Abstract
Field studies on HIV risk suggest that people may rely on impressions they have about the safety of their partner at the dispense of more objective risk protection strategies. In this study, ERP recordings were used to investigate the brain mechanisms that give rise to such impressions. First, in an implicit condition, participants viewed a series of photographs of unacquainted persons while performing a task that did not mention HIV risk. Second, in an explicit condition, participants estimated the HIV risk for each presented person. Dense sensor EEG was recorded during the implicit and explicit conditions. In the analysis, explicit risk ratings were used to categorize ERP data from the implicit and explicit conditions into low and high HIV risk categories. The results reveal implicit ERP differences on the basis of subsequent ratings of HIV risk. Specifically, the processing of risky individuals was associated with an early occipital negativity (240–300 ms) and a subsequent central positivity between 430 and 530 ms compared to safe. A similar ERP modulation emerged in the explicit condition for the central positivity component between 430 and 530 ms. A subsequent late positive potential component between 550 and 800 ms was specifically enhanced for risky persons in the explicit rating condition while not modulated in the implicit condition. Furthermore, ratings of HIV risk correlated substantially with ratings of trustworthiness and responsibility. Taken together, these observations provide evidence for theories of intuitive risk perception, which, in the case of HIV risk, seem to operate via appearance-based stereotypic inferences.
Keywords: risk perception, HIV, intuition, ERP, LPP
Introduction
Studies on peoples’ perception of HIV risk have revealed a peculiar phenomenon: Although knowledge about HIV is high among sexually active individuals in Europe and the US, condoms are used far less often than necessary (Gardner et al., 1999; Civic, 2000). For example, Keller (1993) reported that as much as 85% of a college sample stated that they did not use condoms consistently. Such findings have motivated health psychologists to investigate how people estimate their personal HIV risk. Preliminary evidence suggests that because of the inherently social nature of HIV risk, peoples’ personal risk perceptions are tied to how risky they perceive their sexual partner, the potential carrier of the virus, to be. Field studies have found that people generally consider their partners to be safe (Maticka-Tyndale, 1991; Gold et al., 1992; Swann et al., 1995; Thompson et al., 1999). This has led to the concern that people may be deceived by a partner's safe appearance, which could nurture an illusory feeling of control and thereby undermine consistent condom use (Williams et al., 1992). Indeed, most people who had unsafe intercourse report that they were under the impression that their partners were safe (Gold et al., 1992; Keller, 1993). Overall, the situation is reminiscent of the sometimes surprising effects that the a person's appearance exerts upon real-world decisions, such as voting, employment, or sentencing (Zebrowitz and McDonald, 1991; Hamermesh and Biddle, 1994; Todorov et al., 2005).
Based on the large role of implicit processes in day-to-day thinking (e.g., Bargh and Chartrand, 1999; Frith and Frith, 2008), particularly with respect to the social and sexual domain (Norton et al., 2005; Ariely and Loewenstein, 2006; Ditto et al., 2006; Stacy et al., 2006), we assume that impressions of partner safety may stem from implicit stimulus processing. Here, we use the term “implicit” to refer to fast and largely automatic processing routines which are often ascribed to an intuitive or reflexive system within dual-process views (Strack and Deutsch, 2004; Lieberman et al., 2002; Evans, 2008; Frith and Frith, 2008). In this view, unless alerted by an intuitive alarm signal that a particular partner could be risky, people may not reflect on HIV risk and simply follow their habits (which may or may not include the use of condoms). It is noteworthy that risk perception as conceived here represents a mundane and highly contextualized phenomenon. This stands in contrast to classical theories of risk, which are still the dominant model in risk perception literature and which emphasize the role of relatively stable cognitive estimates of risk probability and severity (Loewenstein et al., 2001; Renner and Schupp, 2011). The hypothesis that such implicit processing is a critical part of risk perception has only been examined in few empirical studies, and thus, primarily due to the difficulties in measuring implicit aspects of information processing, has only received little empirical support to date.
Using HIV as a model system, we attempted to make the implicit processes involved in risk perception amenable to scientific investigation. The innovation of our approach is that neural recordings allow light to be shed on the most elusive aspect of risk impressions: their incidental and spur-of-the-moment-like nature. A few studies have examined the links between partner perception and inferences about HIV risk based on self-report measures. In brief, participants were shown pictures of unacquainted persons and asked to rate the likelihood that the person is HIV positive (Agocha and Cooper, 1999; Dijkstra et al., 2000; Thompson et al., 2002). We devised a paradigm that combines neuroscientific tools with a suitably modified version of this behavioral task in order to explore central, aspects of HIV risk perception that have, until now, been unreachable. Specifically, we set up an implicit condition, in which participants viewed photographs of unacquainted persons while performing a person-recognition task. Importantly, at this stage of the experiment, participants were not informed that the study actually investigated the perception of HIV risk. To capture neural processes unfolding during the first sight of the stimulus persons, we measured high-density ERP. After recording immediate brain responses in the implicit condition, participants viewed the stimulus materials a second time, but with the explicit task of evaluating the HIV risk of each presented person (see Materials and Methods for details). Thus, the paradigm allows the assessment of both implicit and explicit processes relating to HIV risk perception.
In the present research, we hypothesized that impressions of HIV risk are based on an intuitive mode of reasoning (Lieberman, 2000; Loewenstein et al., 2001; Slovic and Peters, 2006). The main aim of this study was to determine whether brain responses during the implicit condition differ as a function of subsequent explicit reports of perceived HIV risk. Methodologically, this is similar to the subsequent memory paradigm (e.g., Wagner et al., 1998), where, in order to uncover encoding processes associated with successful remembering, neural epochs are classified depending on whether an item is remembered or forgotten. The notion of intuitive risk perception predicts that the brain should react differently to risky-looking individuals even in a context unrelated to HIV risk. Intuitive processes are presumed to operate within split seconds, which sets them apart from the slower operations required for deliberation and elaborate analysis. Accordingly, brain responses were expected to be sensitive to perceived HIV risk at processing stages that are too early to be the product of elaborate stimulus analysis (i.e., between 250 and 500 ms; cf. Neely, 1977; Thorpe et al., 1996). A further hypothesis was derived from previous research examining emotional stimulus processing. Specifically, in the time range between 150 and 300 ms, emotional stimuli elicit a relative negative potential over occipito-temporal regions subsequently followed by increased late positive potentials over centro-parietal regions (i.e., between 300 and 700 ms; cf. Schupp et al., 2006). Given that HIV risk is a potential threat for health, similar ERP modulations were expected when comparing high and low HIV risk persons. Furthermore, the design of the study allows the comparison of processing differences between the implicit and explicit condition. Addressing this issue stems from the notion that fast and obligatory implicit processes provide the informational basis for slower, decision-, or deliberation-related explicit processing (Strack and Deutsch, 2004; Frith and Frith, 2008). For instance, inferences about gender and age can occur without intention. When explicitly questioned, however, explicit reports on gender or age rely on the output of these implicit assessments (e.g., Mouchetant-Rostaing and Giard, 2003). Accordingly, the unique effects of explicit HIV risk judgments should appear subsequent to automatic processes of person perception.
