Abstract
Background. Highly pathogenic avian influenza H5N1 viruses remain a threat to human health, with potential to become pandemic agents.
Methods. This phase III, placebo-controlled, observer-blinded study evaluated the immunogenicity, cross-reactivity, safety, and lot consistency of 2 doses of oil-in-water (AS03A) adjuvanted H5N1 A/Indonesia/05/2005 (3.75 μg hemagglutinin antigen) prepandemic candidate vaccine in 4561 adults aged 18–91 years.
Results. Humoral antibody responses in the H5N1 vaccine groups fulfilled US and European immunogenicity licensure criteria for pandemic vaccines in all age strata 21 days after the second dose. At 6 months after the administration of the primary dose, serum antibody seroconversion rates continued to fulfill licensure criteria. Neutralizing cross-clade immune responses were demonstrated against clade 1 A/Vietnam/1194/2004. Consistency was demonstrated for 3 consecutive H5N1 vaccine lots. Temporary injection-site pain was more frequent with H5N1 vaccine than placebo (89.3% and 70.7% in the 18–64 and ≥65 years strata vs 22.2% and 14.4% in the placebo groups). Unsolicited adverse event frequency, including medically attended and serious events, was similar between groups through day 364.
Conclusions. In adults and elderly adults, AS03A-adjuvanted H5N1 candidate vaccine was highly immunogenic for A/Indonesia/05/2005, with cross-reactivity against A/Vietnam/1194/2004. Temporary injection site reactions were more frequent with H5N1 vaccine than placebo, although the H5N1 vaccine was well tolerated overall.
Clinical Trials Registration. NCT00616928.
Avian-origin highly pathogenic influenza A(H5N1) viruses remain a threat. Antigenic and phylogenetic analyses of A(H5N1) viruses reported by the World Health Organization in February 2009 indicated that multiple clade 2 subclades were responsible for the majority of human cases since the reemergence of avian-origin influenza in 2003 [1].
Adjuvanted inactivated split-virion influenza vaccines containing the avian-origin H5N1 hemagglutinin antigen (HA) have been shown to be highly immunogenic and well tolerated in children and adults [2–5]. Reduction in the amount of antigen needed per dose, which is paramount to meet demand for vaccine during a pandemic, was achieved by formulation with an Adjuvant System (AS03) containing α-tocopherol and squalene in an oil-in-water emulsion [4]. The AS03 Adjuvant System enhances the immune response by triggering the transient production of cytokines at the injection site and in the lymph nodes and by promoting antigen presentation by mononuclear phagocytes [6]. While experience during the swine-origin H1N1 pandemic demonstrated that a single 3.75 μg HA dose of AS03A-adjuvanted vaccine was sufficiently immunogenic in adults and children, data concerning AS03A-adjuvanted avian-origin H5N1 vaccines have repeatedly shown that two 3.75 μg doses of HA (A/Vietnam/119/2004 or A/Indonesia/05/2005) were needed to fulfill immunogenicity licensure criteria [3, 4]. In this phase III, randomized, placebo-controlled, observer-blinded study, we assessed the immunogenicity, safety, and lot-to-lot consistency of an AS03A-adjuvanted A/Indonesia/05/2005 (clade 2.1) influenza vaccine in adults aged 18–64 years and in adults aged ≥65 years.
METHODS
Design
This was a multicenter, randomized, placebo-controlled, observer-blinded study conducted in North America. The objective was to assess the immunogenicity and safety of 2 doses of an AS03A-adjuvanted H5N1 A/Indonesia/05/2005 influenza vaccine in adults aged ≥18 years. The equivalence of immunogenicity between 3 different lots of antigen combined with 3 lots of adjuvant was also examined.
Eligible participants were healthy or had controlled chronic illness. Women of child-bearing age were not pregnant and agreed to use reliable methods of contraception. All participants provided informed written consent. The protocol was approved by research ethics boards or local or central institutional review boards and was conducted in accordance with Good Clinical Practice, the Declaration of Helsinki, the US Code of Federal Regulations for the Protection of Human Subjects, the Canadian TriCouncil Policy Statement on Ethical Conduct for Research Involving Humans, and all relevant Canadian and US regulations.
Vaccines and Schedule
GlaxoSmithKline (GSK) Biologicals manufactured the H5N1 vaccine antigen in Ste-Foy, Quebec, Canada. Each dose contained 3.75 μg HA of A/Indonesia/05/2005 (IBCDC-RG2; Centers for Disease Control and Prevention). The adjuvant (AS03A) was a 10% (by volume) DL-α-tocopherol–based oil-in-water emulsion. The placebo control was phosphate-buffered saline.
A randomization list was generated by GSK Biologicals using a blocking scheme. Participants were randomized 3:1 to receive vaccine or placebo, and vaccine recipients were further randomized 1:1:1 to receive 1 of 3 lots. A minimization algorithm was used to balance the randomization by site and age strata (18–30, 31–49, 50–64, 65–74, and ≥75 years).
Participants received 1 dose of vaccine or placebo intramuscularly on day 0 (deltoid, nondominant arm) and a second dose on day 21 (deltoid, dominant arm). The test articles were prepared and administered by unblinded staff who took no further part in the study. Vaccine and placebo injections were administered in overwrapped syringes to obscure contents to other study staff and participants. Participants attended study sites for screening (days –21 to 0) and on days 0 (dose 1), 21 (dose 2), 42, and 182. Telephone interviews were conducted on day 84. A site visit or telephone interview was conducted on day 364.
