Abstract
(See the editorial commentary by Yin and Overton, on pages 1705-7.)
Background. Long-term effects of abacavir (ABC)–lamivudine (3TC), compared with tenofovir (TDF)–emtricitabine (FTC) with efavirenz (EFV) or atazanavir plus ritonavir (ATV/r), on bone mineral density (BMD) have not been analyzed.
Methods. A5224s was a substudy of A5202, in which HIV-infected treatment-naive participants were randomized and blinded to receive ABC-3TC or TDF-FTC with open-label EFV or ATV/r. Primary bone end points included Dual-emission X-ray absorbtiometry (DXA)-measured percent changes in spine and hip BMD at week 96. Primary analyses were intent-to-treat. Statistical tests used the factorial design and included linear regression, 2-sample t, log-rank, and Fisher's exact tests.
Results. Two hundred sixty-nine persons randomized to 4 arms of ABC-3TC or TDF-FTC with EFV or ATV/r. At baseline, 85% were male, and 47% were white non-Hispanic; the median HIV-1 RNA load was 4.6 log10 copies/mL, the median age was 38 years, the median weight was 76 kg, and the median CD4 cell count was 233 cells/μL. At week 96, the mean percentage changes from baseline in spine and hip BMD for ABC-3TC versus TDF-FTC were -1.3% and -3.3% (P = .004) and -2.6% and -4.0% (P = .024), respectively; and for EFV versus ATV/r were -1.7% and -3.1% (P = .035) and -3.1% and -3.4% (P = .61), respectively. Bone fracture was observed in 5.6% of participants. The probability of bone fractures and time to first fracture were not different across components.
Conclusions. Compared with ABC-3TC, TDF-FTC–treated participants had significantly greater decreases in spine and hip BMD, whereas ATV/r led to more significant losses in spine, but not hip, BMD than EFV.
Clinical Trials Registration. NCT00118898.
With the advent of potent antiretroviral therapy (ART), significant comorbidities have emerged, including osteoporosis and increased risk of fractures. Low bone mineral density (BMD) has been reported in studies of HIV-infected individuals; in a meta-analysis, the prevalence of osteoporosis was 3 times higher in HIV-infected patients than HIV-uninfected control subjects [1]. Studies have shown that BMD decreases by 2%–6% within the first 2 years of ART initiation, regardless of the choice of therapy [2–5], with a long-term study showing that this initial decrease is not progressive [3]. Studies reporting increased fracture rates in HIV-infected individuals are emerging [6–9].
Treatment with the nucleotide analogue reverse-transcriptase inhibitor tenofovir disoproxil fumarate (TDF) has been associated with an initial decrease in BMD [2]. In addition, there was more bone loss in virologically suppressed persons who switched to TDF, compared with switching to the nucleoside analogue reverse-transcriptase inhibitor (NRTI) abacavir (ABC) [10]. To date, there has been a single report of a 48-week prospective study of participants initiating their first ART with TDF-emtricitabine (FTC) or ABC-lamivudine (3TC), combined with the nonnucleoside reverse-transcriptase inhibitor (NNRTI) efavirenz (EFV) [11]. A significantly greater decrease in spine and hip BMD was seen with TDF-FTC. To date, there has been no study comparing the effects on bone of EFV compared with those of atazanavir-ritonavir (ATV/r), a protease inhibitor (PI) combination with few metabolic effects [12, 13].
METHODS
A5224s was a substudy of AIDS Clinical Trials Group (ACTG) A5202, in which ART-naive persons aged ≥16 years and with an HIV-1 RNA load >1000 copies/mL were randomized in a double-blinded fashion to receive coformulated TDF-FTC or ABC-3TC, along with open-labeled EFV or ATV/r at standard doses. A coprimary objective of A5224s was to compare the effects of initiating ABC-3TC with those of TDF-FTC on spine and hip BMD. The second coprimary objective was to assess the effect of these drugs on body fat; results of these analyses will be reported elsewhere. A5224s secondary objectives were to compare BMD changes between EFV and ATV/r arms, to compare TDF-FTC with ABC-3TC and EFV with ATV/r on BMD changes at week 48, and to compare the proportion of participants with bone fractures during study. Specific A5224s exclusion criteria were uncontrolled thyroid disease or hypogonadism; endocrine diseases, including Cushing's syndrome, diabetes mellitus, and the use of growth hormone, anabolic steroids, glucocorticoids, or osteoporosis medications; or the intent to start bone-related treatment. The duration of the study was 96 weeks after the last participant enrolled.
Any participant enrolling in A5202 at one of ACTG sites participating in A5224s and meeting criteria for A5224s was eligible to enroll. Each participant signed a written informed consent before enrollment. The study was approved by the local institutional review board at each site.
At baseline, a complete history, including history of fractures, was obtained, and participants underwent a physical examination, including measurement of height and weight. Substudy evaluation included BMD measurement by dual-energy absorptiometry (DXA) in the anteroposterior view (using Hologic or Lunar scanners) of the lumbar spine (from L1-L4) and hip at baseline and at weeks 24, 48, 96, 144, and 192. To assess for osteopenia at baseline, we used t scores (standard deviations from the mean value in young normal individuals) at the spine or hip, based on the manufacturers' sex- and ethnicity-specific reference populations. Technicians were instructed to scan the same hip of each participant and use the same machine on the same participant throughout the study. All DXAs were standardized at the participating sites, then centrally read (Tufts) by blinded personnel. On 18 February 2008 [14], the parent study A5202 team was notified of the Data Safety and Monitoring Board (DSMB) recommendation to unblind the NRTI assignment for participants with screening HIV-1 RNA loads ≥100,000 copies/mL because of excess virologic failures seen in this subgroup who were receiving ABC-3TC regimens.
Statistical Analysis
The primary DXA objectives were to compare, between pooled, randomized NRTI components (ABC-3TC vs TDF-FTC), changes from baseline to week 96 in spine and hip BMD. Other objectives and analyses were considered to be secondary. A5224s was originally powered as a factorial analysis. With a sample size of 125 participants per component, there was 98% power to detect the prespecified 2% between 2 groups difference in BMD percentage change.
