Abstract
(See the editorial commentary by Finelli and Chaves, on pages 1701-4.)
Background. Previous studies identifying associations between influenza and acute cardiac events may have been confounded by climatic factors. Differing seasonal patterns of influenza activity in Hong Kong and England and Wales provide a natural experiment to examine associations with myocardial infarction (MI) independent of cold weather effects.
Methods. Weekly clinical and laboratory influenza surveillance data, environmental temperature and humidity data, and counts of MI-associated hospitalizations and deaths were obtained for England and Wales and for Hong Kong for the period 1998–2008. We used Poisson regression models that included environmental and seasonal variables to investigate the relationship between influenza and MI.
Results. There were ≥1.2 million MI-associated hospitalizations and 410,204 MI-associated deaths in England and Wales, with a marked peak in the winter season. In Hong Kong, the incidence of MI, on the basis of 65,108 hospitalizations and 18,780 deaths, had a large winter and smaller summer peak, mirroring patterns of influenza activity. There was strong evidence for a link between influenza and MI both in England and Wales, where 3.1%–3.4% of MI-associated deaths (P < .001) and 0.7%–1.2% of MI-associated hospitalizations (P < .001) were attributable to influenza, and in Hong Kong, where the corresponding figures were 3.9%–5.6% (P = .018) and 3.0%–3.3% (P = .002).
Conclusions. Influenza was associated with an increase in MI-associated deaths and hospitalizations in 2 contrasting settings.
Influenza causes a heavy burden of morbidity and mortality throughout the world in both temperate and subtropical zones [1, 2]. This burden is likely to be underestimated, because influenza may play a role in hospitalizations and deaths due to other conditions, such as cardiovascular disease [3]. Some studies suggest links between influenza and acute cardiac events, such as myocardial infarction (MI), although this relationship has not not been well characterized [4–7].
In temperate zones, such as the United Kingdom, both influenza and deaths due to MI demonstrate a clear seasonal pattern, with peaks in winter [8]. Environmental factors common during winter, such as low temperature and humidity, may affect blood clotting and predispose persons to thrombotic states [9, 10]. Because influenza levels are collinear in time with winter, confounding by cold weather conditions has traditionally been difficult to explore. However, in Hong Kong's subtropical climate, winters are milder and patterns of influenza seasonality more variable [11]: There are typically 2 periods of peak influenza circulation every year.
We assessed the relationship between population levels of influenza circulation and acute MI–associated admissions and deaths in England and Wales and in Hong Kong, where the summer influenza peak provided a natural experiment to examine any relationship with MI independent of cold weather effects.
METHODS
Data on Clinical Outcomes
Data were obtained on MI-associated hospitalizations and deaths occurring during the period January 1999 through December 2008 classified using the International Classification of Diseases, Ninth Revision (ICD-9 code 410) and Tenth Revision (ICD-10 codes I-21, I-22 and I-23). In England, numbers of MI-associated hospitalizations based on discharge diagnosis of finished consultant episodes by fiscal week of admission, age group, sex, and region were obtained from Hospital Episode Statistics (the NHS Information Centre for health and social care), and daily MI mortality data by age group and sex in England and Wales were obtained from the Office for National Statistics (the NHS Information Centre for health and social care). Equivalent MI data for Hong Kong for 1998–2008 were obtained from the Hospital Authority of Hong Kong (hospitalizations) and the Hong Kong Special Administrative Region Census and Statistics Department (deaths). Numbers of MI-associated hospitalizations and deaths in both regions were aggregated by influenza surveillance week. Age-standardized MI rates were calculated with reference to the World Health Organization World Standard Population. Hospitalization data for 2 counterfactual conditions—colon cancer (ICD-9 codes 153 and 154) and fractured neck of femur (ICD-9 820; chosen due to its increased incidence in winter)—were also obtained.
Influenza Surveillance Data
Weekly influenza surveillance data from sentinel surveillance schemes were obtained for both countries. In England and Wales, the Weekly Returns Service provides rates of general practitioner (GP) consultations for influenza-like illness (ILI) per 100,000 persons. ILI diagnoses are clinically based, although no formal definition is used. These data for 1999–2008 were obtained from the Royal College of General Practitioners network. The weekly proportions of nose and throat swabs testing positive for influenza virus during the influenza season (week 40–week 20) were from the Health Protection Agency/RCGP swabbing scheme, in which ∼50 general practices in England obtained nose and throat swabs from patients presenting with ILI. In Hong Kong, weekly rates of ILI consultations per 1000 persons reported by sentinel General Practitioner and General Out-Patient Clinics (GOPC) were obtained from the Centre for Health Protection [12]. ILI was defined as fever plus either cough or sore throat. Laboratory surveillance data obtained from the same source comprised monthly proportions of specimens that tested positive for influenza obtained from patients who presented to GPs or GOPC settings with ILI, as well as from patients hospitalized for acute respiratory diseases. Linear interpolation was used to generate weekly proportions of specimens testing positive from these monthly data. In sensitivity analysis, monthly proportions of influenza virus–positive specimens were interpolated to weekly proportions using spline functions.