Materials and Methods
Participants
Forty-two volunteers (23 female) were recruited on the campus of the University of Konstanz. Participants received either money or course credits as compensation for participation and provided written consent to the study protocol, which was approved by the local Review Board. Four participants were excluded from the analyses of electrophysiological data because of excessive EEG artifacts or insufficient numbers of trials in one of the conditions. The age range of the final sample was between 20 and 27 years (M = 23.7, SD = 2.3).
Stimulus materials
The stimulus set used in this study comprised of photographs of persons in daily life scenes. The photographs were retrieved with permission from a popular online photo-sharing community (www.flickr.com). To assure high ecological validity, selection of stimuli was based on the following criteria: (1) Single persons in the foreground, (2) with faces clearly visible, (3) fully colored, (4) young adult, (5) caucasian appearance, (6) Attire, other socioeconomic status cues, and situational context features were purposely shown in order to resemble naturalistic viewing conditions and to facilitate impression formation. Two stimulus sets were obtained, consisting of 120 female and 120 male persons. Each set of 120 pictures, complemented by 15 additional pictures, comprised the stimuli related to the person-recognition task in the implicit condition. To increase ecological validity, each participant viewed 120 opposite sex persons.
Task and procedure
Implicit condition (person-recognition task)
As illustrated in Figure 1, implicit risk perception was assessed in the context of a person-recognition task unrelated to HIV risk (cf. Engell et al., 2007). The task consisted of 10 blocks. Within each block, 12 photographs from the 120 exemplar stimulus set and one photograph from the task stimulus set were presented followed by a test stimulus drawn from the task stimulus set. Thus, each stimulus of the 120 exemplar stimulus set was presented once. The stimuli were presented for 2000 ms each, preceded by a fixation cross shown for 1000 ms, and followed by an inter trial interval (ITI) of 3500 ms. The participant's task was to indicate whether the test photograph had been contained in the previous series of photographs. Old/new persons occurred with a probability of 50% across blocks. The sequence of picture presentation was fully randomized for every participant. EEG was continuously recorded as described below.
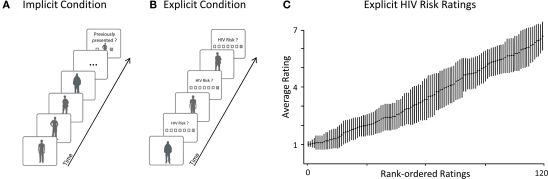
Figure 1.
Example trials and HIV risk rating results. (A) Implicit Condition: Participants viewed naturalistic pictures of unacquainted persons while high-density EEG was recorded. After a series of stimulus persons, participants had to indicate whether a target person had been presented previously. This task helped to maintain a constant level of attention while spontaneous brain reactions for all presented persons were recorded. To avoid confounds, none of the target persons to be recognized were included in the later analyses. Importantly, participants were unaware that they had to evaluate HIV risk in the subsequent explicit condition. (B) Explicit Condition: Upon completion of the implicit condition, participants were instructed to rate the perceived HIV risk for each stimulus person. Ratings of HIV risk were assessed using a seven-point scale. To capture the neural signature of explicit risk processing, high-density EEG was also recorded during this condition. In the analysis, we used these explicit ratings of HIV risk for to sort the EEG epochs from both – implicit and explicit – conditions into safe and risky categories. (C) Results from the explicit condition confirm that participants’ explicit ratings of HIV risk varied across the full range of the scale (1 – safe; 7 – risky): This plot shows average ratings of HIV risk and associated SE after rank-ordering each participant's ratings by HIV risk.
Explicit condition (HIV risk perception task)
During the explicit condition, which immediately followed the implicit condition, participants were asked to report their first impression of HIV risk (see Figure 1). All 120 pictures were presented for 2 s, preceded by a fixation cross (1 s). After a delay period of 1 s, participants were asked to evaluate how likely it is that the presented person is infected with HIV on a seven-point rating scale ranging from “very unlikely” [1] to “very likely” [7]. The next trial was initiated after an ITI of 3.5 s. Pictures again appeared in random order and EEG was also recorded throughout this part of the study.
Electrophysiological recording and analysis
Electrophysiological data were collected from the scalp using a 257 lead HydroCel Geodesic Sensor Net (EGI: Electrical Geodesics, Inc., Eugene, OR, USA). The EEG was recorded continuously with a sampling rate of 250 Hz, with the vertex sensor as reference electrode, and online filtered from 0.1 to 100 Hz using Netstation acquisition software and EGI amplifiers. Impedances were kept below 50 kΩ, as recommended for this type of amplifier. Off-line analyses were performed using EMEGS (Junghöfer and Peyk, 2004) and EEGLAB (Delorme and Makeig, 2004) software packages. Data editing and artifact rejection were based on a method for statistical control of artifacts specifically devised for the analysis of dense sensor EEG recordings (Junghöfer et al., 2000). Preprocessing steps included low-pass filtering at 40 Hz, artifact detection, ocular artifact correction based on a multiple regression method (Miller et al., 1988), bad sensor interpolation, baseline-correction for pre-stimulus (100 ms) ERP activity, and conversion to an average reference (Junghöfer et al., 2000).
Risk categorization
The stimulus persons were categorized according to idiosyncratic risk ratings, whereby those receiving risk ratings between 1 and 3 were coded as representing low risk (“safe”) and those receiving risk ratings between 5 and 7 were coded as representing high risk (“risky”). In order to determine whether ERPs recorded during the implicit or explicit condition evince systematic differences associated with HIV risk perception, the EEG epochs from both conditions were analyzed according to the ratings provided during the explicit condition. For every sensor and participant, average ERP waveforms were calculated for low- and high-risk categories. The assignment of each EEG epoch to low- vs. high-risk categories was based on a post hoc sorting of each participant's idiosyncratic risk ratings for each stimulus person. This procedure resulted in a set of 2 × 2 ERPs for every participant (low- and high-risk; implicit and explicit condition).
Statistical procedure for ERP analysis
ERP components sensitive to risk were identified by visual inspection and single sensor waveform analysis (cf. Schupp et al., 2003). To statistically assess these effects, mean amplitudes from representative occipital and central sensor clusters were averaged and subjected to conventional ANOVAs including the factors “Task” (implicit vs. explicit), “Risk” (risky vs. safe), “Gender” (male vs. female), and “Laterality” (left vs. right).
