z-statistic were tallied. Z-maximum was defined as the highest value within the paired ROIs. This was done separately for each task (language, praxis) and each brain region (BA44/45, BA40). Because voxel counts can be sensitive to thresholding, they were recalculated at 6 different z-maximum thresholds (40%–90%, in increments of 10). Separate laterality indices (LIs) based on these counts were then computed by using the formula (L-R/L+R)*100. A score of +100 indicates complete left laterality, −100 complete right laterality, and 0 an equally balanced engagement of both ROIs. Mean LIs were subsequently calculated across values obtained at each z-maximum threshold. Participants S1 through S5 (open circles) displayed atypical language or praxis dominance. Horizontal error bars indicate within-subject standard error of the mean language LI. Vertical error bars indicate within-subject standard error of the mean praxis LI, collapsing across two hands and two gesture subtypes. (B) Language and praxis LIs based on the magnitude of the fMRI signal change expressed as Beta weights. The mean Betas were derived from all activated voxels (having positive z-values) within an ROI. Notably, participants S1–S5 (open squares) again showed evidence of atypical language and praxis organization with this method. There are no horizontal error bars for language LIs because there is only one measurement for each participant. Vertical error bars indicate within-subject standard error of the mean obtained by collapsing the praxis LIs across the two gesture subtypes and two hands. Note that although LIs based on beta weights provide less information about hemispheric dominance, they show how language and praxis vary across individuals.
Specifically, for each individual, LIs were calculated as [(L–R)/(L+R)]*100, where L (Left) and R (Right) captured the number of suprathreshold voxels in the respective ROI. These LIs can range from +100 to −100, with 0 indicating an equal number of activated voxels in the left and right ROIs. The values of +100 through +33.3 indicate a strong to weak left-hemispheric dominance, and −33.3 through −100 reflect a weak to strong right-hemispheric dominance. Subsequently, LIs were also calculated using Beta Weights (parameter estimates) as inputs to the formula above (hence BEWLIs), again from the same anatomically-defined ROIs. Although the range of values for BEWLIs is narrower3 it has a more even distribution. Therefore, the use of BEWLIs provides a reliability check for the POMLIs and allows evaluating how the lateralization of task-related neural activity varies across participants (Jansen, et al., 2006).
To test directly if there are any relations between the lateralization of language and praxis, we first collapsed the praxis LIs across the two gestures categories and hands. Subsequently, correlations between language and praxis LIs were assessed with SPSS version 15.0 (Chicago, IL).
Results
As expected, we found strong correlations between individuals’ language and praxis LIs, both with POMLIs and BEWLIs (r = 0.73, p < 0.01, in each case).
As shown in Fig. 2, the majority of our left-handed participants demonstrated the typical left-hemisphere dominance both for language in BA44/45 and praxis in BA40 (67% falling in the upper right quadrant of Fig. 2A&B). More importantly, the 27% of individuals who showed either a weak left-hemisphere advantage (S4), atypical bilateral (S2, S3) or a weak right lateralized (S1) language organization in BA44/45 also demonstrated bilateral (S1, S4) or right lateralized (S2, S3) gesture representations in BA40 (Figs. 2A&B). Only a single participant (S5) showed evidence for bilateral language organization based just on signal amplitude, and still exhibited some evidence of the right hemisphere advantage for praxis4.
It is worth emphasizing that Fig. 2 depicts gesture and hand-independent laterality indices (i.e., praxis LIs collapsed across the two gestures categories and hands) because, with the exception of few subjects, these LIs were virtually identical or very similar in majority of our participants. This was indeed confirmed by significant correlations between all praxis LIs independent of whether they were based on activation extent or signal amplitude (see Table 1 & 2).
Tables 1 and 2.
Correlations between praxis laterality indices (LIs)
|
1) Correlations between POMLIs for all gestures | ||||
|---|---|---|---|---|
| A | B | C | D | |
| A. Transitive Left Hand | 1 | 0.81** | 0.72** | 0.67** |
| B. Intransitive Left Hand | 1 | 0.83** | 0.64* | |
| C. Transitive Right Hand | 1 | 0.92** | ||
| D. Intransitive Right Hand | 1 | |||
|
2) Correlations between BEWLIs for all gestures | ||||
|---|---|---|---|---|
| A | B | C | D | |
| A. Transitive Left Hand | 1 | 0.72** | 0.60** | 0.67** |
| B. Intransitive Left Hand | 1 | 0.85** | 0.52* | |
| C. Transitive Right Hand | 1 | 0.65** | ||
| D. Intransitive Right Hand | 1 | |||
Significant at the 0.01 level
Significant at the 0.05 level
**, * Pearson (2-tailed) correlations between laterality indices for both gesture types, irrespective of the hand used during gesture planning. POMLIs – laterality indices based on the percentage of maximum z-value. BEWLIs – laterality indices based on Beta Weights.