Immunogenicity Assessments
Immunogenicity outcome measures were hemagglutination inhibition (HAI) titers and microneutralizing (MN) antibody titers. The coprimary immunogenicity objectives were (1) to evaluate vaccine-homologous HAI responses in both age strata for fulfillment of US Food and Drug Administration Center for Biologic Evaluation and Research (CBER) licensure criteria for the accelerated approval of pandemic influenza vaccines [7], and (2) to test the equivalence of vaccine-homologous HAI geometric mean titers (GMTs) of 3 consecutive vaccine lots in participants aged 18–49 years at day 42. Secondary endpoints included HAI responses at day 182 and in participants aged 18–60 and ≥61 years (European Union Committee for Medicinal Products for Human Use [CHMP] age strata [8]) and in participants aged ≥75 years, and day 42 MN antibody responses against A/Indonesia/05/2005 and clade 1 A/Vietnam/1194/2004.
HAI responses were measured using an established assay method modified for horse rather than avian erythrocytes [9–12], and MN assays were performed according to previously described methods [9, 11]. All serum samples were tested in duplicate by blinded personnel. The 50% neutralization titers were calculated using the Reed and Muench method [13].
The HAI endpoints were seroconversion rate, defined as the percentage of participants who had pre- and postvaccination titers of <1:10 and ≥1:40, respectively, or showed a significant increase in antibody titer (a prevaccination titer of ≥1:10 and ≥4-fold increase in postvaccination titer); and seroprotection rate, defined as the percentage of participants with titers of ≥1:40. All values were calculated with 95% confidence intervals (CIs). CBER licensure criteria require the lower limits of the 95% CIs for seroconversion rate to be ≥40% and ≥30% for participants aged 18–64 and ≥65 years, respectively, and for seroprotection rate to be ≥70% and ≥60% for participants aged 18–64 and ≥65 years, respectively.
For the analysis of CHMP criteria, point estimates of the seroconversion rate needed to be >40% and >30% for participants aged 18–60 and ≥61 years, respectively, and the seroprotection rate needed to be >70% and >60% for subjects aged 18–60 and ≥61 years, respectively [8]. The geometric mean fold rise (GMFR) was defined as the geometric mean of the within-subject ratios of pre- and postvaccination reciprocal HAI titers. GMFRs of >2.5 and >2.0 were required in the 18–60 and ≥61-year-old age groups, respectively, to fulfill the CHMP licensure criterion [8].
For the 3 H5N1 vaccine lots, group GMT ratios were calculated using an analysis of covariance model; vaccine lots were considered equivalent if the 2-sided 95% CIs for all of the GMT ratios were between 0.67 and 1.5.
MN assays were performed on a subgroup of subjects selected at randomization and exploratory analyses presented descriptively. Participants with an antibody titer of <1:28 were considered seronegative. The MN vaccine response rate was defined as the percentage of participants achieving ≥4-fold increase in titer relative to the prevaccination titer. MN GMTs were described, and 95% CIs presented for all values.
Safety Assessments
The coprimary safety objectives were to describe solicited and unsolicited adverse events (AEs). Solicited local and general symptoms were recorded by participants using diary cards for 7 days after each dose and graded using a standard scale [5]. Solicited local events were presumed to be vaccine related; investigators provided causality assessments for solicited general events. Assessments of lymph node enlargement and tenderness were conducted using standard grading definitions at baseline and days 21 and 42. In addition, the following were assessed prospectively: all reports of spontaneously offered AEs (termed unsolicited AEs) from day 0 to day 84, and serious AEs, medically attended events, and adverse events of special interest/potentially immune-mediated disorders (AESIs/pIMDs) from day 0 to day 364 (visit window day 349 to day 379). All AEs were coded by preferred term and primary system organ class [14]. A protocol amendment (8 July 2008) required participants to give additional informed written consent at the day 364 safety assessment. On 20 October 2008, in response to a request from CBER, the list of AESI/pIMDs included in the analysis plan was extended.
Analyses
The target sample size was 4400 subjects, 3300 receiving H5N1 vaccine and 1100 receiving placebo. Sample size calculations to support the primary hypothesis tests indicated that there would be 90% power to meet the coprimary objectives if a total of 1569 participants aged 18–64 years, 399 participants aged ≥65 years, and 399 participants aged 18–49 years in each vaccine lot had evaluable results, and a subset of subjects preselected at randomization were tested by HAI to provide these numbers (assuming ≤5% attrition).
The primary safety analyses were performed on the total vaccinated cohort, including subjects who received ≥1 dose of vaccine or placebo for whom any postvaccination data were available. The immunogenicity analysis was performed on the according-to-protocol immunogenicity cohort, including subjects with complete data for the primary immunogenicity endpoints and not fulfilling any elimination criterion. The lot-to-lot vaccine equivalence analysis was performed on participants aged18–49 years in the according-to-protocol immunogenicity cohort who received H5N1 vaccine. The descriptive MN antibody analysis was performed on a randomly selected subset of H5N1 vaccine recipients.
Solicited AEs were tabulated per subject, including severity scores and duration of symptoms. Unsolicited AEs were coded by preferred term and primary system organ class using the Medical Dictionary for Regulatory Activities [14]. Descriptive summaries included participants with any solicited event, with grade 2 and grade 3 events, and with unsolicited AEs, with 95% CIs.