The first significant effect was observed between 240 and 300 ms and assessed in two bilateral occipito-temporal channel groups including EGI sensors #106, 107, 108, 113, 114, 115, 116, 117, 121, 122, 123, 124, 125, 134, 135, 136, 138, 139, and 146 (left) and #148, 149, 150, 151, 156, 157, 158, 159, 160, 166, 167, 168, 169, 175, and 176 (right). Sensors were located in the vicinity of O1, O2, P9, P10, T5-P7, and T6-P8 according to the international 10/10 system (Chatrian et al., 1985). Albeit less pronounced, due to polarity reversal, mirror effects were observed over fronto-central regions, which are not reported for brevity.
Subsequently, a central positivity component was scored in two separate intervals between 430–530 ms and 550–800 ms post stimulus, including EGI sensors #9, 17, 24, 42, 43, 44, 45, 51, 52, 53, 58, 59, 60, 65, 66, 71, 72, 76, 77, 78, 79, 80, 86, 87, 88, 89, 98, 99, 100, and 110 (left) and #128, 129, 130, 131, 132, 141, 142, 143, 144, 152, 153, 154, 155, 162, 163, 164, 172, 173, 181, 182, 183, 184, 185, 186, 195, 196, 197, 198, 206, and 207 (right). Sensors were located in the vicinity of FC1, FC2, FC3, FC4, C1, C2, C3, C4, CP1, CP2, CP3, CP4, CP5, CP6, P1, P2, P3, P4, P5, and P6. Where appropriate, degrees of freedom were adjusted using the Greenhouse–Geisser method to correct for violations of sphericity.
Results
Behavioral results
To verify that participants attended to the stimuli in the implicit condition, we examined the accuracy-level of the person- recognition task. Accuracy was very high and averaged at 93%, from which we conclude that the person-recognition task fulfilled its purpose. Of note, persons that were targets in the person-recognition task, were not considered in the ERP analysis to avoid confounds associated with target processing.
Next, the distribution of HIV risk ratings was examined to verify that the ratings showed substantial variance. In one analysis, risk ratings were rank ordered for each participant and mean responses at each rank were calculated across participants. As shown in Figure 1C, mean risk ratings increased linearly from very low (minimum = 1.02) to very high (maximum = 6.7). In a further analysis, variance and range of risk ratings were calculated for each subject. On average, risk ratings showed substantial variance () and participants used the full range of the risk scale (mean range ). These analyses demonstrate that our naturalistic stimuli produced broad variations in perceived HIV risk and provided an adequate basis for contrasting ERPs toward safe and risky persons in the implicit and explicit condition.
ERP results
To test for possible ERP differences between risky and safe persons, even without instruction (implicit condition) or before the judgments were made (explicit condition), we aggregated EEG epochs from both conditions based on whether the persons were subsequently judged as being risky or safe in terms of HIV risk. For every sensor and participant, we calculated average ERP waveforms for low and high risk categories in both conditions. Three ERP components were sensitive to HIV risk and submitted to ANOVA analysis comprising the factors Task (implicit vs. explicit), HIV Risk (risky vs. safe), Gender (male vs. female), and Laterality (left vs. right).
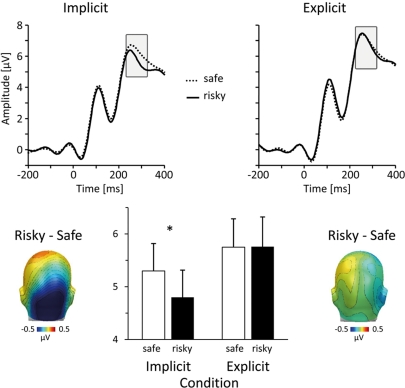
A first difference in processing risky and safe persons occurred between 240 and 300 ms. Most importantly, as illustrated in Figure 2, this effect was specific to the implicit condition, consisting of an occipital negativity toward stimulus persons that were later evaluated as carrying high HIV risk. To assess this effect, mean ERP amplitudes from occipito-temporal sensor clusters were averaged over a time interval from 240 to 300 ms and submitted to ANOVA analysis. A significant interaction between the factors Task and HIV Risk, F(1,36) = 4.39, p < 0.05, indicated differential effects of HIV risk in the implicit and explicit condition. To follow up on this interaction, effects of HIV risk were assessed separately for implicit and explicit conditions. Risky as compared to safe individuals elicited a significantly larger occipito-temporal negativity in the implicit condition, t(37) = 2.83, p < 0.01. In contrast, there was no significant effect of HIV risk in the explicit condition, t(37) = 0.05; ns. As can be seen in Figure 2, the explicit task prompted in general larger negativities over occipito-temporal sites, resulting in a significant main effect of the task factor, F(1,36) = 15.3, p < 0.001.
Figure 2.
Early ERP differences during the implicit condition. In the implicit condition, viewing persons that were subsequently judged as risky elicited an early occipito-temporal negativity. The top panel shows representative grand-average ERP waveforms for risky (solid lines) and safe (dotted lines) persons (sensor #151, near O2 in the 10/20 system) in the implicit (left) and explicit (right) condition. Scalp difference maps at the bottom (left: implicit condition; right: explicit condition) illustrate the topographical distribution of differential activity associated with HIV risk (risky–safe) in the time window between 240 and 300 ms post stimulus. The bar plot in the middle summarizes the mean ERP amplitudes, which were captured over occipito-temporal sites and between 240 and 300 ms. Unfilled bars represent mean ERP amplitudes toward safe persons, filled bars toward risky persons.
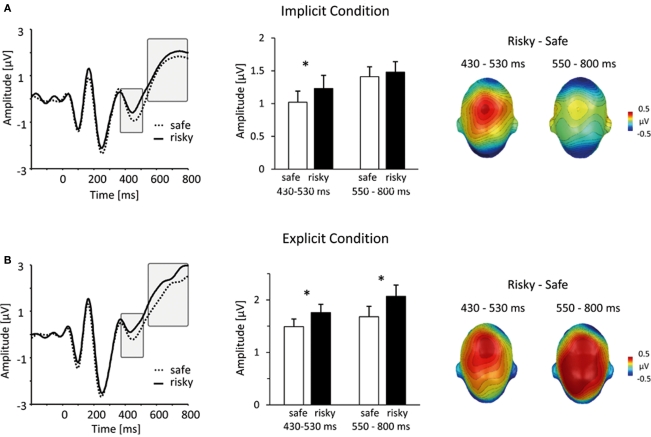
Between 430 and 530 ms post stimulus, processing of stimulus persons, who were later evaluated as risky, elicited an increased central positivity (see Figure 3), F(1,36) = 8.72, p < 0.01. This main effect of HIV risk was present in both, the implicit as well as the explicit condition, Task × HIV Risk F(1,36) = 0.19, p = 0.66, ns. Separate tests of both conditions confirmed the significant effect of HIV risk in the implicit [t(37) = 2.13; p < 0.05] and explicit [t(37) = 2.48; p < 0.05] condition. Inspection of Figure 3 reveals similarities between the implicit and explicit condition. First, ERP waveforms from the implicit and explicit condition evince a highly similar morphology, reflecting the fact that they represent ERP averages to identical visual stimuli and the high reliability of ERPs in our large sample. More important, the spatial pattern of differences between ERPs for risky and safe persons is highly similar in both conditions. The observed overlap in ERP modulations in terms of (a) amplitude differences (risky larger than safe) and (b) spatial distribution (positive modulations over central areas) is remarkable because the relative noise level increases with the calculation of contrasts (risky–safe). Furthermore, similar to the 240- to 300-ms time window, the peak amplitudes were generally larger in the explicit condition, as revealed by the Task main effect, F(1,36) = 10.25, p < 0.001, presumably reflecting the increased allocation of attentional resources during deliberative processing.