All analyses were initially performed using intent-to-treat (ITT) principles based on randomized treatment assignment in which all available data were used and modifications to randomized treatment and missing values were ignored. Supplemental as-treated (AT) analyses were performed in which values were censored after a change in the randomized NRTI component (when comparing NRTI components) or NNRTI/PI component (when comparing NNRTI/PI components). P values <.05 were interpreted as statistically significant, and nominal values are reported without adjustment for multiple comparisons. Analyses were performed using SAS, version 9.1.3 (SAS Institute).
Comparisons between regimen components used 2-sample t, Fisher's exact, or log-rank tests, as appropriate. Analyses that adjusted for baseline factors and explored associations with baseline factors used linear regression. Mixed model analysis of variance with an unstructured correlation structure was used to test for differences in change from baseline between components over time. Time was modeled using piecewise variables, in which one variable captured the linear slope for changes from baseline to week 48 and the second variable captured the linear slope for changes from week 48 to 192. The week-48 separation time point was chosen on the basis of consultation with study chairs, after visual inspection of the data.
RESULTS
Participant Characteristics
A total of 271 participants from 37 ACTG sites in the United States and Puerto Rico intended to participate in A5224s and were randomized to receive ART; of these, 2 were excluded from the analysis when found to have had an eligibility violation. Enrollment spanned from 5 October 2005 through 7 November 2007 with 69 participants randomized to receive EFV plus TDF-FTC, 70 to EFV plus ABC-3TC, 65 to ATV/r plus TDF-FTC, and 65 to ATV/r plus ABC-3TC. Baseline characteristics are summarized in Table 1. Overall, 85% of participants were male and 47% were non-Hispanic white persons. The median age was 38 years, body mass index (BMI; measured as the weight in kilograms divided by the square of the height in meters) was 24.9, CD4 cell count was 233 cells/μL, and HIV-1 RNA load was 4.62 log10 copies/mL. One hundred sixty participants (59%) enrolled had an HIV-1 RNA load <100,000 copies/mL at study screening. Overall, 3% were hepatitis B surface antigen positive, 9% was hepatitis C antibody positive, 32% reported a history of fracture, and 39% had osteopenia (t score ≤-1 at spine or hip) at study entry. The baseline characteristics were balanced across arms.
Table 1.
Baseline Characteristics of Study Participants, by Randomized Arms
| Characteristic | EFV + TDF-FTC (n = 69) | EFV + ABC-3TC (n = 70) | ATV/r + TDF-FTC (n = 65) | ATV/r + ABC-3TC (n = 65) | Total (n = 269) | |
| Age (years) | Median (Q1-Q3) | 40 (33-44) | 39 (31-46) | 38 (30-44) | 37 (29-43) | 38 (31-44) |
| Sex | Male | 58 (84%) | 56 (80%) | 56 (86%) | 59 (91%) | 229 (85%) |
| Female | 11 (16%) | 14 (20%) | 9 (14%) | 6 (9%) | 40 (15%) | |
| Race/Ethnicity | White non-Hispanic | 37 (54%) | 34 (49%) | 26 (40%) | 29 (45%) | 126 (47%) |
| Black non-Hispanic | 22 (32%) | 20 (29%) | 21 (32%) | 27 (42%) | 90 (33%) | |
| Hispanic (regardless of race) | 8 (12%) | 14 (20%) | 14 (22%) | 8 (12%) | 44 (16%) | |
| Other | 2 (3%) | 2 (3%) | 4 (6%) | 1 (2%) | 9 (3%) | |
| BMI (kg/m2) | Median (Q1-Q3) | 24.9 (21.6-27.1) | 24.7 (22.6-28.3) | 24.9 (21.8-28.8) | 25.3 (21.8-28.9) | 24.9 (21.8-28.2) |
| CD4 category (cells/μL) | Median (Q1-Q3) | 250 (132-334) | 213 (106-350) | 247 (114-319) | 222 (75-332) | 233 (106-334) |
| HIV-1 RNA (log10 copies/mL) | Median (Q1-Q3) | 4.7 (4.2-4.9) | 4.7 (4.2-4.9) | 4.5 (4.2-4.9) | 4.6 (4.3-5.1) | 4.6 (4.2-4.9) |
| HIV-1 RNA (copies/mL) | < 100,000 copies/mL | 56 (81%) | 59 (84%) | 52 (80%) | 48 (74%) | 215 (80%) |
| ≥ 100,000 copies/mL | 13 (19%) | 11 (16%) | 13 (20%) | 17 (26%) | 54 (20%) | |
| History of bone fracture | Yes | 22 (32%) | 24 (34%) | 18 (28%) | 22 (34%) | 86 (32%) |
| No | 47 (68%) | 46 (66%) | 46 (71%) | 43 (66%) | 182 (68%) | |
| Not Evaluated | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (<1%) | |
| Lumbar spine BMD (g/cm2) | Median (Q1-Q3) | 1.12 (1.00-1.23) | 1.08 (.97-1.23) | 1.13 (1.03-1.24) | 1.13 (1.04-1.23) | 1.12 (.99-1.23) |
| Hip BMD (g/cm2) | Median (Q1-Q3) | 0.99 (.92-1.07) | 1.02 (.93-1.11) | 1.05 (.98-1.18) | 1.02 (.97-1.13) | 1.02 (.94-1.11) |
| Lumbar spine t-score | > -1 | 45 (67%) | 38 (58%) | 39 (64%) | 46 (72%) | 168 (65%) |
| > -2.5 to ≤ -1 | 19 (28%) | 23 (35%) | 18 (30%) | 13 (20%) | 73 (28%) | |
| ≤ -2.5 | 3 (4%) | 5 (8%) | 4 (7%) | 5 (8%) | 17 (7%) | |
| Hip t-score | > -1 | 50 (75%) | 48 (75%) | 47 (80%) | 51 (80%) | 196 (77%) |
| > -2.5 to ≤ -1 | 17 (25%) | 15 (23%) | 11 (19%) | 13 (20%) | 56 (22%) | |
| ≤ -2.5 | 0 (0%) | 1 (2%) | 1 (2%) | 0 (0%) | 2 (1%) | |
The baseline characteristics of the A5224s participants were compared with those of the 1588 A5202 persons who did not participate in the substudy; no statistically significant differences were found for age, BMI, CD4 cell count, HIV-1 RNA load, or history of fractures. However the non-A5224s group included significantly more Hispanic persons (24% vs 16%; P = .005).