Discussion
These results provide compelling evidence for a link between the cerebral organization of language and praxis representations in the healthy brain, even when the linguistic demands of gesture stimulus processing are controlled. Across a sample of healthy, left-handed adults with considerable natural variation, we find that cerebral asymmetries in frontal representations of language are highly predictive of laterality in parietal representations of gestures. This relationship is consistent with the hypothesis that these fundamental behaviors exploit a common cerebral specialization. Our data do not resolve the nature of this processing specialization, but do suggest that it is not exclusive to the left hemisphere. Possibilities include the ability to construct symbolic representations (Duffy & Liles, 1979), and/or to represent motor sequences (Kimura & Archibald, 1974) or sequential hierarchies (Greenfield, 1991). Regardless of its identity, we suggest that in the minority of cases, this functional specialization is characteristic of processing in the right, or both, cerebral hemispheres (e.g., S1 in Figs. 2A & 2B) 5. Therefore, individuals can show consistent atypical patterns for both language and gesture representations. Further, our findings predict that the vast majority of individuals with atypical language organization should also have difficulties with gestural assessments of praxis following right-hemisphere lesions. Yet, because the strength of this relationship varies across participants, one also needs to be sensitive to the issue of individual differences and the underlying causes (cf. Margolin, 1980, and S5 in Fig. 2A).
Consistent with a putative evolutionary progression from manual to verbal abilities (Bradshaw & Nettleton, 1982; Greenfield, 1991), a recent report showed a close relationship between hand preference for precision grasping and hemispheric dominance for language processing (Gonzalez & Goodale, 2009). Our data, however, suggest that there is a hand-independent relationship between the lateralization of higher-order praxis representations involved in planning and language functions. Interestingly, these two studies demonstrate that laterality for language, precision grasping or gesture can differ from that of hand dominance, which in left-handers reflects a specialization of the right hemisphere. This dissociation would not be expected if human language and skilled manual actions both depended on mechanisms underlying hand dominance (Greenfield, 1991).
Using functional Doppler ultrasonography, Knecht et al. (2000) determined that a sizable minority (29%) of strong left-handers who also had a left-handed parent exhibited right-hemisphere language dominance. Although our study employed fMRI, the frequency of atypical lateralization of language (27% or 33%, depending on the analytical method used) is quite consistent. Atypical lateralization of functions may result from early brain damage (Kimura, 1983). However, our sample consisted of healthy adults without known history of neurological and psychiatric illnesses. Therefore, these findings likely represent natural variation in functional asymmetries. A greater understanding of the interrelationships between seemingly disparate brain functions (in our case language and praxis), as well as more accurate models of lateralization, will entail greater appreciation of the range or normal variability in the population.
Acknowledgments
This research was supported by a grant (#NS053962) from NIH/NINDS to S.H.F.
We thank David Carey and anonymous reviewers for their constructive feedback on earlier versions of this manuscript.
Footnotes
The categorization of gestures into transitive and intransitive has a long history and figures prominently in modern theories of praxis skills (Gonzalez Rothi, Ochipa, & Heilman, 1991; see also Kroliczak & Frey, 2009).
This paper provides an English translation and discussion of Finkelnburg’s 1870 lecture. Heilman & Rothi, 1997 provide a historical review of opposing perspectives, including Liepmann’s conviction that apraxia is not a disorder of symbolic processing.
The values of +100 or −100 for BEWLIs would be obtained only if all voxels within one of the corresponding anatomical ROIs had no activation or were deactivated (i.e., z-values ≤ 0). We did not have such a case.
Conflicting findings on the lateralization of functions using the two methods of LI calculation, most evident here for language in S5, can occur when the activation map in one hemisphere has both higher signal amplitude and smaller spatial extent than in the other hemisphere. This case illustrates the importance of considering both signal amplitude and spatial extent when using LIs.