RESULTS
Participants
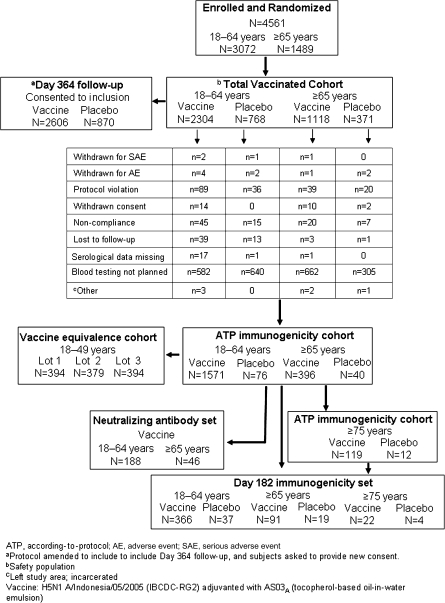
Of 4561 participants randomized, all received H5N1 vaccine or placebo (Figure 1). There were 3072 participants in the 18–64-year-old stratum and 1489 in the ≥65-year-old stratum. A sufficient number of particiants were enrolled to provide a safety database of ≥3000 H5N1 vaccine recipients. Baseline characteristics were balanced in the vaccine and placebo groups (Table 1). Vaccination began 28 January 2008, and the last participant completed the day 42 visit on 22 April 2008. The last day 364 safety assessment was performed on 25 November 2009.
Figure 1.
Study flow diagram.
Table 1.
Patient Demographics and Characteristics in the Total Vaccinated Cohort
| Characteristic | Aged 18–64 years |
Aged ≥65 years |
||
| Vaccine (n = 2304) | Placebo (n = 768) | Vaccine (n = 1118) | Placebo (n = 371) | |
| Age, mean years (range) | 38.5 (18–64) | 38.7 (18–64) | 71.9 (65–91) | 72.1 (65–89) |
| Age stratum, no. (%) | ||||
| 18–49 years | 1707 (74.1) | 568 (74.0) | … | … |
| 50–64 years | 597 (25.9) | 200 (26.0) | … | … |
| 65–74 years | … | … | 783 (70.0) | 261 (70.4) |
| ≥75 years | … | … | 335 (30.0) | 110 (29.6) |
| Female sex, no. (%) | 1328 (57.6) | 424 (55.2) | 621 (55.5) | 196 (52.8) |
| Race, n (%) | ||||
| White; European heritage | 1980 (85.9) | 647 (84.2) | 1050 (93.3) | 345 (93) |
| African heritage/African American | 219 (9.5) | 90 (11.7) | 40 (3.6) | 14 (3.8) |
| White; Arabic/North African heritage | 30 (1.3) | 8 (1.0) | 19 (1.7) | 7 (1.9) |
| American Indian or Alaskan native | 10 (0.4) | 7 (0.9) | 1 (0.1) | 0 |
| Asian | ||||
| Central/South | 7 (0.3) | 0 | 1 (0.1) | 1 (0.3) |
| East | 6 (0.3) | 1 (0.1) | 2 (0.2) | 2 (0.5) |
| Japanese | 2 (0.1) | 1 (0.1) | 0 | 0 |
| Southeast | 9 (0.4) | 6 (0.8) | 2 (0.2) | 1 (0.3) |
| Native Hawaiian/other Pacific Islander | 5 (0.2) | 2 (0.3) | 0 | 0 |
| Weight, mean kg (SD) | 82.6 (21.15) | 81.8 (20.64) | 80.9 (17.51) | 80.8 (17.97) |
NOTE. The candidate vaccine was H5N1 A/Indonesia/05/2005 (IBCDC-RG2) adjuvanted with AS03A (tocopherol-based oil-in-water emulsion). SD, standard deviation.
Immunogenicity
HAI assay.
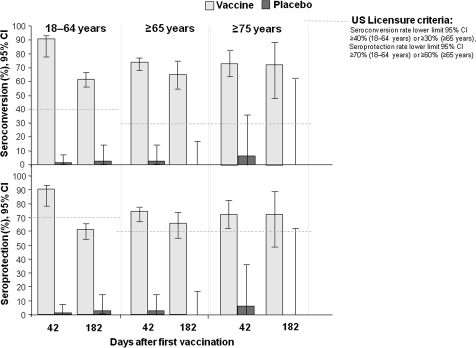
The CBER and CHMP targets for vaccine-homologous HAI responses were exceeded in all age strata at day 42 in H5N1 vaccine recipients (Figure 2). Day 42 seroconversion rates and seroprotection rates with H5N1 vaccine were both 90.8% (95% CI, 89.3%–92.2%) in the 18–64-year-old stratum and were 74.0% (95% CI, 69.4%–78.2%) and 74.5% (95% CI, 69.9%–78.7%), respectively, in the ≥65-year-old stratum. Similar responses were seen in the CHMP age strata. GMFRs at day 42 fulfilled targets in the 18–60 and ≥61 years strata (Table 2). Immune responses persisted at day 182 in H5N1 vaccine recipients. Seroconversion rates and seroprotection rates in the placebo groups were low, at 0%–8.3%.
Figure 2.
Hemagglutination inhibition assay responses against the vaccine-homologous strain in the according-to-protocol immunogenicity cohort (day 42) and the day 182 immunogenicity cohort. The candidate vaccine was H5N1 A/Indonesia/05/2005 (IBCDC-RG2) adjuvanted with AS03A (tocopherol-based oil-in-water emulsion).
Table 2.