Participant Disposition
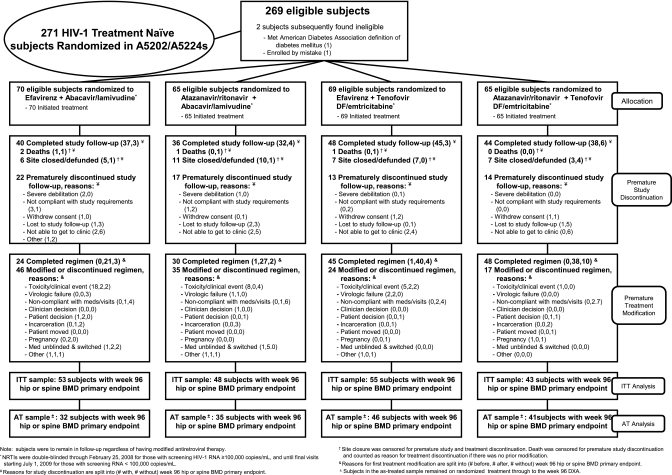
Figure 1 details the disposition of all participants. Overall, 66 (25%) of the A5224s participants prematurely discontinued the substudy, and 4 (1%) died. In addition, 31 participants (12%) discontinued, because their sites were defunded during the study. There was no statistically significant difference in time to premature study discontinuation between NRTI components (P = .13, site closure and death censored) or NNRTI-PI components (P = .86). The median time from randomization to the last clinic visit was 165 weeks.
Figure 1.
Details of disposition and outcome of study participants.
Percentage Changes in Spine BMD.
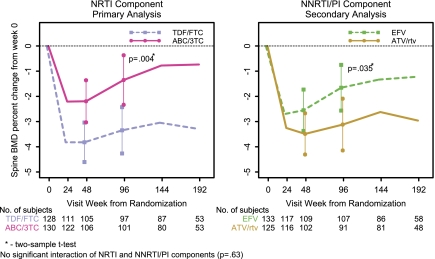
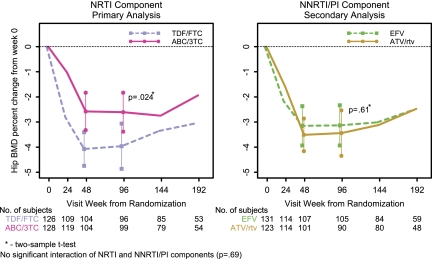
The first coprimary analysis assessed the difference in mean percentage change in spine BMD at week 96 between ABC-3TC and TDF-FTC. Table 2 summarizes the estimated mean percentage change over time in spine and hip BMD by all regimens. Figures 2 and 3 plot the mean percentage change over time in spine and hip BMD by NRTI and NNRTI-PI components.
Table 2.
Percent Changes in Lumbar Spine and Hip BMD for All 4 Treatment Arms. The Duration of the Study Was 96 Weeks Since the Last Subject Enrolled, Thus the Smaller n in Later Time Points
| EFV + TDF/FTC (N=69) | EFV + ABC/3TC (N=70) | ATV/r + TDF/FTC (N=65) | ATV/r + ABC/3TC (N=65) | Total (N=269) | ||
| Change in lumbar spine BMD (%), week 0–24 | N | 57 | 60 | 54 | 62 | 233 |
| Mean (SD) | -3.28 (2.71) | -2.15 (2.69) | -4.40 (3.58) | -2.26 (3.28) | -2.98 (3.19) | |
| week 0–48 | N | 56 | 53 | 49 | 53 | 211 |
| Mean (SD) | -3.46 (4.06) | -1.59 (4.42) | -4.23 (4.03) | -2.80 (4.20) | -3.00 (4.26) | |
| P value | <.001 | .012 | <.001 | <.001 | <.001 | |
| week 0–96 | N | 54 | 53 | 43 | 48 | 198 |
| Mean (SD) | -2.52 (4.08) | -.78 (5.20) | -4.38 (4.95) | -1.99 (4.69) | -2.33 (4.87) | |
| P value | <.001 | .28 | <.001 | .005 | <.001 | |
| week 0–144 | N | 46 | 40 | 41 | 40 | 167 |
| Mean (SD) | -2.60 (4.58) | 0.12 (5.92) | -3.55 (5.25) | -1.67 (3.93) | -1.96 (5.10) | |
| week 0–192 | N | 30 | 28 | 23 | 25 | 106 |
| Mean (SD) | -2.02 (3.92) | -0.37 (6.67) | -4.93 (5.76) | -1.15 (4.32) | -2.01 (5.45) | |
| Change in hip BMD (%), week 0–24 | N | 57 | 57 | 52 | 62 | 228 |
| Mean (SD) | -3.18 (5.13) | -1.15 (2.58) | -2.41 (2.59) | -.90 (2.45) | -1.88 (3.49) | |
| week 0–48 | N | 56 | 51 | 48 | 53 | 208 |
| Mean (SD) | -3.78 (3.63) | -2.46 (4.46) | -4.42 (3.21) | -2.69 (3.17) | -3.33 (3.71) | |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | |
| week 0–96 | N | 54 | 51 | 42 | 48 | 195 |
| Mean (SD) | -3.69 (3.81) | -2.54 (4.40) | -4.31 (5.17) | -2.68 (3.30) | -3.28 (4.22) | |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | |
| week 0–144 | N | 45 | 39 | 40 | 40 | 164 |
| Mean (SD) | -3.28 (3.74) | -2.71 (4.90) | -3.44 (5.63) | -2.79 (3.86) | -3.06 (4.54) | |
| week 0–192 | N | 30 | 29 | 23 | 25 | 107 |
| Mean (SD) | -2.65 (4.17) | -2.34 (3.98) | -3.56 (6.04) | -1.47 (4.12) | -2.49 (4.57) | |
Figure 2.
Mean percentage change in lumbar spine BMD by ITT analysis.
Figure 3.
Mean percentage change in hip BMD by ITT analysis.
The estimated mean percentage change in spine BMD for all participants was −3.0% at week 48 and −2.3% at week 96. The comparison of ABC-3TC (n = 135) and TDF-FTC (n = 134) with EFV and ATV/r combined (factorial analysis) was performed, because there was no significant evidence that the treatment effect between these drugs differed at 96 weeks by the NNRTI-PI component (P = .63). Similarly, the comparison of EFV (n = 139) and ATV/r (n = 130) with ABC-3TC and TDF-FTC combined was performed.