Cf. Goldenberg, Hartmann, & Schlott, 2003; who question asymbolia/apraxia, but also consider a general left-hemisphere function.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18(2):423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, et al. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space--the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22(1):42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Nettleton NC. Language lateralization to the dominant hemisphere: Tool use, gesture and language in hominid evolution. Curr Psychol Rev. 1982;2(No 2):171–192. [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33(2):430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Chlebus P, Mikl M, Brazdil M, Pazourkova M, Krupa P, Rektor I. fMRI evaluation of hemispheric language dominance using various methods of laterality index calculation. Exp Brain Res. 2007;179(3):365–374. doi: 10.1007/s00221-006-0794-y. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130(Pt 5):1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- Duffy RJ, Liles BZ. A translation of Finkelnburg’s (1870) lecture on aphasia as “asymbolia” with commentary. J Speech Hear Disord. 1979;44(2):156–168. doi: 10.1044/jshd.4402.156. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Fischer RS, Alexander MP, Gabriel C, Gould E, Milione J. Reversed lateralization of cognitive functions in right handers. Exceptions to classical aphasiology. Brain. 1991;114(Pt 1A):245–261. [PubMed] [Google Scholar]
- Goldenberg G, Hartmann K, Schlott I. Defective pantomime of object use in left brain damage: apraxia or asymbolia? Neuropsychologia. 2003;41(12):1565–1573. doi: 10.1016/s0028-3932(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez CL, Goodale MA. Hand preference for precision grasping predicts language lateralization. Neuropsychologia. 2009;47(14):3182–3189. doi: 10.1016/j.neuropsychologia.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Gonzalez Rothi LJ, Ochipa C, Heilman KM. A cognitive neuropsychological model of limb praxis. Cognitive Neuropsychology. 1991;8(6):443–458. [Google Scholar]
- Goodglass H, Kaplan E. Disturbance of Gesture and Pantomime in Aphasia. Brain. 1963;86:703–720. doi: 10.1093/brain/86.4.703. [DOI] [PubMed] [Google Scholar]
- Greenfield PM. Language, tools and brain: The ontogeny and phylogeny of hierarchically organized sequential behavior. Behav Brain Sci. 1991;14:531–595. [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123:2306–2313. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Rothi LJ, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32(4):342–346. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Rothi LJG. Limb apraxia: A look back. In: Rothi LJG, Heilman KM, editors. Apraxia: The neuropsychology of action. Hove, England: Psychology Press/Erlbaum (UK) Taylor & Francis; 1997. pp. 7–18. [Google Scholar]
- Jansen A, Menke R, Sommer J, Forster AF, Bruchmann S, Hempleman J, et al. The assessment of hemispheric lateralization in functional MRI--robustness and reproducibility. Neuroimage. 2006;33(1):204–217. doi: 10.1016/j.neuroimage.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Junque C, Litvan I, Vendrell P. Does reversed laterality really exist in dextrals? A case study. Neuropsychologia. 1986;24(2):241–254. doi: 10.1016/0028-3932(86)90056-4. [DOI] [PubMed] [Google Scholar]
- Kimura D. Speech representation in an unbiased sample of left-handers. Hum Neurobiol. 1983;2(3):147–154. [PubMed] [Google Scholar]
- Kimura D, Archibald Y. Motor functions of the left hemisphere. Brain. 1974;97(2):337–350. doi: 10.1093/brain/97.1.337. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Kroliczak G, Frey SH. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cereb Cortex. 2009;19(10):2396–2410. doi: 10.1093/cercor/bhn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin DI. Right hemisphere dominance for praxis and left hemisphere dominance for speech in a left-hander. Neuropsychologia. 1980;18(6):715–719. doi: 10.1016/0028-3932(80)90114-1. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Loring DW, Lee K, Hughes M, Lee G, Nichols M, et al. Cerebral lateralization: relationship of language and ideomotor praxis. Neurology. 1999;53(9):2028–2031. doi: 10.1212/wnl.53.9.2028. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Passman LJ, Cunha FC, Souza-Lima F, Andreiuolo PA. Functional MRI correlates of real and imagined tool-use pantomimes. Neurology. 2000;54(6):1331–1336. doi: 10.1212/wnl.54.6.1331. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papagno C, Della Sala S, Basso A. Ideomotor apraxia without aphasia and aphasia without apraxia: the anatomical support for a double dissociation. Journal of Neurology, Neurosurgery and Psychiatry. 1993;56(3):286–289. doi: 10.1136/jnnp.56.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, et al. Neural basis of pantomiming the use of visually presented objects. Neuroimage. 2004;21(4):1224–1231. doi: 10.1016/j.neuroimage.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]