Environmental Variables
Daily minimum, mean, and maximum temperatures in central England were obtained from the British Atmospheric Data Centre. These cover a roughly triangular area bounded by Bristol, Lancashire, and London. The MIDAS Land Surface Observation Stations dataset was used to provide daily data on relative humidity for an approximately equivalent area (incorporating weather stations in Somerset, Lancashire, and London). For Hong Kong, daily data on minimum, mean, and maximum temperature and mean daily relative humidity were obtained from the Hong Kong Observatory. The mean of each daily temperature and humidity parameter was calculated across influenza surveillance weeks.
Statistical Methods
The weekly number of MI-associated events in each country was modeled using a Poisson regression model with a scale parameter set to the Pearson χ2 statistic divided by the residual degrees of freedom to model over-dispersion. We adjusted for long-term trends in MI using both a linear and quadratic term for calendar year. In Hong Kong, data from 2003 were excluded from analysis, because the 2003 outbreak of severe acute respiratory syndrome (SARS) substantially affected both health-seeking behavior and the reliability of reporting. We controlled for seasonality using Fourier terms with 6 harmonics per year. In sensitivity analysis, we used an indicator variable for month to model MI seasonality in place of Fourier terms. We also modeled both seasonality and long-term trends using spline functions.
The primary exposure was weekly levels of influenza. In England and Wales, weekly GP consultations for ILI were used to represent circulating influenza: In temperate zones, weeks with highest ILI rates correspond to or closely track weeks with the highest proportion of samples testing positive for influenza virus [13]. In Hong Kong, the primary measure of influenza used was weekly proportion of specimens testing positive for influenza virus: Patterns of influenza seasonality are less clear in subtropical climates, so ILI data are thought to be less specific for influenza than in temperate zones [14]. Influenza surveillance data are potentially affected by delays in both consulting and reporting and thus may lag behind the true community incidence of infection. Therefore, we performed separate regressions with the exposure variable lagged up to 4 weeks in either direction. Results are presented as an incidence rate ratio (IRR) for MI-associated hospitalization or death for a 10th–90th percentile change in influenza circulation. Final models were chosen with reference to the lowest Akaike information criterion [15].
Models also included mean weekly temperature and relative humidity, both modeled as 4-knot natural cubic splines to allow for nonlinearity. Sensitivity analyses included use of weekly mean temperature modeled as a linear term and as a low threshold effect, with the cutoff based on graphs showing where the predicted risk ratio of MI-associated death or hospitalization rose to >1, and use of daily minimum and maximum temperatures averaged separately by week and included in models as natural cubic splines.
We examined the partial autocorrelation function to investigate the presence of any residual autocorrelation. All models were fitted with a term for residuals lagged by 1 week, because some degree of autocorrelation at a lag of 1 week remained after adjusting for yearly and seasonal patterns.
We repeated analyses using 2 different outcomes unlikely to be associated with influenza circulation: hospital admissions for colon cancer and fractured neck of femur. We also conducted an exploratory analysis to examine the relationship between influenza and MI by age and sex. Finally, we calculated the proportion of MIs attributed to influenza by predicting the number of MIs under the final model (X) and under a model assuming zero circulating influenza (Y) as (X − Y)/X. This calculation was repeated for weeks of high influenza circulation (≥90th percentile of ILI consultations or proportion of specimens testing positive). All analyses were performed using Stata software, version 11.0 (Stata Corp).
RESULTS
During the period from January 1999 through December 2008, there were 1,219,150 MI-associated hospitalizations (median, 2421 per week; interquartile range [IQR], 2112–2578) in England, of which 62.5% occurred in male patients. The median weekly age-standardized rate was 2.81 cases per 100,000 persons. Over the same time period 410,204 MI-associated deaths (median, 777 deaths per week; IQR, 639–908 deaths per week) were reported in England and Wales. Both MI-associated deaths and hospitalizations demonstrated a marked winter peak. GP consultation rates for ILI varied from 0.8 to 270.8 consultations per 100,000 persons per week (mean, 16.2 consultations per 100,000 persons per week) and were highest in 1998–1999 and 1999–2000, corresponding to circulation of the A/Sydney/5/97 strain of influenza A H3N2 subtype. ILI consultations showed a similar distribution to the weekly percentage of specimens testing positive for influenza virus during the influenza season, which ranged from 0% to 100% (mean, 18.1%).