Changes by NRTI Components: Primary Analysis.
By ITT at week 96, there was a significant decrease in mean percentage change in spine BMD for all arms except ABC-3TC plus EFV, but significantly less for ABC-3TC (estimated mean of −1.3%) than for TDF-FTC (−3.3%; difference [Δ] = 2.0%; 95% confidence interval [CI], .7%–3.3%; P = .004). The AT analysis showed similar results, with the mean percentage change in ABC-3TC– and TDF-FTC–treated participants being –1.0% and −3.2%, (Δ = 2.2%; 95% CI, .6%–3.7%; P = .006). The difference between the NRTI components in the mean percentage change in spine BMD was already evident at week 48, at which point the ABC-3TC arms had an estimated mean percentage change of 1.6% (95% CI, .5%–2.8%) smaller than that in the TDF-FTC arms (P = .005).
At week 96, among participants assigned to receive EFV, there was a trend toward a greater decrease in mean percentage change in spine BMD when combined with TDF-FTC than when combined with ABC-3TC (Δ, 1.7%; 95% CI, .04%–3.5%; P = .056). In ATV/r-treated arms, there was a significantly greater decrease in mean percentage change in spine BMD when combined with TDF-FTC than when combined with ABC/3TC (Δ, 2.4%; 95% CI, .4%–4.4%; P = .020, by ITT).
Changes by NNRTI-PI Component: Secondary Analysis.
At week 96, by ITT analysis, the mean percentage change in spine BMD was significantly greater in those assigned to ATV/r (−3.1%) than in those in the EFV arm (−1.7%; Δ, −1.5%; 95% CI, −2.8% to −.1%; P = .035). Similar results were seen in the AT analysis. However, at 48 weeks, the mean percentage change was not significantly different between those treated with ATV/r (−3.5%) and those treated with EFV (−2.6%; Δ = −.9%; 95% CI, −2.1% to .2%; P = .11).
Percentage Changes in Hip BMD
The second coprimary analysis involved the mean percentage change in hip BMD at week 96 between the ABC-3TC and the TDF-FTC arms. The estimated mean percentage change in hip BMD for all participants was −3.3% at both weeks 48 and 96 (Table 2). A comparison of ABC-3TC (n = 135) and TDF-FTC (n = 134) with EFV and ATV/r combined was performed, because there was no significant evidence that the treatment effect between these drugs differed at 96 weeks by the NNRTI-PI component (P = .69). Similarly, a comparison of EFV (n = 139) and ATV/r (n = 130) with ABC-3TC and TDF-FTC combined was performed.
Changes by NRTI Components: Primary Analysis.
At week 96, ITT analysis showed that the ABC-3TC arms had a significantly smaller decrease in mean percentage change in hip BMD, compared with the TDF-FTC arms (−2.6% vs −4.0%; Δ, 1.4%; 95% CI, .2%–2.5%; P = .024). The AT analysis showed similar results; at week 96, the mean percentage change in hip BMD in the the ABC-3TC arms was −2.6%, compared with −3.9% for TDF-FTC (Δ, = 1.3%; 95% CI, .02%–2.6%; P = .046). The difference between the NRTI components in the mean percentage change in hip BMD was already evident at week 48, with an estimated mean change of −2.6% for ABC-3TC and −4.1% for TDF-FTC (Δ, 1.5%; 95% CI, .5%–2.5%; P = .003).
For persons assigned to receive EFV, at 96 weeks, the mean percentage change in hip BMD was not statistically significantly different between the NRTI components, compared with those assigned to receive ABC-3TC; the estimated mean change was −2.5%, compared with −3.7% for those given TDF-FTC (Δ, 1.2%; 95% CI, −.4% to 2.7%; P = .15). There was a trend toward a smaller decrease in mean percentage change in hip BMD for persons given ATV/r with ABC-3TC (−2.7%), compared with those given TDF-FTC (−4.3%; Δ, 1.6%; 95% CI, .2%–3.4%; P = .075).
Changes by NNRTI-PI Component: Secondary Analysis.
At week 96 and by ITT analysis, the mean percnetage change in hip BMD was not statistically significantly different between EFV and ATV/r (Δ, −.3%; 95% CI, −1.5% to .9%; P = .61). Similar results were seen in the AT analysis and at week 48.
Changes in Spine and Hip BMD Adjusted for Baseline Covariates
The ITT analyses of mean percentage change from entry to week 96 of spine and hip BMD were adjusted for the following prespecified baseline covariates that could affect BMD, first individually and then jointly, with use of linear regression: NNRTI-PI (or NRTI components for the NNRTI-PI analyses), spine BMD (or hip BMD for corresponding analysis), sex, age, race/ethnicity, log10 HIV-1 RNA load, CD4 cell count, and BMI. For analyses of the NRTI component effect or the NNRTI-PI component effect, all of the adjusted models led to results similar to those of the unadjusted analyses.
Association Between Baseline Factors and Changes in BMD at 96 Weeks
Table 3 summarizes the linear regression analyses that were performed to assess the baseline factors associated with 96-week percentage change in spine and hip BMD. The covariates included in the model were the same as the ones mentioned in the previous paragraph. For spine BMD, in addition to the significant ABC-3TC and ATV/r effects, in both univariate and multivariable models, higher baseline CD4 cell count was independently associated with significant increases, and higher baseline log10 HIV-1 RNA load was independently associated with significant decreases in spine BMD at 96 weeks. For hip BMD, in addition to the significant ABC-3TC effect, in univariate and multivariable models, higher baseline BMI was independently associated with significant increases at 96 weeks.
Table 3.