Hemagglutination Inhibition (HAI) Assay: Geometric Mean Titers (GMT) and Geometric Mean Fold Rise (GMFR) in the According-to-Protocol Immunogenicity Cohort (Day 42) and the Day 182 Immunogenicity Set
| HAI GMT n (95% CI) |
HAI GMFR n (95% CI) |
|||||
| Age | Treatment group | Prevaccinationa | Day 42 | Day 182 | Day 42 | Day 182 |
| 18–64 years | Vaccine | 1571 5.0 (5.0–5.0) | 1571 249.0 (231.8–267.5) | 366 36.2 (31.0–42.2) | 1571 49.6 (46.2–53.3) | 366 7.2 (6.2–8.4) |
| Placebo | 76 5.0 (5.0–5.0) | 76 5.1 (4.9–5.4) | 37 5.5 (4.8–6.5) | 76 1.0 (1.0–1.1) | 37 1.1 (1.0–1.3) | |
| ≥65 years | Vaccine | 396 5.2 (5.1–5.3) | 396 81.9 (69.7–96.2) | 91 44.8 (33.3–60.4) | 396 15.8 (13.4–18.5) | 91 8.8 (6.5–11.9) |
| Placebo | 40 5.0–(5.0–5.0) | 40 5.5 (4.5–6.8) | 19 5.4 (4.6–6.3) | 40 1.1 (0.9–1.4) | 19 1.1 (0.9–1.3) | |
| 18–60 years | Vaccine | 1488 5.0 (5.0–5.0) | 1488 258.0 (239.7–277.7) | 353 37.5 (31.8–43.6) | 1488 51.4 (47.8–55.3) | 353 7.4 (6.3–8.7) |
| Placebo | 68 5.0 (5.0–5.0) | 68 5.2 (4.9–5.5) | 29 5.7 (4.7–6.9) | 68 1.0 (1.0–1.1) | 29 1.1 (0.9–1.4) | |
| ≥61 years | Vaccine | 479 5.2 (5.0–5.3) | 479 89.0 (77.1–102.7) | 104 39.6 (29.9–52.5) | 479 17.2 (14.9–19.9) | 104 7.8 (5.9–10.4) |
| Placebo | 48 5.0 (5.0–5.0) | 48 5.5 (4.6–6.5) | 27 5.3 (4.7–5.8) | 48 1.1 (0.9–1.3) | 27 1.1 (0.9–1.2) | |
| ≥75 years | Vaccine | 119 5.1 (5.0–5.2) | 119 75.2 (55.7–101.5) | 22 45.4 (25.2–81.8) | 119 14.8 (11.0–20.0) | 22 9.1 (5.0–16.4) |
| Placebo | 12 5.0 (5.0–5.0) | 12 7.1 (3.3–15.2) | 4 5.0 (5.0–5.0) | 12 1.4 (0.7–3.0) | 4 1.0 (1.0–1.0) | |
NOTE. GMFR is defined as the mean of the within-subject ratios of pre- and postvaccination reciprocal HAI titers; to fulfill the European licensure criterion, GMFR >2.5 (18–60 years of age) and >2.0 (≥61 years of age); there was no US criterion for GMFR. CI, confidence interval.
According-to-protocol immunogenicity cohort.
The equivalence of 3 consecutive lots of H5N1 vaccine was revealed at day 42. The adjusted GMTs for lots 1, 2, and 3 were 275.8, 291.7, and 333.5, respectively. The adjusted GMT ratio was 0.95 (95% CI, 0.78–1.15) for lots 1 and 2; 0.83 (95% CI, 0.68–1.00) for lots 1 and 3; and 0.87 (95% CI, 0.72–1.06) for lots 2 and 3.
MN antibody assay.
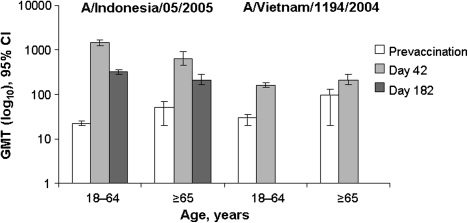
Before vaccination, 72% (n = 136) and 28.3% (n = 13) of participants aged 18–64 and ≥65 years, respectively, were seronegative for vaccine-homologous MN antibodies, and 59.7% (n = 108) and 18.2% (n = 8), respectively, were seronegative for MN antibodies to clade 1 A/Vietnam/1194/2004. In the H5N1 vaccine groups, MN antibodies developed at day 42 against the vaccine-homologous virus and the clade 1 A/Vietnam strain (Figure 3). At day 182, total MN vaccine response rates against the vaccine-homologous strain in the 18–64 years and ≥65 years strata were 85.6% (95% CI, 79.7%–90.4%) and 51.1% (95% CI, 35.8%–66.3%), respectively.
Figure 3.
Neutralizing antibody geometric mean titers against vaccine-homologous and vaccine-heterologous strains in the neutralizing antibody set. The candidate vaccine was H5N1 A/Indonesia/05/2005 (IBCDC-RG2) adjuvanted with AS03A (tocopherol-based oil-in-water emulsion).
Exploratory analyses showed a strong and highly significant positive linear correlation between log-transformed vaccine-homologous MN and HAI responses at day 42 and day 182 in both the 18–64 and ≥65 years strata and a weaker but still significant correlation between A/Indonesia/5/2005 HAI responses and cross-reactive MN antibody responses to A/Vietnam/1194/2004 (data not shown).
Reactogenicity and Safety
Solicited adverse events.
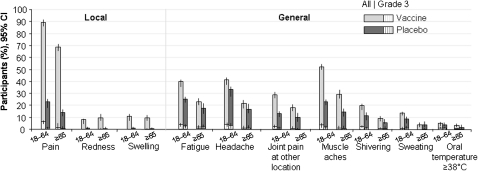
The frequency of solicited local and general AEs during the 7-day postvaccination periods is seen in Figure 4. Pain was the most common local symptom in all groups and was reported by 2024 (89.3%) of 2267 H5N1 vaccine recipients aged 18–64 years, compared with 171 (22.2%) of 754 in the placebo group, and by 784 (70.7%) of 1109 H5N1 vaccine recipients aged ≥65 years, compared with 53 (14.4%) of 368 in the placebo group. Pain was reported to be grade 1 or 2 in the majority of participants; grade 3 pain in the H5N1 vaccine groups was reported by 141 (6.2%) of 2267 in the 18–64 years stratum, compared with 6/754 (0.8%) in the placebo group; and by 15 (1.4%) of 1109 in the ≥65 years stratum, compared with 2 (0.5%) of 368 in the placebo group. Pain lasted a mean of 2.8 days in the H5N1 vaccine group and 1.9 days in the placebo group (the standard deviation for pain duration in H5N1 group is 1.34, and is 1.21 in the Placebo group).