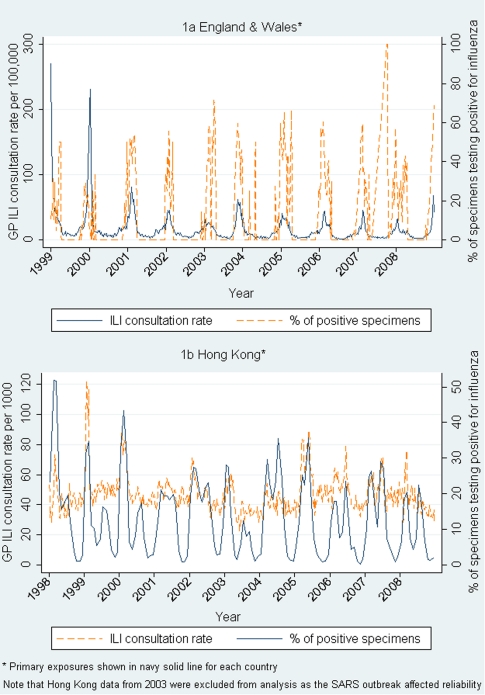
In Hong Kong, during the period from January 1998 through December 2008, there were 65,108 MI-associated hospitalizations (median, 110 per week [IQR, 97–126]; median weekly age-standardized rate, 1.11 cases per 100,000 persons), with 59.6% occurring in male patients and 18,780 MI-associated deaths (median, 32 deaths per week; IQR, 27–38 deaths per week). There was a large winter peak, as well as a smaller summer increase in the number of MIs. The percentage of specimens testing positive for influenza virus (measured throughout the year) varied from 0.3% to 51.9% (mean, 13%) per week. Corresponding ILI consultation rates in GP and outpatient clinics are shown in Table 1, as are additional descriptions of exposure variables. Figure 1 demonstrates the weaker correlation in Hong Kong between clinical ILI and laboratory isolation rates.
Table 1.
Description of Influenza and Meteorological Variables in England and Wales and Hong Kong Over the Study Period
| Site, variable | No. of weeks | Mean ± SD | Minimum | P10 | P25 | P50 | P75 | P90 | Maximum |
| England and Wales | |||||||||
| Influenza GP consultation rate for ILI, per 100,000 persons Specimens testing positive for influenza virus, % |
520 325† |
16.2 ± 24.1 18.1 ± 20.9 |
0.8 0 |
3.0 0 |
5.1 0 |
9.5 9.6 |
18.2 33.3 |
34.2 50 |
270.8 100 |
| Meteorological variables Mean temperature, °C Relative humidity, % |
520 520 |
10.4 ± 4.8 80.6 ± 5.3 |
-0.1 61.8 |
4.2 73.4 |
6.4 76.8 |
10.2 81.1 |
14.5 84.6 |
16.7 87.2 |
22.0 93.6 |
| Hong Kong | |||||||||
| Influenza GP consultation rate for ILI, per 1000 persons GOPC consultation rate for ILI, per 1000 persons Specimens testing positive for influenza, % |
571 571 570 |
47.1 ± 12.4 5.4 ± 2.6 13.0 ± 10.3 |
22.9 1.0 0.3 |
35.0 2.8 1.8 |
38.9 3.7 4.1 |
45.3 4.8 10.7 |
52.0 6.5 19.4 |
60.3 8.3 27.0 |
123.0 19.7 51.9 |
| Meteorological variables Mean temperature, °C Relative humidity, % |
571 571 |
23.6 ± 4.8 78.0 ± 7.9 |
11.4 40.6 |
16.9 66.9 |
19.7 74.3 |
24.7 79.4 |
27.7 83.4 |
29.0 86.6 |
30.5 93.6 |
NOTE. Laboratory data for England and Wales on the proportion of specimens testing positive for influenza were available only during the influenza surveillance season (weeks 40–20); the mean weekly number of specimens was 37. For Hong Kong the mean weekly number of specimens was 508 (or 2192 per month). GOPC, general outpatient clinics; GP, general practitioner; ILI, influenza-like illness; SD, standard deviation; P, percentile.
Figure 1.
Weekly general practitioner (GP) consultation rates for influenza-like illness and percentage of positive specimens. SARS, severe acute respiratory syndrome.
Association Between Influenza and MI-Associated Deaths
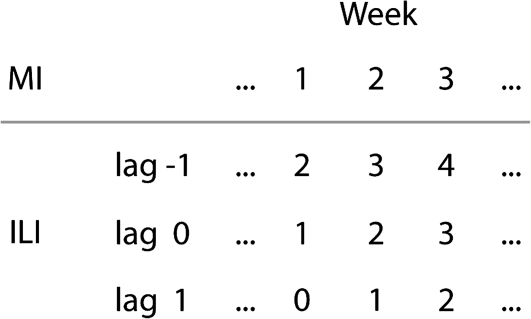
In England and Wales, a strong association was seen between GP consultations for ILI and MI-associated deaths (IRR, 1.051; 95% confidence interval [CI], 1.043–1.058; P < .001 for a 10th–90th percentile change in ILI consultations occurring 1 week later) after adjusting for environmental temperature and humidity. The best-fitting models included lags of either −1 week (as above) or −2 weeks (IRR, 1.056; 95% CI 1.049–1.064; P < .001), although a significant association remained in the model with no lag time (IRR, 1.036; 95% CI, 1.028–1.043; P < .001). An additional description of the lag time between ILI consultations and MI-associated deaths is shown in Figure 2.
Figure 2.