Results of the Regression Analysis
| Variable | No. of participants | Parameter estimate | 95% CI | P valuea |
| 96-week percentage change in lumbar spine BMD, univariate analysesb | ||||
| ABC-3TC (vs TDF-FTC) | 198 | 2.00 | (.66–3.33) | .004 |
| ATV/r (vs EFV) | 198 | -1.46 | (-2.82–.10) | .035 |
| Baseline HIV-1 RNA (per log10 copies/mL) | 198 | -2.00 | (-3.00–1.01) | <.001 |
| Baseline CD4 cell count (per 50 cells/μL) | 198 | 0.48 | (.28–.68) | <.001 |
| 96-week percentage change in lumbar spine BMD, multivariable analysisc | ||||
| ABC-3TC (vs TDF-FTC) | 198 | 1.90 | (.64–3.17) | .003 |
| ATV/r (vs EFV) | 198 | -1.38 | (-2.70–.07) | .039 |
| Baseline HIV-1 RNA (per log10 copies/mL) | 198 | -1.17 | (-2.30–.05) | .041 |
| Baseline CD4 cell count (per 50 cells/μL) | 198 | 0.37 | (.14–.59) | .001 |
| 96-week percentage change in hip BMD, univariate analysesb | ||||
| ABC-3TC (vs TDF-FTC) | 195 | 1.35 | (.18–2.53) | .024 |
| Baseline BMI (per kg/m2) | 195 | 0.16 | (.02–.29) | .021 |
| 96-week percentage change in hip BMD, multivariable analysisc | ||||
| ABC-3TC (vs TDF-FTC) | 195 | 1.28 | (.10–2.46) | .033 |
| Baseline BMI (per kg/m2) | 195 | 0.18 | (.04–.32) | .013 |
NOTE.
Only P values < .050 are presented.
Individually assessed sex, age, race/ethnicity, log10 HIV-1 RNA, CD4 count, BMI, ABC-3TC, and ATV/r.
Jointly assessed sex, age, race/ethnicity, log10 HIV-1 RNA load, CD4 cell count, BMI, ABC-3TC, and ATV/r.
Timing of BMD Changes: Repeated Measures Analyses.
To understand the dynamics of BMD change over time, an analysis of the slopes of changes in the early phase (0–48 weeks) and late phase (48–192 weeks) was explored in and between study components. For spine BMD, as shown in Table 4, there was a statistically significant difference between the NRTIs in the slope of BMD change during both the early and the late phase, favoring ABC-3TC. Of interest, ABC-3TC arms, but not TDF-FTC, had a significant positive spine BMD percentage change per year during the late phase. For NNRTI-PI components, there was no statistically significant difference in the slopes between NNRTI and PI arms in either phase, with both arms having decreasing BMD during the early phase and only EFV significantly increasing spine BMD in the late phase.
Table 4.
Repeated Measures Analysis, BMD (Percentage Change)
| BMD site | Time interval | Mean percentage change/year | P value | 95% CI | |
| Lumbar spine | Entry to week 48 | TDF/FTC | −4.09 | <.001 | (−4.87–3.30) |
| ABC/3TC | −2.43 | <.001 | (−3.25–1.60) | ||
| Difference (ABC/3TC - TDF/FTC) | 1.66 | .005 | (.52–2.80) | ||
| Week 48 to week 192 | TDF/FTC | 0.00 | 1.00 | (−.34–0.34) | |
| ABC/3TC | 0.60 | .002 | (.22–.98) | ||
| Difference (ABC/3TC - TDF/FTC) | 0.60 | .022 | (.09–1.11) | ||
| Entry to week 48 | EFV | −2.77 | <.001 | (−3.58–1.95) | |
| ATV/r | −3.70 | <.001 | (−4.52–2.88) | ||
| Difference (ATV/r - EFV) | −0.93 | .11 | (−2.09–.22) | ||
| Week 48 to week 192 | EFV | 0.44 | .016 | (.08–.79) | |
| ATV/r | 0.12 | .51 | (−.24–.49) | ||
| Difference (ATV/r - EFV) | −0.31 | .23 | (−.83–.20) | ||
| Hip | Entry to week 48 | TDF/FTC | −4.29 | <.001 | (−5.02–3.56) |
| ABC/3TC | −2.86 | <.001 | (−3.60–2.11) | ||
| Difference (ABC/3TC - TDF/FTC) | 1.43 | .007 | (.39–2.47) | ||
| Week 48 to week 192 | TDF/FTC | 0.27 | .14 | (−.09–.62) | |
| ABC/3TC | 0.41 | .017 | (.07–.74) | ||
| Difference (ABC/3TC - TDF/FTC) | 0.14 | .56 | (−.34–.63) | ||
| Entry to week 48 | EFV | −3.38 | <.001 | (−4.19–2.57) | |
| ATV/r | −3.74 | <.001 | (−4.42–3.06) | ||
| Difference (ATV/r - EFV) | −0.36 | .50 | (−1.42–.69) | ||
| Week 48 to week 192 | EFV | 0.30 | .065 | (−.02–.62) | |
| ATV/r | 0.36 | .061 | (−.02–.73) | ||
| Difference (ATV/r - EFV) | 0.06 | .82 | (−.44–.55) |
For hip BMD, the treatment differences and kinetics of bone loss were similar, with most of the BMD loss occurring during the first 48 weeks in both NRTI arms. During the late phase, ABC-3TC arms again showed a significant gain in hip BMD. Both EFV and ATV/r arms lost bone in the first phase, but the slope of the late phase did not reach statistical significance in either arms.
On-Study Bone Fractures
On-study bone fractures were collected in A5224s and in the A5202 parent study (n = 1857). In the substudy, 15 participants (5.6%) reported a bone fracture, all of which were a result of trauma, with 10 occurring in the EFV arms. There were no statistically significant differences in the number of fractures between the NRTIs (P = 1.00) or the NNRTI and PI study arms (P = .29). Similarly, there was no statistically significant difference in time to first bone fracture between NRTI (P = .76) or NNRTI/PI study arms (P = .27).
In the parent study-A5202, 80 participants (4.3%) reported at least one bone fracture on study (ABC-3TC plus EFV, 4.7%; ABC-3TC plus ATV/r, 3.5%; TDF-FTC plus EFV, 4.5%; and TDF-FTC plus ATV/r, 4.5%). Among these, 10 (12.7%) were atraumatic. The bone fractures were balanced across the study arms, with no statistically significant differences between the NRTI (P = .73) or the NNRTI and PI components (P = .57). No statistically significant difference in time to first bone fracture was seen between the NRTIs (P = .71) or the NNRTI and PI components (P = .49). Similarly, incidence rates were similar across arms (ABC-3TC plus EFV, 1.9 cases per 100 patient-years; ABC-3TC plus ATV/r, 1.4 cases per 100 patient-years; TDF-FTC plus EFV, 1.8 cases per 100 patient-years; and TDF-FTC plus ATV/r, 1.8 cases per 100 patient-years).