Figure 4.
Solicited adverse events during the 7-day postvaccination period (doses 1 and 2 pooled) in the total vaccinated cohort.
Muscle ache was the most common solicited general event and was reported by 1526 (45.2%) of 3375 H5N1 vaccine recipients and 231 (20.6%) of 1123 placebo recipients. Grade 3 general events occurred in 0.8%–3.2% of H5N1 vaccine recipients and 0.9%–2.4% of placebo recipients. Oral temperatures of ≥39°C were reported by <1% of participants in each group.
The incidence of AEs after the first and second doses was 87.4% and 80.3%, respectively, for H5N1 vaccine and 46.0% and 32.6%, respectively, for placebo in the 18–64 years stratum, and 69.0% and 65.9%, respectively, for H5N1 vaccine, and 32.3% and 22.9%, respectively, for placebo in the ≥65 years stratum.
Unsolicited adverse events.
From day 0 to day 84, ≥1 unsolicited AEs were reported by 1017 (44.1%) of 2304 participants aged 18–64 years in the H5N1 vaccine group and 321 (41.8%) of 768 participants aged 18–64 years in the placebo group and by 467 (41.8%) of 1118 participants aged ≥65 years in the H5N1 vaccine group and 130 (35.0%) of 371 participants aged ≥65 years in the placebo group (Table 3). Transient, and generally mild, axillary discomfort was reported by 0.3% of H5N1 vaccine recipients and no placebo recipients, but physician-observed lymphadenopathy was uncommon.
Table 3.
Most Frequent (≥1% in a Treatment Group) Spontaneously Reported (Unsolicited) Adverse Events From Day 0 to Day 84 in the Total Vaccinated Cohort
| 18–64 Years of age |
≥65 Years of age |
|||
| Adverse event | Vaccine (n = 2304) no. (%; 95% CI) | Placebo (n = 768) no. (%; 95% CI) | Vaccine (n = 1118) no. (%; 95% CI) | Placebo (n = 371) no. (%; 95% CI) |
| ≥1 unsolicited symptom | 1017 (44.1; 42.1–46.2) | 321 (41.8; 38.3–45.4) | 467 (41.8; 38.9–44.7) | 130 (35.0; 30.2–40.1) |
| Nasopharyngitis | 116 (5.0; 4.2–6.0) | 29 (3.8; 2.5–5.4) | 40 (3.6; 2.6–4.8) | 11 (3.0; 1.5–5.2) |
| Oropharyngeal pain | 91 (3.9; 3.2–4.8) | 39 (5.1; 3.6–6.9) | 34 (3.0; 2.1–4.2) | 12 (3.2; 1.7–5.6) |
| Headache | 73 (3.2; 2.5–4.0) | 31 (4.0; 2.8–5.7) | 28 (2.5; 1.7–3.6) | 8 (2.2; 0.9–4.2) |
| Nausea | 78 (3.4; 2.7–4.2) | 20 (2.6; 1.6–4.0) | 20 (1.8; 1.1–2.7) | 4 (1.1; 0.3–2.7) |
| Upper respiratory tract infection | 73 (3.2; 2.5–4.0) | 25 (3.3; 2.1–4.8) | 27 (2.4; 1.6–3.5) | 13 (3.5; 1.9–5.9) |
| Cough | 66 (2.9; 2.2–3.6) | 28 (3.6; 2.4–5.2) | 29 (2.6; 1.7–3.7) | 6 (1.6; 0.6–3.5) |
| Nasal congestion | 59 (2.6; 2.0–3.3) | 20 (2.6; 1.6–4.0) | 11 (1.0; 0.5–1.8) | 2 (0.5; 0.1–1.9) |
| Diarrhoea | 57 (2.5; 1.9–3.2) | 14 (1.8; 1.0–3.0) | 34 (3.0; 2.1–4.2) | 11 (3.0; 1.5–5.2) |
| Back pain | 43 (1.9; 1.4–2.5) | 19 (2.5; 1.5–3.8) | 21 (1.9; 1.2–2.9) | 3 (0.8; 0.2–2.3) |
| Sinusitis | 56 (2.4; 1.8–3.1) | 13 (1.7; 0.9–2.9) | 17 (1.5; 0.9–2.4) | 5 (1.3; 0.4–3.1) |
| Injection site pruritus | 56 (2.4; 1.8–3.1) | 13 (1.7; 0.9–2.9) | 23 (2.1; 1.3–3.1) | 1 (0.3; 0–1.5) |
| Pain in extremity | 25 (1.1; 0.7–1.6) | 6 (0.8; 0.3–1.7) | 19 (1.7; 1.0–2.6) | 4 (1.1; 0.3–2.7) |
| Lymphadenopathy | 22 (1.0; 0.6–1.4) | 14 (1.8; 1.0–3.0) | 3 (0.3; 0.1–0.8) | 1 (0.3; 0–1.5) |
| Rhinorrhoea | 28 (1.2; 0.8–1.8) | 14 (1.8; 1.0–3.0) | 12 (1.2; 0.6–2.0) | 3 (0.8; 0.2–2.3) |
| Influenza like illness | 38 (1.6; 1.2–2.3) | 12 (1.6; 0.8–2.7) | 8 (0.7; 0.3–1.4) | 8 (2.2; 0.9–4.2) |
| Bronchitis | 31 (1.3; 0.9–1.9) | 8 (1.0; 0.5–2.0) | 12 (1.3; 0.7–2.1) | 7 (1.9; 0.8–3.8) |
| Musculoskeletal pain | 12 (0.5; 0.3–0.9) | 5 (0.7; 0.2–1.5) | 15 (1.3; 0.8–2.2) | 5 (1.3; 0.4–3.1) |
NOTE. The candidate vaccine was H5N1 A/Indonesia/05/2005 (IBCDC-RG2) adjuvanted with AS03A (tocopherol-based oil-in-water emulsion). CI, confidence interval.