Schematic illustration of the interpretation of lag times in the analysis of associations between myocardial infarction (MI)–associated death and influenza-like illness (ILI) consultations. For example in the analysis with a lag time of −1 week, MI-associated deaths in week 1 are correlated with ILI consultations in week 2, etc.
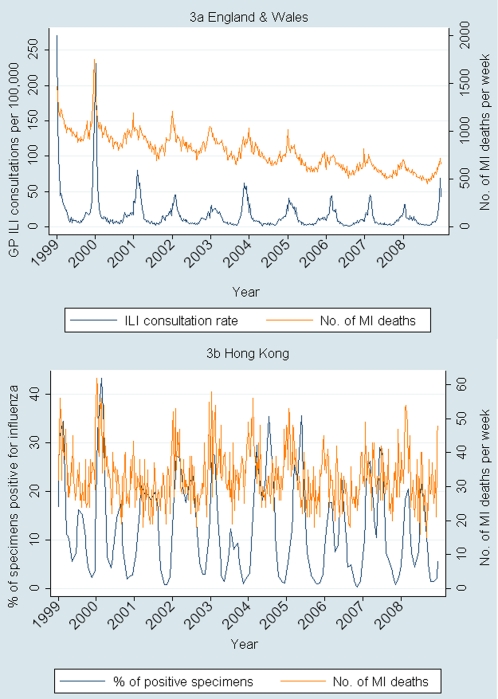
In Hong Kong, there was a similarly robust association between the proportion of specimens testing positive for influenza virus and MI-associated deaths occurring in the same week (IRR, 1.077; 95% CI, 1.013–1.145; P = .018) for a 10th–90th percentile change in proportion of positive specimens after adjusting for temperature and relative humidity. The best model fits were seen around lag 0, with similar results given by models including lags of −1 week (IRR, 1.076; 95% CI, 1.012–1.144; P = .02) and +1 week (IRR, 1.074; 95% CI 1.010–1.142; P = .023). Seasonal patterns of influenza circulation and MI-associated deaths are shown in Figure 3 for the 2 countries.
Figure 3.
Weekly influenza circulation and number of myocardial infarction (MI)–associated deaths. ILI, influenza-like illness.
Association Between Influenza and MI-Associated Hospitalizations
In England and Wales, ILI consultations lagged by −1 to −3 weeks (representing the best model fits) were associated with MI-associated hospitalization after adjusting for environmental variables. There was strong evidence of a small effect (IRR for a lag of −1 week, 1.009 [95% CI, 1.003–1.015; P = .004]; IRR for a lag of −2 weeks, 1.013 [95% CI, 1.008–1.019; P < .001]; IRR for a lag of −3 weeks, 1.012 [95% CI, 1.006–1.019; P < .001]). There was no association between ILI consultation rates and MI-associated hospitalizations reported in the same week (adjusted IRR, 1.002; 95% CI, 0.996–1.003; P = .59).
In Hong Kong, an association was seen between the proportion of influenza positive specimens and MI-associated hospitalizations in the same week (IRR, 1.066; 95% CI, 1.024–1.109; P = .002) after adjustment for environmental variables. Similar model fits and results were given by models including lag times of −1 week (IRR, 1.067; 95% CI 1.025–1.110; P = .001) and +1 week (IRR, 1.066; 95% CI 1.024–1.109; P = .002). Table 2 shows the effect of including different lag times for influenza reporting on MI-associated hospitalizations.
Table 2.
Associations Between Myocardial Infarction (MI) Events (Hospitalizations or Deaths) and Influenza Circulation, Lagged by Differing Numbers of Weeks, in England and Wales and in Hong Kong
| Lag time, weeksa | England and Wales |
Hong Kong |
||
| Adjusted IRR (95% CI)b | P | Adjusted IRR (95% CI)c | P | |
| MI-associated hospitalizations | ||||
| –4 –3 –2 –1 0 +1 +2 +3 |
1.006 (1.000–1.012) 1.012 (1.006–1.019) 1.013 (1.008–1.019) 1.009 (1.003–1.015) 1.002 (0.996–1.008) 0.996 (0.990–1.002) 0.994 (0.988–1.000) 0.997 (0.991–1.003) |
.05 <.001 <.001 .004 .59 .21 .04 .35 |
1.032 (0.991–1.074) 1.043 (1.002–1.086) 1.057 (1.015–1.100) 1.067 (1.025–1.110) 1.066 (1.024–1.109) 1.066 (1.024–1.109) 1.059 (1.017–1.102) 1.035 (0.994–1.078) |
.13 .04 .007 .001 .002 .002 .005 .09 |
| MI-associated deaths | ||||
| –4 –3 –2 –1 0 +1 +2 +3 |
1.020 (1.012–1.028) 1.042 (1.033–1.050) 1.056 (1.049–1.064) 1.051 (1.043–1.058) 1.036 (1.028–1.043) 1.021 (1.013–1.029) 1.013 (1.005–1.021) 1.010 (1.002–1.018) |
<.001 <.001 <.001 <.001 <.001 <.001 .002 .015 |
1.010 (0.950–1.074) 1.027 (0.966–1.092) 1.043 (0.981–1.110) 1.076 (1.012–1.144) 1.077 (1.013–1.145) 1.074 (1.010–1.141) 1.068 (1.004–1.136) 1.056 (0.992–1.124) |
.75 .40 .18 .020 .018 .023 .037 .089 |
NOTE. CI, confidence interval; IRR, incidence rate ratio.
aNote a lag time of, for example, -2 weeks refers to influenza activity occurring 2 weeks after MI events in week 0, whereas a lag of +2 weeks refers to ILI consultations taking place 2 weeks before MI events in week 0.