DISCUSSION
This report details changes in BMD in participants randomized to receive 1 of 4 frequently used regimens for treatment of HIV infection. As shown in prior studies, we demonstrated that ART initiation led to a large initial decrease in BMD, with ABC-3TC plus EFV being the only regimen studied that did not lead to a statistically significant decrease in spine BMD at week 96. We also found that TDF-FTC led to greater decreases in spine and hip BMD than did ABC-3TC and that ATV/r induced a significantly greater decrease in the spine BMD than did EFV. Our results are robust, because correcting for potential confounders and/or imbalances, including traditional bone risk factors and HIV disease characteristics, did not affect these results. AT analyses yielded results similar to those of ITT analyses. The incidence of fractures did not differ significantly between the regimen components.
The present study adds to a body of literature demonstrating a greater effect on reducing BMD with TDF-based therapies, compared with other regimens [11, 15]. A previous randomized clinical trial involving ART-naive participants compared TDF-FTC with ABC-3TC, both with EFV [11]. At 48 weeks, decreases in spine and hip BMD were significantly greater with TDF-FTC. Our data are consistent with these results and extend the observation to 96 weeks and to the use of both EFV and ATV/r.
The role of PI therapy in HIV-associated osteoporosis has been debated. Our study revealed a greater decrease in BMD with ATV/r regimens, compared with EFV, but only at the spine. A trend toward greater decrease in total body BMD with another ritonavir-boosted PI (lopinavir/r), compared with EFV, was observed in another randomized trial [16]. By contrast, other studies have not shown an effect of PI on BMD [17, 18]. Some of these discrepancies may be related to the use of whole-body DXA instead of using the more sensitive site-specific bone DXAs. Our study showed that the effect of ART varies by site, supporting the use of site-specific DXA. This site differential effect could be attributable to the trabecular nature of vertebral bone, which is more active and more subject to bone turnover and remodeling, compared with cortical (eg, hip) bone. In addition, different PIs may have differential effects on bone, analogous to their variable effect in the drug class on lipid changes.
The mechanisms involved in bone loss after initiation of ART are not well understood. TDF may affect bone through proximal tubule toxicity, resulting in phosphate wasting and increased bone turnover [19]. EFV and PIs may affect BMD indirectly through vitamin D metabolism [20–26]. In multivariable analysis exploring the factors associated with BMD changes at 96 weeks, we found that, in addition to TDF-FTC and ATV/r each leading to greater spine BMD decrease, compared with ABC-3TC and EFV, respectively (also in hip BMD for TDF-FTC), other baseline factors were associated with BMD loss. Some of these (eg, lower BMI) are also associated with BMD decreases in the general population. Relevant HIV-specific factors that decreased BMD include higher baseline HIV-1 RNA load and lower CD4 cell count, corroborating findings of other studies showing a greater risk of osteopenia and/or osteoporosis in those with longer HIV infection duration [27–29]. These observations support the fact that HIV infection or immunologic factors linked to HIV infection play a role in bone loss after treatment initiation. Indeed, HIV proteins can increase osteoclastic activity [30] and promote osteoblast apoptosis [31, 32]. Furthermore, cytokines, such as IL-6 and TNF-α, may stimulate osteoclast activity [33–35].
Because most of our study participants were young (median age, 38 years), with a relatively low risk of falls, it was not surprising that we did not observe an increased rate of fractures with specific ART regimens. However, the degree of BMD loss and the between-component differences should not be perceived as clinically insignificant. Indeed, these decreases are similar in magnitude to the BMD losses sustained during the first 2 years of menopause [36]. Furthermore, our study population was young and mostly (85%) male, a group typically spared significant loss of BMD. In the general population, the mean 2-year change in BMD in men 20–49 years of age is −0.8% at the hip and −0.3% at the lumbar spine [37]. Even at the most vulnerable skeletal time in women (during the first 2 years of menopause), the loss of BMD accelerates, with mean annual rates of bone loss of 1.2% –1.6%. This magnitude of bone loss is equivalent to the point estimates of the mean differences shown between the ABC-3TC and TDF-FTC regimens at the hip and spine and between EFV and ATV/r at the spine, although the confidence intervals are consistent with smaller differences in the means.
Our study is notable for the observations regarding timing of BMD changes after ART initiation. Large early reductions in spine and hip BMD were observed within the first 48 weeks after ART initiation with all regimens. After the initial 48 weeks (cutoff chosen by inspection), BMD did not change or even improved slightly with some of the regimens. This is consistent with prior ART initiation studies and with longitudinal studies of treatment-experienced participants that have shown stability in BMD over time [3, 28, 38, 39].
Our study has several limitations. First, the duration of follow-up for study of bone end points was relatively short. Nevertheless, to our knowledge, our study has the longest follow-up of published prospective longitudinal studies of BMD after ART initiation. Second, the changes in the NRTI backbone of the regimen that resulted from the outcome of the DSMB review of A5202 were relatively frequent. However, our ITT results were consistent with our AT results. Other limitations are that the NNRTI-PI component was provided in an open-labeled fashion and that there was a high amount of missing data, which is not unusual for large multicentered studies. Finally, the study did not collect smoking and alcohol status, which could affect BMD.