AEs by system organ class showed a numerically higher incidence of “gastrointestinal disorders” among H5N1 vaccine recipients than among placebo recipients, although 95% CIs overlapped for each individual AE term: nausea, 2.9% and 2.1%; diarrhea, 2.7% and 2.2%; and vomiting, 1.1% and 0.9%, respectively. Four participants in the H5N1 vaccine group reported injection site reactions (pain n = 2, pruritus n = 2) that occurred beyond the 7-day postvaccination period, and 1 of these participants reported injection site pain occurring more than 14 days after vaccination. The incidence of grade 3 unsolicited AEs was 6.9% (236/3422) among H5N1 vaccine recipients and 6.8% (78/1139) among placebo recipients.
Serious AEs, medically attended events, and AESI/pIMDs.
Serious AEs, medically attended events, and AESI/pIMDs were assessed from day 0 to the day 364 visit window in the total vaccinated cohort. One or more serious AEs were reported by 111 (3.2%) of 3422 vaccine recipients and 45 (4.0%) of 1139 placebo recipients, and there was no differential temporal clustering among vaccine recipients, compared with placebo recipients. One or more medically attended events were reported by 1027 (30%) of 3422 vaccine recipients and 346 (30.4%) of 1139 in the placebo group. No medically attended events were reported by >2.1% of subjects for any preferred term in either group.
There were 4 deaths (0.1%) in the vaccine group (myocardial infarction, ovarian carcinoma with metastases to the liver, malignant neoplasm, and diabetes mellitus/liver disease) and 7 deaths (0.6%) in the placebo group (malignant brain neoplasm, cardiomegaly, cardiac disorder prior to motor vehicle accident, gunshot, malignant neoplasm of the tongue, pneumonia, and a report of death without a specified diagnosis in an 89-year-old woman) during the follow-up period. Three deaths (1 vaccinee and 2 placebo recipients) occurred within 3 weeks of vaccine exposure.
Twelve participants (0.4%) in the vaccine group and 1 participant (0.1%) in the placebo group reported AESIs/pIMDs. Eight subjects were aged 18–64 years, and 5 were >64 years. In the H5N1 vaccine group, 2 subjects reported psoriasis and 2 reported polymyalgia rheumatica, and there was 1 report each of celiac disease, Crohn’s disease, autoimmune hepatitis, rheumatoid arthritis, facial palsy, erythema nodosum, radiculitis, and fourth cranial nerve palsy. One subject with polymyalgia rheumatica also was diagnosed with temporal arteritis. These events were not temporally clustered, and none were assessed as vaccine related by the investigators.
DISCUSSION
In this large, multicenter, phase III study, a 2-dose schedule of 3.75 μg HA AS03A-adjuvanted H5N1 A/Indonesia/05/2005 influenza vaccine induced vaccine-homologous HAI antibody titers that fulfilled licensure criteria for seroconversion and seroprotection in adults aged 18–64 and ≥65 years (US licensure age strata) [7], and in adults aged 18–60 and ≥61 years (European licensure age strata) [8], at 42 days after the primary dose. The majority of participants in all age strata retained A/Indonesia/05/2005 HAI titers of ≥1:40 at 6 months. In addition, the immunogenic consistency of 3 consecutive lots of antigen, combined with 3 consecutive lots of adjuvant, was revealed by adjusted GMT ratios at day 42. These observations validate the selection of an AS03A-adjuvanted formulation previously based on phase I/II data [3].
In addition to developing antigen-sparing pandemic vaccines, it has been suggested that national pandemic and prepandemic planning incorporate vaccination strategies whereby a population is primed with stockpiled avian influenza vaccine, then subsequently vaccinated with a pandemic vaccine matched to the emergent influenza strain [15–17]. Such a strategy would require vaccines that induce cross-reactivity against drift variant viruses, since influenza viruses can evolve into phylogenetically and antigenically distinct clades, and stockpiled vaccine might not exactly match the eventual pandemic strain [1]. Protective cross-reactive responses have been demonstrated in preclinical studies in which ferrets that received AS03-adjuvanted A/Vietnam/1194/2004 vaccine subsequently survived a lethal vaccine-heterologous challenge with A/Indonesia/05/2005 [18], and clinical studies have shown that a 2-dose series of AS03A-adjuvanted A/Vietnam/1194/2004 vaccine elicits cross-reactive immune responses against clade 2 strains when doses are given 21 days apart, and 6 or 12 months apart [4, 19–22]. This study provides additional evidence of cross-reactive MN immune responses against clade 1 A/Vietnam/1194/2004 following administration of AS03A-adjuvanted A/Indonesia/05/2005 vaccine.
None of the 18–64-year-old group and 0.3% of the ≥65-year-old group had HAI antibody titers of >1:10 against the vaccine strain at baseline. However, >70% of participants aged ≥65 years were seropositive for MN antibodies against the vaccine-homologous and/or drift-variant strain before vaccination, including 11 of 12 participants aged ≥75 years who were seropositive for A/Vietnam/1194/2004. This phenomenon has been observed in previous studies, and it is thought that elderly people with prolonged natural exposure to seasonal influenza viruses and/or multiple lifetime vaccinations may develop antibodies with antigenic cross-reactivity with H5N1 strains [23, 24].