IRR for a 10th–90th percentile change in GP influenza-like illness consultations adjusted for seasonality and long-term trends, weekly mean temperature, weekly mean relative humidity, and residual autocorrelation.
IRR for a 10th–90th percentile change in proportion of specimens testing positive for influenza adjusted for the same factors as above.
Sensitivity Analyses
Adjustments were made to the final model to test the robustness of our effect estimates. Modeling MI seasonality using alternative methods, such as spline functions or indicator variables, for month with a linear year variable had little effect on the magnitude and direction of influenza effect estimates. Including weekly mean temperature modeled as a linear term and as a low threshold effect gave similar results to the final model in which temperature was included as a natural cubic spline. The best model fits were seen at temperature lags of either 0 or 1 week, which gave similar results. Use of the mean of weekly maximum and then of weekly minimum temperatures modeled as natural cubic splines made little difference to effect estimates. In Hong Kong, use of the weekly percentage of positive specimens interpolated using a spline function rather than simple linear interpolation gave slightly lower point estimates but similar effects for both MI-associated deaths and MI-associated hospitalizations. Results of the main sensitivity analyses are shown in table 3.
Table 3.
Sensitivity Analyses for Models of the Association Between Influenza and Myocardial Infarction (MI) Hospitalizations or Deaths in England and Wales and in Hong Kong Showing the Effect of Varying Seasonality, Temperature, and Measures of Exposure
| Sensitivity analyses | England and Wales |
Hong Kong |
||||||
| MI-associated hospitalizations |
MI-associated deaths |
MI-associated hospitalizations |
MI-associated deaths |
|||||
| IRR (95% CI) | P | IRR (95% CI) | P | IRR (95% CI) | P | IRR (95% CI) | P | |
| Final model | 1.013 (1.008–1.019)* | <.001 | 1.056 (1.049–1.064)* | <.001 | 1.066 (1.024–1.109)† | .002 | 1.077 (1.013–1.145)† | .018 |
| Seasonality Indicator month variable Splines (3 or 5 knots per year) |
1.016 (1.010–1.022) 1.025 (1.016–1.034) |
<.001 <.001 |
1.063 (1.055–1.071) 1.063 (1.052–1.073) |
<.001 <.001 |
1.059 (1.019–1.101) 1.066 (1.016–1.118) |
.004 .009 |
1.088 (1.025–1.155) 1.113 (1.030–1.201) |
.006 .006 |
| Temperature Linear term for mean temperature Low threshold effect Ncs of maximum temperature Ncs of minimum temperature Ncsφ of mean temperature averaged across weeks 0 and 1 |
1.014 (1.008–1.020) 1.014 (1.008–1.020) 1.014 (1.008–1.020) 1.013 (1.007–1.019) 1.012 (1.006–1.017) |
<.001 <.001 <.001 <.001 <.001 |
1.057 (1.050–1.065) 1.057 (1.050–1.065) 1.056 (1.049–1.064) 1.056 (1.048–1.063) 1.056 (1.049–1.063) |
<.001 <.001 <.001 <.001 <.001 |
1.066 (1.024–1.109) 1.061 (1.019–1.104) 1.065 (1.023–1.109) 1.065 (1.024–1.109) 1.067 (1.027–1.108) |
.002 .004 .002 .002 .001 |
1.076 (1.012–1.145) 1.073 (1.009–1.141) 1.076 (1.012–1.144) 1.076 (1.012–1.144) 1.074 (1.010–1.141) |
.019 .026 .020 .020 .021 |
| Percentage of positive specimens from spline interpolation of monthly data |
… | … | … | … | 1.058 (1.021–1.097) | .002 | 1.048 (.993–1.107) | .090 |
NOTE. CI, confidence interval; IRR, incidence rate ratio; Ncs, natural cubic spline.
IRR for the effect of a 10th–90th percentile change in weekly GP influenza-like illness consultations lagged by -2 weeks on MI, adjusted for seasonality and long-term trends, weekly mean temperature, weekly mean relative humidity, and residual autocorrelation.
IRR for the effect of a 10th–90th percentile change in weekly proportion of specimens testing positive for influenza virus on MI (with no lag), adjusted for seasonality and long-term trends, weekly mean temperature, weekly mean relative humidity, and residual autocorrelation.
Hospitalizations for colon cancer (adjusted IRR, 0.98; 95% CI, 0.94–1.01; P = .21) and fractured neck of femur (adjusted IRR, 0.99; 95% CI, 0.96–1.02; P = .68) were not associated with influenza circulation. In both countries, the strongest associations between influenza and MI were seen in the oldest age groups (ie, age of >80 years and, to a lesser extent, age of 60–79 years); see Appendix Table 4.