Figure 3.
Similarities and differences between implicit and explicit conditions. (A) In the implicit condition, viewing persons that were subsequently judged as risky elicited a mid-latency central positivity. The left panel shows representative grand-average ERP waveforms for risky (solid lines) and safe (dotted lines) persons (sensor #257, corresponding to Cz in the 10/20 system). The bar plot in the middle panel summarizes mean ERP amplitudes, captured over a central cluster at an earlier (430–530 ms) and later (550–800 ms) interval (unfilled bars: safe persons, filled bars: risky persons). The right panel illustrates the scalp topography of differences between risky and safe ERPs, projected on a model head (top view). (B) This panel shows the same information for the results from the explicit condition. Notably, between 430 and 530 ms, implicit and explicit conditions revealed a similar differentiation between risky and safe persons. In contrast, in the time window between 550 and 800, larger central positivities for risky persons were specific to the explicit condition.
Finally, in the time period between 550 and 800 ms, persons of high levels of HIV risk were associated with an increased positive potential over central regions. As shown in Figure 3, this effect was specific to the explicit condition. Supporting these impressions, statistical analysis revealed a significant Task × HIV Risk interaction, F(1,36) = 11.64, p < 0.01. Separate analysis within the explicit condition revealed a pronounced main effect of HIV Risk, t(37) = 3.95, p < 0.001, while there was no significant main effect of risk within the implicit condition, t(37) = 0.69, ns. Similar enhancements of sustained centro-parietal positivities have been classically observed in explicit attention and categorization tasks as well as in studies investigating emotional stimulus processing (e.g., Picton and Hillyard, 1988; Schupp et al., 2006). Accordingly, significant stimuli are presumed to draw more processing resources at a processing stage associated with working memory and conscious recognition (e.g., Sergent et al., 2005; Del Cul et al., 2007). Thus, based on the assumption that participants attempt to detect signs of HIV-riskiness in the explicit condition, the identification of such signs may lead to larger late positive potentials after these signs have been detected via spontaneous person perception processes.
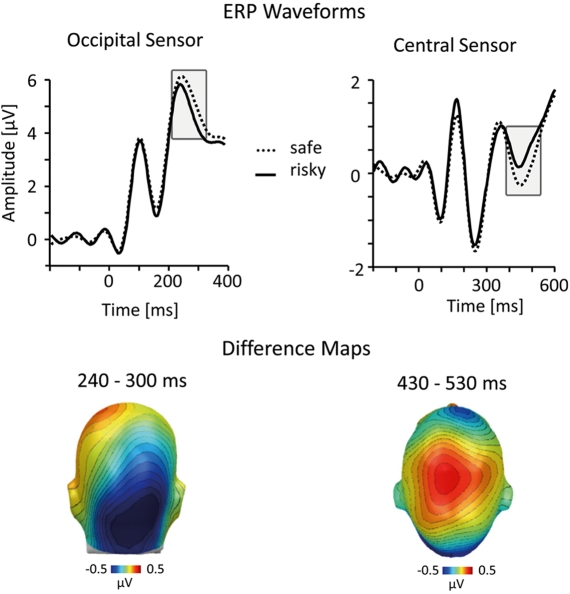
To ensure that the observed results are stable across alternative methods for categorizing risky and safe persons, we performed the same analysis with different schemes for categorizing ERP data based on self-report ratings. The main results reported above treated stimulus persons with risk ratings between one and three as “safe” and stimulus persons with risk ratings between 5 and 7 as “risky.” In an alternative analysis, all risk ratings were z-standardized within each participant and ERPs were calculated corresponding to low and high z-standardized risk ratings. This analysis is better suited to dealing with individual response tendencies (e.g., a tendency to report low risk for all stimulus persons), but is still sensitive to variations from the idiosyncratic mean. Statistical analysis of this z-score-based ERP categorization replicated the main results based on the dichotomized raw scores (see Figure 4).
Figure 4.
Analysis incorporating z-scored HIV risk ratings. To account for possible response tendencies, we performed a z-score transformation of the HIV risk ratings before sorting ERP epochs into risky and safe categories. Representative ERP waveforms (top panel) and difference maps (bottom; both from the implicit condition) illustrate that this analysis confirmed results of the normative analysis (see Figures 2 and 3).
Correlations between ratings of HIV risk and other person characteristics
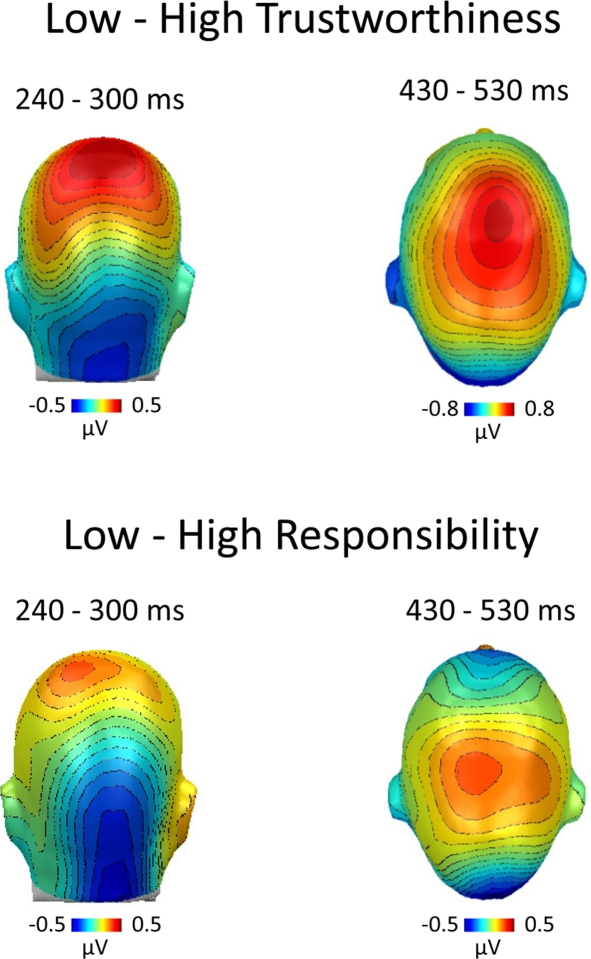
The results strongly support the notion that explicit ratings of HIV risk are antedated by implicit processing differences in an experimental context, i.e., person recognition, unrelated to HIV risk. This is remarkable since HIV risk is not related to observable and valid cues in the appearance of a person (Thompson et al., 2002). HIV risk perception based on appearance may accordingly be embedded in a broader web of person characteristics, and the first glance at an unacquainted person may access these associative knowledge structures in an implicit manner. Previous research identified key characteristics of a tacit HIV risk stereotype. Specifically, a low sense of responsibility was reliably named as a cardinal feature characterizing persons with high HIV risk (Renner and Schwarzer, 2003). Furthermore, there is evidence that trust is perceived with minimal processing time and subject to spontaneous inferences (Bar et al., 2006; Willis and Todorov, 2006). Hence, we tested whether these characteristics were associated with HIV risk in our naturalistic risk perception task. Specifically, in a further experimental session conducted after the main experiment, we assessed additional trait ratings for all presented stimuli. Correlation analysis supported the notion that observable person characteristics accompany perceived HIV risk. Judgments of HIV risk showed a strong negative association with the perceived sense of responsibility (average r = −0.56) and trustworthiness (r = −0.57). This observation prompted us to categorize the implicit EEG epochs according to ratings of trustworthiness (with ratings of 1–3 corresponding to untrustworthy and ratings between 5 and 7 corresponding to trustworthy persons). ERP analyses revealed very similar results compared to the main analysis based on HIV risk ratings. As shown in Figure 5, individuals who were perceived as untrustworthy elicited larger negativity over posterior sites between 240 and 300 ms and increased central positivity between 430 and 530 ms. Corresponding results were also obtained using ratings of responsibility to categorize EEG epochs (Figure 5). These findings have profound implications: Key information of the HIV risk stereotype of young adults is extracted spontaneously, and may lead to an immediate impression that a given person is safe or risky. Signals of safety, in turn, presumably counter thoughts regarding effective means of HIV prevention and participants may simply follow their habits. This hypothesis provides a mechanism for the finding of retrospective studies that people who engaged in unprotected sex generally assumed that their partners were safe or did not consider the risk they were taking (e.g., Gold, 1993; Keller, 1993).