Previous exposure to seasonal influenza vaccination has been reported to reduce immune responses to subsequent pandemic influenza vaccination [25–29]. Recent experience with AS03A-adjuvanted H1N1 pandemic influenza vaccine showed that although licensure criteria for immunogenicity against the vaccine strain were consistently fulfilled, postvaccination antibody titers were lower in subjects who had recently received trivalent seasonal influenza vaccination, compared with those who had not [30]. The influence of preexisting antibody levels, previous influenza vaccination, or intercurrent seasonal influenza on immune responses to pandemic influenza vaccine was beyond the scope of this study. The substantial immune responses in both age strata suggest that preexisting cross-reactive antibody does not have a dominating impact on immunogenicity to AS03A-adjuvanted H5N1 vaccine that would impede its general use to address an advancing pandemic. While we cannot evaluate the possibility that intercurrent seasonal influenza might have negatively influenced immune responses in some subjects, the virtual absence of antibody increases in the concurrent placebo group suggests that intercurrent seasonal influenza infections did not inflate our estimates of vaccine immunogenicity. Moreover, although elderly people are noted to have reduced seroconversion to influenza vaccines, all immunogenicity criteria were met for older persons in this study, albeit with lower GMTs than achieved in younger adults.
The acceptability of vaccine programs, even in the absence of severe or substantial AEs, must be considered in the development of new vaccines. The incidences of injection site pain, muscle aches, headache, and fatigue were higher among H5N1 vaccine recipients than among placebo recipients, although about 20% of placebo recipients also reported these AEs. The duration of these transient reactions was typically 2–3 days, and grade 3 reactions were uncommon; adherence to second doses was ≥95%. Rates of all unsolicited AEs, objectively assessed enlargement of the axillary and/or supraclavicular nodes, and symptomatic lymphadenopathy did not differ meaningfully between treatment groups. Nine participants discontinued participation because of an AE, and these were balanced between the H5N1 vaccine and placebo groups. Serious AEs were uncommon, occurring in 4% and 3.2% of placebo and H5N1 vaccine recipients, respectively. Thirty percent of participants in each group experienced at least 1 medically attended event.
Twelve H5N1 vaccine recipients and 1 placebo recipient had 1 of a heterogenous group of AESI/pIMDs, with 1 subject in the H5N1 vaccine group reporting both polymyalgia rheumatica and temporal arteritis. The disease process predated receipt of the vaccine or had a potential alternative etiology in at least one-third of participants. The overall rate of AESI/pIMDs was <0.3%, and this clinical trial did not have sufficient power to evaluate any potential association of these rare events with the vaccine. Although these events will be closely monitored in future trials, large postmarketing surveillance databases would likely be needed to detect such associations given the low background incidence of these diagnoses, their heterogeneous pathophysiology, and the need to account for factors such as age, sex, and temporal and geographical clustering [31].
In summary, a 2-dose schedule of AS03A-adjuvanted 3.75 μg A/Indonesia/05/2005 HA elicited immune responses that fulfilled licensure criteria in adults and elderly adults, including participants aged ≥75 years. The vaccine was associated with a higher rate of transient injection site reactions and systemic symptoms than was placebo.
Acknowledgments
We are grateful to the National Institute for Biological Standards and Control (Potters Bar, UK) for providing virus strains for in-vitro testing and also to the Centers for Disease Control and Prevention (Atlanta, GA) for supplying the A/Indonesia/05/2005 (IBCDC-RG2) vaccine strain. The guidance and support of the US Department of Health and Human Services was greatly appreciated. The authors are indebted to the participating study volunteers, clinicians, coordinators, and laboratory technicians at the study sites and to the sponsor's project staff for their support and contributions throughout the study, in particular to Drs Nathan Bennett, Laurence Chu, Matthew Davis, Darrell Herrington, Robert Jeanfreau, Casey Johnson, William Seger, Stephan Sharp, François Blouin, Peter Dzongowski, and Dennis Reich as investigators, to Kim Cerenze, Caroline Gesualdi, Dawn Hall, and Eleanor Esperjo for study coordination, to Stephanie Cooley for preparation of the study protocol and related study documentation, and to Drs Harry Seifert and Dorothy Slavin (safety physicians). We are grateful to Dr Roger Bernhard and his team, who performed the serological laboratory work at GSK in Dresden, Germany, and to Thierry Ollinger, Laurence Baufays, and Pascal Gerard for coordination at GSK in Rixensart, Belgium.We thank Dr Annick Moon (Independent, UK; on behalf of GlaxoSmithKline Biologicals), who provided medical writing services and developed the manuscript according to the recommendations, documentation, and outline provided by the lead authors, ie, Joanne Langley and Louis Fries. Finally, we thank Dr Isabelle Gautherot (GSK Biologicals) and Dr Géraldine Verplancke (Keyrus BioPharma; on behalf of GlaxoSmithKline Biologicals), who provided support to coordinate the circulation of the manuscript to all coauthors, to collect comments received from coauthors, and to make sure that International Committee of Medical Journal Editors recommendations were fulfilled.
Author contributions: All authors had full access to the data. All authors participated in the design or implementation or analysis and interpretation of the study. They all critically reviewed the proposed drafts of the manuscript, and their comments were taken into account and incorporated. All authors approved the content of the final version of the manuscript before it was submitted by the corresponding author. D. V. and L. Fries led the clinical team at GlaxoSmithKline. J. M. L., H. C., C. F., L. Frenette, D. R., L. Ferguson, and M.S. served as principal site investigators. E. S., M. D., M. B., B. B., and T.P. contributed as site investigators. L. G., G. R., M. C., and D. B. coordinated the study at the investigator site. P. L. was responsible for the statistical analyses.