In conclusion, we revealed that the initiation of ART leads to prompt reductions in spine and hip BMD observed within the first 48 weeks, independent of ART type. At week 96, TDF-FTC, both in the spine and hip, and ATV/r in the spine produced significantly more bone loss than did ABC-3TC– or EFV-based regimens. Studies investigating the mechanisms behind the bone loss with ART initiation are needed.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (U01AI068636, AI68634, AI38855, and AI069501). GlaxoSmithKline and Gilead funded the DXA and CT scans. Study medications were provided by Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Acknowledgment Appendix for A5224s
Sadia Shaik, M.D. and Ruben Lopez, M.D.- Harbor-UCLA Medical Center (Site 603) CTU Grant #:AI069424, RR00425
Susan L. Koletar, MD and Diane Gochnour, RN- The Ohio State University Medical Center (Site 2301) CTU Grant # AI069474
Geyoul Kim, RN and Mark Rodriguez, RN- Washington University (Site 2101) CTU Grant #:U01AI069495; GCRC Grant: UL1 RR024992
Elizabeth Lindsey, RN and Tamara James, BS - Alabama Therapeutics CRS (Site 5801) CTU Grant #: U01 AI069452
Ann C. Collier, MD and Jeffrey Schouten, MD, JD- University of Washington (Site 1401) CTU Grant #: AI069434; UL1 RR025014
Jorge L. Santana Bagur,MD and Santiago Marrero,MD- Puerto Rico-AIDS Clinical Trials Unit (Site 5401) CTU Grant # 5 U0I AI069415-03
Jenifer Baer, RN, BSN and Carl Fichtenbaum, MD- University of Cincinnati (Site 2401) CTU Grant # AI069513
Patricia Walton BSN RN and Barbara Philpotts BSN RN- Case Western Reserve (Site 2501) CTU Grant #: AI69501
Princy Kumar, M.D. and Joseph Timpone, M.D.- Georgetown University (Site 1008) CTU Grant#: ACTG grant # 5U01AI069494
Donna Pittard RN BSN and David Currin RN- University of North Carolina (Site 3201) CTU Grant #: 5 - U01 AI069423-03; UNC CFAR #: P30 AI050410(-11); UNC CTRC #: UL 1RR 025747
Julie Hoffman, R.N. and Edward Seefried, R.N.- San Diego Medical Center UC (Site 701) CTU Grant # AI69432
Susan Swindells MBBS and Frances Van Meter APRN- University of Nebraska (Site 1505) CTU Grant #: AI 27661
Deborah McMahon, MD and Barbara Rutecki, MSN, MPH, CRNP- University of Pittsburgh (Site 1001) CTU Grant #: 1 U01 AI069494-01
Michael P. Dube, M.D. and Martha Greenwald, R.N., M.S.N- Indiana University (Site 2601) CTU Grant #: 5U01AI025859; GCRC #: M01 RR00750
Ilene Wiggins, RN, and Eric Zimmerman, RN- Johns Hopkins University (Site 201) CTU Grant #: AI27668; CTSA Grant # UL1 RR025005
Judith. Aberg, M.D. and Margarita Vasquez R.N.- New York University/NYC HHC at Bellevue Hospital Center (Site 401) CTU Grant #: AI27665, New grant number: AI069532
Martin McCarter and M. Graham Ray, R.N., M.S.N. - Colorado AIDS Clinical Trials Unit, (Site 6101) CTU Grant # AI69450; RR025780
Mamta Jain, MD -PI and Tianna Petersen, MS- University of Texas Southwestern Medical Center (Site 3751) CTU Grant #: 3U01AI046376-05S4
Emily Stumm, BS and Pablo Tebas MD- University of Pennsylvania, Philadelphia (Site 6201) CTU Grant #: P30-AI0450008-11; CFAR Grant #: UO1-AI069467-04
Mary Albrecht, MD and Neah Kim, NP- Beth Israel Deaconess (Partners/Harvard) CRS (Site 103) CTU Grant # U01 AI069472-04
Paul Edward Sax, M.D. and Joanne Delaney RN- Brigham and Women's Hospital (Site 107) CTU Grant # UOI AI 069472
Christine Hurley, RN and Roberto Corales, DO- AIDS Care (Site 1108) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160
Keith Henry, MD and Bette Bordenave, RN- Hennepin County Medical Center (Site 1502) CTU Grant #: N01 AI72626
Wendy Armstrong, MD and Ericka R. Patrick, RN, MSN, CCRC- Emory University HIV/AIDS Clinical Trails Unit (Site 5802) CTU Grant #: UO1Al69418-01/CFAR Grant Number: P30Al050409
Jane Reid RNC MS and Mary Adams RN MPh- University of Rochester (Site 1101) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160
Gene D. Morse, Pharm.D., FCCP, BCPS- SUNY - Buffalo, Erie County Medical Ctr. (Site 1102) CTU Grant # AI27658
Michael P. Dube, M.D. and Martha Greenwald, R.N., M.S.N- Wishard Memorial Hospital Indiana University (Site 2603) CTU Grant #: 5U01AI025859; GCRC #: M01 RR00750
Kimberly Y. Smith, MD, MPH and Joan A. Swiatek, APN- Rush University Medical Center (Site 2702) CTU Grant #: U01 AI069471
Nancy Hanks, RN, and Debra Ogata-Arakaki, RN, -University of Hawaii at Manoa, Leahi Hospital (Site 5201) CTU Grant # AI34853
Ardis Moe, MD and Maria Palmer PA-C- UCLA Medical Center (Site 601) CTU Grant #
1U01AI069424-01
Jeffery Meier, M.D. and Jack T. Stapleton, M.D. - University of Iowa Hospitals and Clinics (Site 1504) CTU Grant #: UL1RR024979
Gary Matthew Cox, MD and Martha Silberman, RN- Duke University Medical Center Adult CRS (Site 1601) CTU Grant # 5U01 AI069 484-02
Cook County Hospital
Gerianne Casey, RN and William O'Brien MD-University of Texas, Galveston (Site 6301) CTU Grant # AI32782
Valery Hughes, FNP and Todd Stroberg, RN- Cornell CRS (Site 7803, 7804) – CTU Grant#: U01 AI069419; CTSC #: UL1 RR024996
Nyef El-Daher MD -McCree McCuller Wellness Center at the Connection (Site 1107) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160
Rebecca J. Basham, B.S. and Husamettin Erdem, M.D.-Vanderbilt Therapeutics CRS (Site 3652) CTU Grant #: AI46339-01; MO1 RR 00095
References
- 1.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–74. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 2.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 3.Cassetti I, Madruga JV, Suleiman JM, et al. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV Clin Trials. 2007;8:164–72. doi: 10.1310/hct0803-164. [DOI] [PubMed] [Google Scholar]
- 4.Brown T, McComsey G. Initiation of antiretroviral therapy with efavirenz associated with decreases in 25 Hydroxyvitamin D. Antivir Ther. 2010;15:425–9. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 5.Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;23:817–24. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 6.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–504. doi: 10.1210/jc.2008-0828. PMCID: 2567857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collin F, Duval X, Le Moing V, et al. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS. 2009;23:1021–4. doi: 10.1097/QAD.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dao C, Young B, Buchacz K, Baker R, Brooks J, Hos IA, editors. Higher and increasing rates of fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared o the general US population, 1994 to 2008. 17th Conference on Retrovirus and Opportunistic Infections. San Francisco, CA: 2010. [Google Scholar]