Predicted Percentage of MIs Attributable to Influenza
Proportions of MI-associated deaths attributed to influenza under the final models ranged from 3.9% to 5.6% for Hong Kong and 3.1% to 3.4% for England and Wales, depending on the model of seasonality used. Proportions of MI-associated hospitalizations attributed to influenza were smaller in both settings: 3.0%–3.3% and 0.7%–1.2%, respectively. In weeks in which influenza circulation was in the ≥90th percentile, 9.7%–13.6% of MI-associated deaths in Hong Kong and 10.7%–11.8% of MI-associated deaths in England and Wales were attributed to influenza. For MI-associated hospitalizations, the corresponding figures were 7.5%–8.2% and 2.5%–4.6%.
DISCUSSION
Our results demonstrate strong associations between population levels of influenza and MI-associated deaths and hospitalizations in both a temperate (England and Wales) and subtropical climate (Hong Kong) after adjustment for seasonality and relevant environmental confounders. We estimate that a small but significant proportion of MIs in both settings may be attributed to influenza, with figures increasing in weeks of highest influenza circulation.
Similar studies have shown rises in related but less specific outcomes, such as deaths due to cardiovascular disease [8, 16, 17], or less sensitive outcomes, such as autopsy-confirmed MI-associated deaths [18], during influenza epidemics. However, we believe that this is the first study to examine the relationship between influenza circulation and national rates of fatal and nonfatal MI in 2 different settings and populations. It has been suggested that systemic prothombotic [19] and pro-inflammatory [20] effects of influenza or other infectious agents may acutely destabilize vulnerable atherosclerotic plaques, leading to MI [21]. Although we showed a stronger association between influenza and MI-associated deaths versus MI-associated hospitalizations, it is not clear whether influenza is likely to trigger cardiac events of greater severity. As with other studies, associations between influenza and adverse events were most marked in the oldest-aged persons [22], who are more likely to have extensive underlying coronary disease. Future work using age-stratified influenza data would help to explore this further while reducing potential for ecological bias.
A strength of our study was the comparison of data from a temperate and a subtropical climate. Although most early ecological studies were done in temperate zones, it has more recently been suggested that influenza-associated mortality in warm regions such as Hong Kong is comparable to that of temperate regions [23]. We saw a slightly greater effect of influenza on both MI-associated deaths and hospitalizations in Hong Kong versus England and Wales, but this should be interpreted with caution, because population size—and, therefore, the numbers of events in Hong Kong—were much lower. Extremes of temperature are known to be associated with deaths due to cardiovascular disease [24]. The use of several methods to control for temperature and the differing relationship of influenza with temperature in Hong Kong, including the presence of a summer influenza peak, reduced the chance that residual confounding by environmental variables was responsible for effects observed.
Weaknesses inherent to using routine surveillance data for research include potential lack of coverage and lack of timeliness [25]. Although underreporting of influenza is common, this would tend to dilute rather than bias the direction of our results. Reporting delays are potentially more problematic. One United Kingdom–based study showed that telephone calls to NHS Direct for colds and influenza preceded GP reports of the same symptoms by 1–3 weeks [26]. This may explain why we saw the best model fits, and greatest estimates of effect, when UK influenza data was lagged by −1 to −3 weeks (representing our assumption that reported ILI consultations represent illness occurring in the community some time earlier). A US study found a 1–2-week lag between internet searches for influenza-associated information and primary care consultations for ILI [27]. A survey of 918 people with an ILI attending GPs in England showed that approximately one-half of persons aged >45 years waited at least 6 days before consulting a GP, with 13% waiting ≥2 weeks [28]. Although reporting delays in surveillance data are the most likely explanation, peaks in MIs might precede GP reports of ILI if triggered by other synchronous environmental events; however, we used multiple sensitivity analyses to control for temperature. Although we considered controlling for other viruses, such as respiratory syncytial virus (RSV), 95% of RSV samples in the United Kingdom are recovered from children [29], and RSV seasonality tends to differ from that of influenza.
In the United Kingdom, although ILI consultations lack specificity for influenza, the highest positive predictive values of ILI occur at times of peak influenza circulation. We did not use influenza virus data, which were limited by relatively small numbers of specimens (mean, 37 per week) and confined to the influenza season. The peak week of influenza virus activity in the United Kingdom tends to precede the peak for ILI consultations by ∼2 weeks [30]. In Hong Kong, where we used influenza virus data as the main exposure, we did not see the same pattern of lag times. Differences in consulting behavior may explain the reduced reporting delay in Hong Kong influenza data. After the SARS outbreak of 2003, official advice was to consult a doctor as soon as influenza-like symptoms are experienced. In contrast, campaigns in the United Kingdom throughout the late 1990s aimed to discourage attendance at the GP for colds and ILI, to try to reduce unnecessary prescriptions of antibiotics [29].