Funding
This project has been funded by GSK biologicals and with federal funds from the US Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response (ASPR), Biomedical Advanced Research and Development Authority (BARDA), under contract No. HHSO100200700029C. GSK Biologicals was involved in all stages of the study conduct and analysis. GSK Biologicals also took in charge all costs associated with the development and the publication of the present paper.
References
- 1.World Health Organization. Antigenic and genetic characteristics of H5N1 viruses and candidate H5N1 vaccine viruses developed for potential use as pre-pandemic vaccines. http://www.who.int/csr/disease/avian_influenza/guidelines/200902_H5VaccineVirusUpdate.pdf. Published 2009. Accessed 15 September 2010. [PubMed] [Google Scholar]
- 2.Díez-Domingo J, Garcés-Sanchez M, Baldó J-M, et al. Immunogenicity and safety of H5N1 A/Vietnam/1194/2004 (clade 1) AS03-adjuvanted pre-pandemic candidate influenza vaccines in children aged 3 to 9 years: a phase II, randomized, open, controlled study. Pediatr Infect Dis J. 2010;29:e35–46. doi: 10.1097/INF.0b013e3181daf921. [DOI] [PubMed] [Google Scholar]
- 3.Langley JM, Frenette L, Ferguson L, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010;201:1644–53. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- 4.Leroux-Roels I, Borkowski A, Vanwolleghem T, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–9. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 5.Rumke HC, Bayas JM, de Juanes JR, et al. Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine. 2008;26:2378–88. doi: 10.1016/j.vaccine.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 6.Moral S, Didierlaurent A, Bourguignon P, et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. vaccine. 2011;29:2461–73. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration. Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines. http://www.fda.gov/cber/gdlns/panfluvac.htm. 2007. Accessed 8 July 2010. [Google Scholar]
- 8.European Committee for Proprietary Medicinal Products. Guideline on influenza vaccine prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context (EMEA/CHMP/VWP/263499/2006) London: European Agency for the Evaluation of Medicinal Products; 2007. [Google Scholar]
- 9.Hehme N, Engelmann H, Kuenzel W, Neumeier E, Saenger R. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Res. 2004;103:163–71. doi: 10.1016/j.virusres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Kendal A, Pereira M, Skehel J. In: Hemagglutination inhibition: concepts and procedures for laboratory-based influenza surveillance. Kendal AP, Pereira MS, Skehel JJ, editors. Atlanta, GA: Centers for Disease Control and Prevention and Pan-American Health Organization; 1982. pp. B17–35. [Google Scholar]
- 11.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 2004;103:91–5. doi: 10.1016/j.virusres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Reed L, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 14.Medical dictionary for regulatory activities. http://www.meddramsso.com/. Accessed 10 September 2010. [Google Scholar]
- 15.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings LC, Monto AS, Chan PK, Szucs TD, Nicholson KG. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect Dis. 2008;8:650–8. doi: 10.1016/S1473-3099(08)70232-9. [DOI] [PubMed] [Google Scholar]
- 17.Osterhaus AD. Pre- or post-pandemic influenza vaccine? Vaccine. 2007;25:4983–4. doi: 10.1016/j.vaccine.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Baras B, Stittelaar KJ, Simon JH, et al. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS One. 2008;3:e1401. doi: 10.1371/journal.pone.0001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz T, Horacek T, Knuf M, et al. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine. 2009;27:6284–90. doi: 10.1016/j.vaccine.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 20.Leroux-Roels G. Prepandemic H5N1 influenza vaccine adjuvanted with AS03: a review of the pre-clinical and clinical data. Expert Opin Biol Ther. 2009;9:1057–71. doi: 10.1517/14712590903066695. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz T, Horacek T, Knuf M. AS03-adjuvanted prepandemic H5N1 vaccine allows highly flexible prime-boost vaccination strategy. In: The 3rd European Influenza Conference. Villamoura. Portugal; 14–17 September 2008. [Google Scholar]
- 22.Schwarz TF, Horacek T, Knuf M, et al. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine. 2009;27:6284–90. doi: 10.1016/j.vaccine.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Banzhoff A, Gasparini R, Laghi-Pasini F, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One. 2009;4:e4384. doi: 10.1371/journal.pone.0004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gioia C, Castilletti C, Tempestilli M, et al. Cross-subtype immunity against avian influenza in persons recently vaccinated for Influenza. Emerg Infect Dis. 2008;14:121–8. doi: 10.3201/eid1401.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals: a randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–5. [PubMed] [Google Scholar]
- 26.Iorio AM, Camilloni B, Basileo M, Neri M, Lepri E, Spighi M. Effects of repeated annual influenza vaccination on antibody responses against unchanged vaccine antigens in elderly frail institutionalized volunteers. Gerontology. 2007;53:411–8. doi: 10.1159/000110579. [DOI] [PubMed] [Google Scholar]
- 27.Leroux-Roels I, Roman F, Forgus S, et al. Priming with AS03(A)-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine. 2010;28:849–57. doi: 10.1016/j.vaccine.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Nabeshima S, Kashiwagi K, Murata M, Kanamoto Y, Furusyo N, Hayashi J. Antibody response to influenza vaccine in adults vaccinated with identical vaccine strains in consecutive years. J Med Virol. 2007;79:320–5. doi: 10.1002/jmv.20801. [DOI] [PubMed] [Google Scholar]
- 29.Nolan T, Richmond PC, Formica NT, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26:6383–91. doi: 10.1016/j.vaccine.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 30.Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis. 2010;51:668–77. doi: 10.1086/655830. [DOI] [PubMed] [Google Scholar]
- 31.Black S, Eskola J, Siegrist CA, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet. 2009;374:2115–22. doi: 10.1016/S0140-6736(09)61877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]