Figure 5.
ERP categorization according to ratings of trustworthiness and responsibility. After the main experiment, participants were asked to rate all stimulus persons on additional person characteristics. HIV risk ratings showed significant negative correlations with trustworthiness and responsibility. Hence, to substantiate our findings, we categorized the ERP data recorded during the implicit condition based on subsequent ratings of trustworthiness and responsibility. The difference maps between ERPs toward untrustworthy–trustworthy persons (top panel) and irresponsible–responsible persons (bottom panel) revealed similar processing differences as observed in the main analysis, which was based on ratings of HIV risk (see Figures 2 and 3).
Prima facie, perceived HIV risk may also relate to the attractiveness of the person. However, in our rather large sample and stimulus set, attractiveness showed only very weak associations to perceived risk (r = −0.14). This corroborates other findings (Agocha and Cooper, 1999; Dijkstra et al., 2000) and is, in principle, consistent with the “beauty is good”-heuristic, although the effect size is very weak (only 2% variance explained). Furthermore, in a subsequent step of analysis, ratings of attractiveness were used to classify brain responses during the implicit condition. ERP waveforms and brain difference maps (attractive–unattractive) showed no effect for attractiveness [240–300 ms: F(1,36) = 0.98; ns; 430–530 ms: F(1,36) = 0.29; ns]. The assumption proposed in previous research that attractive people may be regarded as riskier was therefore not supported by the present data (Dijkstra et al., 2000).
Discussion
Field studies suggest a rather novel perspective on HIV risk perception (Gold et al., 1992; Thompson et al., 1996): Instead of being the result of deliberative reasoning processes, HIV risk may be inferred by relying on implicit processes. Specifically, we hypothesized that risk-related information may be extracted rapidly and with ease, based on parallel processing, and independent from processing goals (Loewenstein et al., 2001). To test this assumption, the implicit processing of unacquainted persons was assessed by EEG measures sorted in high and low risk categories according to subsequent ratings of HIV risk. Two ERP components, an early occipital negativity (240–300 ms) and a mid-latency central positivity (430–530 ms), were significantly larger for risky individuals. Two features of our paradigm are noteworthy. First, great care was taken to assure that participants in the implicit condition were not aware that the purpose of the study was to examine HIV risk perception. Second, the reported ERP differences were observed while participants performed a person-recognition task. Overall, the results suggest that HIV risk-related information is extracted at an implicit level in an experimental context ostensibly unrelated to HIV risk. One may speculate that the increased attention to risky stimuli associated with these implicit processes facilitates deliberative processes and protection motivation. While such a mechanism seems particularly important in situations of sexual arousal and diminished control (Blanton and Gerrard, 1997; Kruse and Fromme, 2005; Ariely and Loewenstein, 2006; Ditto et al., 2006; Shuper and Fisher, 2008), dangerous consequences may arise when the implicit processes lack validity, as in the case of HIV.
Both implicit ERP differentiations (240–300 ms over occipital areas and 430–530 ms over central sites) are similar to what is observed during ERP studies of emotional stimulus processing. Research with highly arousing emotional scenes such as erotica or mutilations revealed these two ERP components to have similar topography, polarity, and latency (cf. Schupp et al., 2003; Junghöfer et al., 2001). These studies also employed passive picture viewing paradigms resembling our implicit condition. More recent studies demonstrated similar effects for low and moderately arousing materials such as emotional faces, gestures, words, or clashing moral statements (Schupp et al., 2004; Kissler et al., 2007; Van Berkum et al., 2009; Flaisch et al., 2011). These findings suggest that risky-looking individuals elicit the brain signature of emotional significance intimately linked to selective visual attention processes, presumably because they pose a potential threat. Furthermore, these findings agree with the principle of a negativity bias in that stimuli signaling danger and riskiness are more effective in engaging affect processing than stimuli signaling safety and low risk (Cacioppo et al., 1999; Baumeister et al., 2001). Nevertheless, as with all neuroimaging measures, the drawing of inferences about the nature of mental processes (in this case “affect”) from the signature of brain activity alone is disputable (Poldrack, 2006). Hence further research (e.g., fMRI) will be needed to bolster the hypothesis that the current ERP findings relate to what has been dubbed as “affect heuristic” in recent conceptions of risk (Slovic and Peters, 2006).