- 9.Womack J, Goulet J, C G, et al., editors. HIV-infection and fragility fracture risk among male Veterans. 17th Conference on Retroviruses and Opportunistic Infections; 2010 February 18. San Francisco, CA: 2010. [Google Scholar]
- 10.Cooper DA, Bloch M, Humphries A, et al. Simplification with fixed-dose tenofovir-emtricitaine or abacavir-lamivudine in adults with suppressed HIV repliation (The Steal Study): a randomized, open-label, 96-week, non-inferiority trial. 16th Conference on Retroviruses and Opportunistic Infections 2009 February 8–11. Montreal, Canada: 2010. [Google Scholar]
- 11.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 12.Jemsek JG, Arathoon E, Arlotti M, et al. Body fat and other metabolic effects of atazanavir and efavirenz, each administered in combination with zidovudine plus lamivudine, in antiretroviral-naive HIV-infected patients. Clin Infect Dis. 2006;42:273–80. doi: 10.1086/498505. [DOI] [PubMed] [Google Scholar]
- 13.Noor MA, Flint OP, Maa JF, Parker RA. Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose uptake and insulin sensitivity: demonstrable differences in vitro and clinically. AIDS. 2006;20:1813–21. doi: 10.1097/01.aids.0000244200.11006.55. [DOI] [PubMed] [Google Scholar]
- 14.Sax PE, Tierney C, Collier AC, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361:2230–40. doi: 10.1056/NEJMoa0906768. PMCID: 2800041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper DA, Bloch M, Humphries A, et al., editors. Simplicication with fixed-dose tenofovir-emtricitaine or abacavir-lamivudine in adults with suppressed HIV replication (The Steal Study): a randomized, open-label, 96-week, non-inferiority trial. 16th Conference on Retroviruses and Opportunistic Infections; 2009 February 8–11. Montreal, Canada: 2009. [Google Scholar]
- 16.Huang J, Hughes M, Riddler SA, Haubrich R, editors. Effects of randomized regimen and nucleoside reverse transcriptase inhibitor (NRTI) selection on 96 week bone mineral density (BMD): results from ACTG 5142. Study Team is a Vienna Meeting. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM. da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–61. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 18.Amiel C, Ostertag A, Slama L, et al. BMD is reduced in HIV-infected men irrespective of treatment. J Bone Miner Res. 2004;19:402–9. doi: 10.1359/JBMR.0301246. [DOI] [PubMed] [Google Scholar]
- 19.Fux CA, Rauch A, Simcock M, et al. Tenofovir use is associated with an increase in serum alkaline phosphatase in the Swiss HIV Cohort Study. Antivir Ther. 2008;13:1077–82. [PubMed] [Google Scholar]
- 20.Ellfolk M, Norlin M, Gyllensten K, Wikvall K. Regulation of human vitamin D(3) 25-hydroxylases in dermal fibroblasts and prostate cancer LNCaP cells. Mol Pharmacol. 2009;75:1392–9. doi: 10.1124/mol.108.053660. [DOI] [PubMed] [Google Scholar]
- 21.Fabbriciani G, De Socio GV. Efavirenz and bone health. AIDS. 2009;23:1181. doi: 10.1097/QAD.0b013e32832bab0f. [DOI] [PubMed] [Google Scholar]
- 22.Herzmann C, Arasteh K. Efavirenz-induced osteomalacia. AIDS. 2009;23:274–5. doi: 10.1097/QAD.0b013e32831f4685. [DOI] [PubMed] [Google Scholar]
- 23.Landriscina M, Altamura SA, Roca L, et al. Reverse transcriptase inhibitors induce cell differentiation and enhance the immunogenic phenotype in human renal clear-cell carcinoma. Int J Cancer. 2008;122:2842–50. doi: 10.1002/ijc.23197. [DOI] [PubMed] [Google Scholar]
- 24.Mouly S, Lown KS, Kornhauser D, et al. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther. 2002;72:1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 25.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17:513–20. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 26.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17:513–20. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 27.Madeddu G, Spanu A, Solinas P, et al. Bone mass loss and vitamin D metabolism impairment in HIV patients receiving highly active antiretroviral therapy. Q J Nucl Med Mol Imaging. 2004;48:39–48. [PubMed] [Google Scholar]
- 28.Mondy K, Yarasheski K, Powderly WG, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–90. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 29.Cazanave C, Dupon M, Lavignolle-Aurillac V, et al. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS. 2008;22:395–402. doi: 10.1097/QAD.0b013e3282f423dd. [DOI] [PubMed] [Google Scholar]
- 30.Fakruddin JM, Laurence J. HIV-1 Vpr enhances production of receptor of activated NF-kappaB ligand (RANKL) via potentiation of glucocorticoid receptor activity. Arch Virol. 2005;150:67–78. doi: 10.1007/s00705-004-0395-7. [DOI] [PubMed] [Google Scholar]
- 31.Borderi M, Gibellini D, Crignis E, et al., editors. HIV-1 induces apoptosis in primary osteoblasts: an alternative mechanism in the development of osteopenia and osteoporosis. 15th Conference on Retroviruses and Opportunistic Infections 2009 February; Montreal, Canada: 2009. [Google Scholar]
- 32.Gibellini D, De Crignis E, Ponti C, et al. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFalpha activation. J Med Virol. 2008;80:1507–14. doi: 10.1002/jmv.21266. [DOI] [PubMed] [Google Scholar]
- 33.Manolagas SC. Role of cytokines in bone resorption. Bone. 1995;17(Suppl 2):63S–7. doi: 10.1016/8756-3282(95)00180-l. [DOI] [PubMed] [Google Scholar]
- 34.Roodman GD. Role of cytokines in the regulation of bone resorption. Calcif Tissue Int. 1993;53(Suppl 1):S94–8. doi: 10.1007/BF01673412. [DOI] [PubMed] [Google Scholar]
- 35.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043–51. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 36.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93:861–8. doi: 10.1210/jc.2007-1876. PMCID: 2266953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–12. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 38.Nolan D, Upton R, McKinnon E, et al. Stable or increasing bone mineral density in HIV-infected patients treated with nelfinavir or indinavir. AIDS. 2001;15:1275–80. doi: 10.1097/00002030-200107060-00009. [DOI] [PubMed] [Google Scholar]
- 39.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2006;91:2938–45. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]