Overall, up to 5.6% of MI-associated deaths in Hong Kong and 3.4% in England and Wales were attributed to influenza (equating to 1052 and 13,947 deaths, respectively). Although this is a relatively small proportion, in England and Wales, over the study period, influenza vaccination rates among persons aged >65 years were around 65%–75% [31]. In Hong Kong, influenza vaccine was not introduced for community-dwelling older people until 2004, with uptake estimated at 31.2% in 2004 and 48.1% in 2005 [32]. The effect of influenza on MI occurred mainly in elderly persons who—especially in England and Wales—are relatively highly vaccinated. Without access to seasonal influenza vaccine, the potential for impact on MI events could be much greater.
In conclusion, we found a consistent association between seasonal influenza circulation and acute MI-associated hospitalizations and deaths in 2 different settings characterized by differing populations, climates, and patterns of health-seeking behavior. Acute cardiac events should be considered when anticipating influenza outbreaks.
Appendix.
Table 4. Age-Specific Associations Between Myocardial Infarction (MI)-Associated Hospitalizations and Influenza Circulation in England and Wales and Hong Kong
| England and Wales |
Hong Kong |
|||
| Age, years | Adjusted IRR (95% CI)a for MI-associated hospitalizations | P | Adjusted IRR (95% CI)b for MI-associated hospitalizations | P |
| <40 | 0.965 (0.931–-1.000) | .05 | 0.892 (0.674–-1.178) | .42 |
| 40–59 | 1.000 (0.985–-1.015) | .99 | 0.982 (0.895–-1.078) | .71 |
| 60–79 | 1.009 (0.995–-1.023) | .21 | 1.057 (1.006–-1.111) | .03 |
| ≥80 | 1.028 (1.010–-1.046) | .002 | 1.161 (1.086–-1.240) | <.001 |
NOTE. CI, confidence interval; GP, general practitioner; ILI, influenza-like illness; IRR, incidence rate ratio.
IRR for the effect of a 10th–90th percentile change in weekly GP ILI consultations lagged by −2 weeks on MI, adjusted for seasonality and long-term trends, weekly mean temperature, weekly mean relative humidity, and residual autocorrelation.
IRR for the effect of a 10th–90th percentile change in weekly proportion of specimens testing positive for influenza virus on MI (with no lag), adjusted for seasonality and long-term trends, weekly mean temperature, weekly mean relative humidity, and residual autocorrelation.
Acknowledgments
Dr Douglas Fleming kindly provided comments on the manuscript. The Weekly Returns Service, which provides clinical ILI data for England and Wales, is run by the Royal College of General Practitioners Birmingham Research Unit. Laboratory data on influenza virus came from the RCGP swabbing scheme, coordinated through the Health Protection Agency Centre for Infections Respiratory Virus Unit. We thank Lincoln Lau for technical assistance.
Funding
This work was supported in part by the Harvard Center for Communicable Disease Dynamics from the US National Institutes of Health models of Infectious Disease Agent Study program (grant 1 U54 GM088558) and the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant AoE/M-12/06). C. W. G. is supported by an MRC Clinical Research Training Fellowship. L. S. holds a Wellcome Trust Senior Clinical Fellowship. A. H. is funded by Camden Primary Care Trust.
References
- 1.Fleming DM. The contribution of influenza to combined acute respiratory infections, hospital admissions, and deaths in winter. Commun Dis Public Health. 2000;3:32–8. [PubMed] [Google Scholar]
- 2.Wong CM, Yang L, Chan KP, et al. Influenza-associated hospitalization in a subtropical city. PLoS Med. 2006;3:e121. doi: 10.1371/journal.pmed.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren-Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9:601–10. doi: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 4.Clayton TC, Thompson M, Meade TW. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2008;29:96–03. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 5.Guan XR, Li X, Xin XM, et al. Influenza virus infection and risk of acute myocardial infarction. Inflammation. 2008;31:266–72. doi: 10.1007/s10753-008-9074-2. [DOI] [PubMed] [Google Scholar]
- 6.Pesonen E, Andsberg E, Grubb A, et al. Elevated infection parameters and infection symptoms predict an acute coronary event. Ther Adv Cardiovasc Dis. 2008;2:419–24. doi: 10.1177/1753944708098695. [DOI] [PubMed] [Google Scholar]
- 7.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–18. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 8.Fleming DM, Cross KW, Pannell RS. Influenza and its relationship to circulatory disorders. Epidemiol Infect. 2005;133:255–62. doi: 10.1017/s0950268804003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider A, Panagiotakos D, Picciotto S, et al. Air temperature and inflammatory responses in myocardial infarction survivors. Epidemiology. 2008;19:391–00. doi: 10.1097/EDE.0b013e31816a4325. [DOI] [PubMed] [Google Scholar]