HIV risk, trust, and responsibility: interrelated dimensions of person characteristics
The present findings raise the issue regarding what kind of information could provide the basis for the observed implicit processing differences. Specifically, HIV infection is not reliably associated with overt signs (Thompson et al., 2002) and the short time since the disease appeared precludes evolution that would endow the organism with immunological defense mechanisms. We speculate that a high-risk stereotype of HIV contains a set of interrelated person characteristics, providing grounds to judge HIV risk based on intuition. Previous research revealed that lack of responsibility is a cardinal feature of the high-risk stereotype in young adults (Renner and Schwarzer, 2003). Consistent with these findings, we observed a significant correlation of HIV risk with ratings of responsibility and trust. Furthermore, ample evidence supports the notion that inferences about trustworthiness are based on immediate person perception. For instance, exposure times as little as 33 ms are sufficient to infer trust or threat (Bar et al., 2006; Willis and Todorov, 2006). In addition, distrust led to the engagement of neural structures implicated in emotion processing (amygdala and insular cortex; Engell et al., 2007; Todorov and Engell, 2008). Interestingly, using trust and responsibility ratings to categorize EEG epochs, we observed a highly similar pattern of ERP differences in the implicit condition as obtained for HIV risk. This suggests that, presumably reflecting common meaning structures, HIV risk, trustworthiness, and responsibility share a substantial part of their variance at the implicit level (Edelman, 1998). In line with this reasoning, recent conceptions about person perception suggest that initial processing steps provide a raw and transitory sketch of a first impression associated with a general disposition for avoidance or approach behavior (Todorov and Engell, 2008; Todorov et al., 2008).
Self-report data regarding how participants derived their risk estimates further support the notion of the intuitive rather than deliberative nature of HIV risk ratings. In a post-experimental questionnaire, participants were asked how they performed the HIV risk judgments, such as relying on specific cues for risk or using rules of thumb. The majority (n = 30) could not explicitly explain how they derived their HIV risk judgments, which is consistent with the fact that the reasons behind intuitive impressions often elude introspection. Only a few participants mentioned information related to a high-risk stereotype, such as “if the person looked irresponsible and endangered, then I gave high HIV risk.” Thus, the significant correlations among HIV risk, trustworthiness, and responsibility are unlikely to reflect representations of explicit language-based knowledge and speak to the implicit nature of HIV risk impressions. This parallels findings from person perception research and can be understood by considering the nature of implicit processes, which are notorious for being associated with feelings of knowing, experiences of self-evident validity, and opacity to the underlying mental processes (Lewicki et al., 1992).
The relationship of implicit and explicit processes in HIV risk perception
The current design allowed evaluation of the functional significance of implicit processes in comparison to an explicit task focus of judging HIV risk. Surprisingly, within the first 500 ms of stimulus processing, the task to explicitly judge HIV risk was not associated with a greater sensitivity towards risky stimuli beyond what was already found during implicit person perception. Specifically, the central positivity to risky persons between 430 and 530 ms was similarly pronounced for the implicit and explicit condition and earlier ERP differences were specific to the implicit condition. One may speculate that processing within the first 500 ms reflects primarily the stimulus-driven engagement of neural cell ensembles, which may serve as an informational basis for explicit judgments of risk. This hypothesis builds upon recent research on conscious stimulus perception. For instance, research on error awareness suggested that early ERP differences (i.e., error related negativity) along with other signals (i.e., autonomic measures) contribute to explicit error detection (Wessel et al., 2011). Future studies may explore the hypothesis that multiple implicit signalsprecede relatively ‘late’ ERP activity associated with explicit risk judgments.
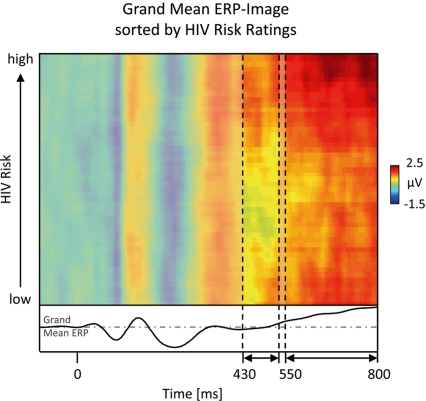
The most obvious difference between implicit and explicit risk processing regards the late positive potential between 550 and 800 ms post stimulus. A direct comparison between the implicit and explicit conditions revealed that the explicit condition risky individuals elicited an increased positivity in this time interval compared to safe. This ERP effect relates to findings that emotional stimuli as well as task-relevant stimuli elicit larger late positive potentials (Picton and Hillyard, 1988; Schupp et al., 2006). Of note, because the task focus specifically enhanced the LPP to risky individuals, only the interaction of both factors, emotional significance and task relevance, can explain the present findings. It has been suggested that fundamental implicit processes spontaneously extract information, and that task-related motivational processes recruit this information to exert further amplifying influences, if the information matches the target template (Strack and Deutsch, 2004). This notion entails the assumption that explicit processes refine and elaborate information extracted at the implicit level providing graded information along the safety-risk continuum. Accordingly, a single-trial ERP-Image analysis (Jung et al., 2001; Delorme et al., 2007) was conducted to explore whether the LPP reflects HIV risk in a graded fashion (see Figure 6). Similar to recent findings (Schmälzle et al., in press), this analysis revealed an almost linear relationship of HIV risk and LPP amplitude with stimuli eliciting the largest LPP receiving highest subsequent risk ratings (see Figure 6). This contrasts with earlier ERP modulations in the explicit and implicit conditions, which did not reveal such a close relationship to gradual HIV risk as observed for the LPP component. Overall, the extraction of risk-related information early in the processing stream is elaborated based on more graded information regarding riskiness when HIV risk is the explicit processing goal.
Figure 6.
Gradual ERP-image analysis. A more fine-grained exploration of the relationship between central ERP amplitudes and explicit HIV risk ratings is provided by ERP-Image analysis. To obtain this picture, single-trial area-measures of the late ERP component were sorted by perceived risk, normalized (adjusting for unequal trial numbers across participants), smoothed, and averaged across participants (Delorme et al., 2007). Horizontal lines represent the color-coded voltage per pseudo-trial, with trials being sorted in ascending order by subsequent HIV risk ratings. It is apparent that in the time windows between 430–530 and 550–800 ms, trials associated with higher risk perception tend to have more reddish colors, indicating larger amplitudes. In particular the 550- to 800-ms interval shows an almost linear relationship between ERP amplitude and HIV risk. To allow the reader to compare this image to the ERP figures (Figures 2 and 4), the waveform at the bottom depicts the grand mean ERP, which is obtained by collapsing across all trials of the ERP-Image and recoding amplitude as height.