- 10.Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Short term effects of temperature on risk of myocardial infarction in England and Wales: time series regression analysis of the Myocardial Ischaemia National Audit Project (MINAP) registry. BMJ. 2010;341:c3823. doi: 10.1136/bmj.c3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowling BJ, Wong IO, Ho LM, Riley S, Leung GM. Methods for monitoring influenza surveillance data. Int J Epidemiol. 2006;35:1314–21. doi: 10.1093/ije/dyl162. [DOI] [PubMed] [Google Scholar]
- 12.Centre for Health Protection Website HK. Influenza surveillance data. http://www.chp.gov.hk/en/sentinel/26/44/292.html. Accessed 11 May 2010. [Google Scholar]
- 13.van den Wijngaard C, van Asten L, van Pelt W, et al. Validation of syndromic surveillance for respiratory pathogen activity. Emerg Infect Dis. 2008;14:917–25. doi: 10.3201/eid1406.071467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan PK, Mok HY, Lee TC, Chu IM, Lam WY, Sung JJ. Seasonal influenza activity in Hong Kong and its association with meteorological variations. J Med Virol. 2009;81:1797–06. doi: 10.1002/jmv.21551. [DOI] [PubMed] [Google Scholar]
- 15.Tang JW, Lai FY, Wong F, Hon KL. Incidence of common respiratory viral infections related to climate factors in hospitalized children in Hong Kong. Epidemiol Infect. 2010;138:226–35. doi: 10.1017/S0950268809990410. [DOI] [PubMed] [Google Scholar]
- 16.Bainton D, Jones GR, Hole D. Influenza and ischaemic heart disease–a possible trigger for acute myocardial infarction? Int J Epidemiol. 1978;7:231–39. doi: 10.1093/ije/7.3.231. [DOI] [PubMed] [Google Scholar]
- 17.Tillett HE, Smith JW, Gooch CD. Excess deaths attributable to influenza in England and Wales: age at death and certified cause. Int J Epidemiol. 1983;12:344–52. doi: 10.1093/ije/12.3.344. [DOI] [PubMed] [Google Scholar]
- 18.Madjid M, Miller CC, Zarubaev VV, et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J. 2007;28:1205–10. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller TT, van der Sluijs KF, de K, et al. Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ Res. 2006;99:1261–69. doi: 10.1161/01.RES.0000250834.29108.1a. [DOI] [PubMed] [Google Scholar]
- 20.Szretter KJ, Gangappa S, Lu X, et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81:2736–44. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation. 1999;100:e20–e28. doi: 10.1161/01.cir.100.4.e20. [DOI] [PubMed] [Google Scholar]
- 22.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 23.Wong CM, Chan KP, Hedley AJ, Peiris JS. Influenza-associated mortality in Hong Kong. Clin Infect Dis. 2004;39:1611–17. doi: 10.1086/425315. [DOI] [PubMed] [Google Scholar]
- 24.Pan WH, Li LA, Tsai MJ. Temperature extremes and mortality from coronary heart disease and cerebral infarction in elderly Chinese. Lancet. 1995;345:353–55. doi: 10.1016/s0140-6736(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 25.Paul M, Held L, Toschke AM. Multivariate modelling of infectious disease surveillance data. Stat Med. 2008;27:6250–67. doi: 10.1002/sim.3440. [DOI] [PubMed] [Google Scholar]
- 26.Doroshenko A, Cooper D, Smith G, et al. Evaluation of syndromic surveillance based on National Health Service Direct derived data–England and Wales. MMWR Morb Mortal Wkly Rep. 2005;54(Suppl.):117–22. [PubMed] [Google Scholar]
- 27.Ginsberg J, Mohebbi MH, Patel RS, Brammer L, Smolinski MS, Brilliant L. Detecting influenza epidemics using search engine query data. Nature. 2009;457:1012–14. doi: 10.1038/nature07634. [DOI] [PubMed] [Google Scholar]
- 28.Ross AM, Kai J, Salter R, Ross J, Fleming DM. Presentation with influenza-like illness in general practice: implications for use of neuraminidase inhibitors. Commun Dis Public Health. 2000;3:256–60. [PubMed] [Google Scholar]
- 29.Fleming DM, Elliot AJ. Lessons from 40 years' surveillance of influenza in England and Wales. Epidemiol Infect. 2008;136:866–75. doi: 10.1017/S0950268807009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijer A, Meerhoff TJ, Meuwissen LE, van der Velden V, Paget WJ. Epidemiological and virological assessment of influenza activity in Europe during the winter 2005-2006. Euro Surveill. 2007;12:E11–12. doi: 10.2807/esm.12.09.00733-en. [DOI] [PubMed] [Google Scholar]
- 31.Health Protection Agency Website. Influenza vaccination statistics. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/SeasonalInfluenza/EpidemiologicalData/15influsInfluenzavaccinationuptakemonitoring/. Accessed 16 July 2010. [Google Scholar]
- 32.Lau JT, Kim JH, Choi KC, Tsui HY, Yang X. Changes in prevalence of influenza vaccination and strength of association of factors predicting influenza vaccination over time–results of two population-based surveys. Vaccine. 2007;25:8279–89. doi: 10.1016/j.vaccine.2007.09.047. [DOI] [PubMed] [Google Scholar]