Risk perception: a broader perspective
Current theories of health behavior assume that people need to perceive themselves as being at risk in order to be motivated to take protective action (Armitage and Conner, 2000; Renner and Schwarzer, 2003). Risk perception in turn has been conceptualized as beliefs about the likelihood that one will be affected by a negative event (e.g., getting infected with HIV) in combination with the severity of the negative event (e.g., lethal; Weinstein, 2000). This “risk as analysis” view has recently been contrasted with the intuitive sensing of risk. The “risk as feeling” perspective (Loewenstein et al., 2001; Slovic and Peters, 2006) holds that risk perception builds upon affective stimulus evaluations (Cacioppo and Berntson, 1994; Lang et al., 1997) that form the basis for authentic experiences of risk as opposed to cognitive inferences. Neural measures may provide important information regarding the operation of such intuitive processes. In particular, the present ERP findings from the implicit condition suggest that high as compared to low HIV risk persons drew increased attentional processing resources within the first 500 ms of stimulus processing. Given the large array of studies that relate these ERP modulations to stimulus significance (Schupp et al., 2006), one interpretation is that these findings may reflect affective stimulus evaluations, which may underlie the impressions of riskiness. From this perspective, the domain of health risk perception may be broadened with a focus on affective evaluations of risk (also see Hogarth et al., 2011), which may be dissociated from knowledge-based probability estimates that are still the dominant way of thinking in risk perception research. Related thoughts have also been put forward from the perspective of evolutionary social psychology, where it has been argued that basic social-cognitive mechanisms may be adaptively attuned to detecting signals of risk, such as aggression (Bar et al., 2006) or contagious diseases (Ackerman et al., 2009; Park and Schaller, 2009).
Current research on health risk perception may also be related to research on risky decision making using behavioral economic paradigms and research on conflict monitoring and feedback processing (Yeung et al., 2004; Mohr et al., 2010). Research implementing response conflicts often report a fronto-central N2 component with onset latencies of ~200–250 ms. While being similar in terms of latency and polarity to the early effect in the implicit condition, the topography of the components differ (fronto-central vs. occipito-temporal), presumably reflecting differences related to task relevance (i.e., explicit vs. implicit processing), stimulus modality, or motor response demands. One approach to more closely relate these areas of research might be to investigate feedback signals regarding HIV risk perception. Thompson et al. (2002) showed that participants were not able to detect HIV positive individuals and that receiving feedback increased condom usage in an intervention design. It would be interesting to see whether feedback changes implicit response and is reflected by a modulation of ERP components associated with feedback processing (Yeung and Sanfey, 2004). Furthermore, hemodynamic measures may shed light on neural structures implicated in HIV risk perception. For instance, a recent meta-analysis implicated the insular and the dorsolateral frontal cortex in economic risk studies (Mohr et al., 2010) and the anterior cingulate cortex has been discussed as a key structure in an intuitive alarm system triggering deliberative thought and control processes (Lieberman, 2000). Overall, neuroimaging measures may allow the revelation of similarities and differences in risk processing across various domains of risk (e.g. health, behavioral economics), and help to decompose underlying mechanisms at the level of distinct processing stages (cf. Schonberg et al., 2011).
Conclusion
The present findings demonstrate the intuitive basis of HIV risk perception. Measuring ERPs while viewing of unacquainted persons allowed for the identification of neural differentiation between risky and safe individuals in both, an implicit and an explicit condition. These findings help to understand how people easily form erroneous beliefs about risk during first encounters. Informing people about these facts may lead to more effective strategies to promote the adoption of effective precautionary behaviors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the German Research Foundation (RE 1583/3-1 granted to Britta Renner and Harald Schupp). Financial support for open access publication costs was provided by the German Research Foundation (DFG).
References
- Ackerman J. M., Becker D. V., Mortensen C. R., Sasaki T., Neuberg S. L., Kenrick D. T. (2009). A pox on the mind: cognitive processing of physical disfigurement. J. Exp. Soc. Psychol. 45, 478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agocha V. B., Cooper M. L. (1999). Risk perception and safer-sex intentions: does a partner's physical attractiveness undermine the use of risk-relevant information. Pers. Soc. Psychol. Bull. 25, 751–765 [Google Scholar]
- Ariely D., Loewenstein G. (2006). The heat of the moment: the effect of sexual arousal on sexual decision making. J. Behav. Decis. Mak. 19, 87–98 [Google Scholar]
- Armitage C. J., Conner M. (2000). Social cognition models and health behavior: a structured review. Psychol. Health, 15, 173–189 [Google Scholar]
- Bar M., Linz H., Neta M. (2006). Very first impressions. Emotion 6, 269–278 10.1037/1528-3542.6.2.269 [DOI] [PubMed] [Google Scholar]
- Bargh J. A., Chartrand T. L. (1999). The unbearable automaticity of being. Am. Psychol. 54, 462–479 [Google Scholar]
- Baumeister R. F., Bratslavsky E., Finkenauer C., Vohs K. D. (2001). Bad is stronger than good. Rev. Gen. Psychol. 5, 323–370 [Google Scholar]
- Blanton H., Gerrard M. (1997). Effect of sexual motivation on men's risk perception for sexually transmitted disease: there must be 50 ways to justify a lover. Health Psychol. 16, 374–379 10.1037/0278-6133.16.4.374 [DOI] [PubMed] [Google Scholar]
- Cacioppo J. T., Berntson G. G. (1994). Relationship between attitudes and evaluative space: a critical review, with emphasis on the separability of positive and negative substrates.Psychol. Bull. 115, 401–423 [Google Scholar]
- Cacioppo J. T., Gardner W. L., Berntson G. G. (1999). The affect system has parallel and integrative processing components: form follows function. J. Pers. Soc. Psychol. 76, 839–855 [Google Scholar]
- Chatrian G. E., Lettich E., Nelson P. L. (1985). Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am. J. EEG Technol. 25, 83–92 [Google Scholar]
- Civic D. (2000). College students’ reasons for nonuse of condoms within dating relationships. J. Sex Marital Ther. 26, 95–105 10.1080/009262300278678 [DOI] [PubMed] [Google Scholar]
- Del Cul A., Baillet S., Dehaene S. (2007). Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol. 5, e260. 10.1371/journal.pbio.0050260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 [DOI] [PubMed] [Google Scholar]
- Delorme A., Westerfield M., Makeig S. (2007). Medial prefrontal theta bursts precede rapid motor responses during visual selective attention. J. Neurosci. 27, 11949–11959 10.1523/JNEUROSCI.3477-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra P., Buunk B. P., Blanton H. (2000). The effect of target's physical attractiveness and dominance on STD-Risk perceptions. J. Appl. Soc. Psychol. 30, 1738–1755 [Google Scholar]
- Ditto P. H., Pizarro D. A., Epstein E. B., Jacobson J. A., MacDonald T. K. (2006). Motivational myopia: visceral influences on risk taking behavior. J. Behav. Decis. Mak. 19, 99–113 [Google Scholar]
- Edelman S. (1998). Representation is representation of similarities. Behav. Brain Sci. 21, 449–498 [DOI] [PubMed] [Google Scholar]
- Engell A. D., Haxby J. V., Todorov A. (2007). Implicit trustworthiness decisions: automatic coding of face properties in human amygdala. J. Cogn. Neurosci. 19, 1508–1519 [DOI] [PubMed] [Google Scholar]
- Evans J. S. (2008). Dual-processing accounts of reasoning, judgment, and social cognition. Annu. Rev. Psychol. 59, 255–278 10.1146/annurev.psych.59.103006.093629 [DOI] [PubMed] [Google Scholar]
- Flaisch T., Häcker F., Renner B., Schupp H. T. (2011). Emotion and the processing of symbolic gestures: an event-related brain potential study. Soc. Cogn. Affect. Neurosci. 6, 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C. D., Frith U. (2008). Implicit and explicit processes in social cognition. Neuron 60, 503–510 10.1016/j.neuron.2008.10.032 [DOI] [PubMed] [Google Scholar]
- Gardner R., Blackburn R., Upadhyay U. (1999). Closing the condom gap. Popul. Rep. H. 9, 1–35 [PubMed] [Google Scholar]
- Gold R. S. (1993). “On the need to mind the gap: on-line versus off-line cognitions underlying sexual risk-taking,” in The Theory of Reasoned Action: Its Application to AIDS Preventive Behavior, eds Terry D., Gallois C., McCamish M. (Oxford: Pergamon Press; ), 227–252 [Google Scholar]
- Gold R. S., Karmiloff-Smith A., Skinner M. J., Morton J. (1992). Situational factors and thought processes associated with unprotected intercourse in heterosexual students. AIDS Care 4, 305–323 10.1080/09540129208253101 [DOI] [PubMed] [Google Scholar]
- Hamermesh D. S., Biddle J. E. (1994). Beauty and the labour market. Am. Econ. Rev. 84, 1174–1194 [Google Scholar]
- Hogarth R. M., Portell M., Cuxart A., Kolev G. I. (2011). Emotion and reason in everyday risk perception. J. Behav. Decis. Mak. 24, 202–222 [Google Scholar]
- Jung T. P., Makeig S., Westerfield W., Townsend J., Courchesne E., Sejnowski T. J. (2001). Analysis and visualization of single-trial event-related potentials. Hum. Brain Mapp. 14, 166–185 10.1002/hbm.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghöfer M., Bradley M. M., Elbert T. R., Lang P. J. (2001). Fleeting images: a new look at early emotion discrimination. Psychophysiology 38, 175–178 10.1111/1469-8986.3820175 [DOI] [PubMed] [Google Scholar]
- Junghöfer M., Elbert T., Tucker D. M., Rockstroh B. (2000). Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology 37, 523–532 10.1111/1469-8986.3740523 [DOI] [PubMed] [Google Scholar]
- Junghöfer M., Peyk P. (2004). Analysis of electrical potentials and magnetic fields of the brain. Matlab Select 2, 24–28 [Google Scholar]
- Keller M. L. (1993). Why don't young adults protect themselves against sexual transmission of HIV? Possible answers to a complex question. AIDS Educ. Prev. 5, 220–233 [PubMed] [Google Scholar]
- Kissler J., Herbert C., Peyk P., Junghöfer M. (2007). Buzzwords: early cortical responses to emotional words during reading. Psychol. Sci. 18, 475–480 [DOI] [PubMed] [Google Scholar]
- Kruse M. I., Fromme K. (2005). Influence of physical attractiveness and alcohol on men's perceptions of potential sexual partners and sexual behavior. Exp. Clin. Psychopharmacol. 13, 146–156 [DOI] [PubMed] [Google Scholar]
- Lang P. J., Bradley M. M., Cuthbert B. N. (1997). “Motivated attention: affect, activation, and action,” in Attention and Orienting: Sensory and Motivational Processes, eds Lang P. J., Simons R. F., Balaban M. (Hillsdale, NJ: Erlbaum; ), 97–135 [Google Scholar]
- Lewicki P., Hill T., Czyzewska M. (1992). Nonconscious acquisition of information. Am. Psychol. 47, 796–801 [DOI] [PubMed] [Google Scholar]
- Lieberman M.D. (2000). Intuition: a social cognitive neuroscience approach. Psychol. Bull. 126, 109–137 [DOI] [PubMed] [Google Scholar]
- Lieberman M. D., Gaunt R., Gilbert D. T., Trope Y. (2002). Reflection and reflexion: a social cognitive neuroscience approach to attributional inference. Adv. Exp. Soc. Psychol. 34, 199–249 [Google Scholar]
- Loewenstein G. F., Weber E. U., Hsee C. K., Welch N. (2001). Risk as feelings. Psychol. Bull. 127, 267–286 [DOI] [PubMed] [Google Scholar]
- Maticka-Tyndale E. (1991). Sexual scripts and AIDS prevention: variations in adherence to safer-sex guidelines by heterosexual adolescents. J. Sex Res. 28, 45–66 10.1080/00224499109551594 [DOI] [Google Scholar]
- Miller G. A., Gratton G., Yee C. M. (1988). Generalized implementation of an eye movement correction procedure. Psychophysiology 25, 241–243 10.1111/j.1469-8986.1988.tb00999.x [DOI] [Google Scholar]
- Mohr P. N. C., Biele G., Heekeren H. R. (2010). Neural processing of risk. J. Neurosci. 30, 6613–6619 10.1523/JNEUROSCI.0003-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchetant-Rostaing Y., Giard M. H. (2003). Electrophysiological correlates of age and gender perception on human faces. J. Cogn. Neurosci. 15, 900–910 [DOI] [PubMed] [Google Scholar]
- Neely J. H. (1977). Semantic priming and retrieval from lexical memory: roles of inhibitionless spreading of activation and limited-capacity attention. J. Exp. Psychol. Gen. 106, 226–254 [Google Scholar]
- Norton T. R., Bogart L. M., Cecil H., Pinkerton S. D. (2005). Primacy of affect over cognition in determining adult men's condom-use behavior: a review. J. Appl. Soc. Psychol. 35, 2493–2534 [Google Scholar]
- Park J. H., Schaller M. (2009). Parasites, minds, and cultures. Psychologist 22, 942–945 [Google Scholar]
- Picton T. W., Hillyard S. A. (1988). “Endogenous event-related potentials,” in Handbook of Electroencephalography and Clinical Neurophysiology, Vol. 3, Human Event-Related Potentials, ed. Picton T. W. (Amsterdam: Elsevier; ), 361–426 [Google Scholar]
- Poldrack R. A. (2006). Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. (Regul. Ed.) 10, 59–63 [DOI] [PubMed] [Google Scholar]
- Renner B., Schupp H. (2011). “The perception of health risk,” in The Oxford Handbook of Health Psychology, ed. Friedman H. S. (New York: Oxford University Press; ), 639–666 [Google Scholar]
- Renner B., Schwarzer R. (2003). Risk stereotypes, risk perception and risk behavior in relation to HIV. J. Health Psychol. 11, 112–121 [Google Scholar]
- Schmälzle R., Renner B., Schupp H. T. (in press). Neural Correlates of Perceived Risk: The case of HIV. Soc. Cog. Aff. Neurosci. [DOI] [PMC free article] [PubMed]
- Schonberg T., Fox C. R., Poldrack R. A. (2011). Mind the gap: bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends Cogn. Sci. (Regul. Ed.) 15, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H. T., Flaisch T., Stockburger J., Junghöfer M. (2006). Emotion and attention: event-related brain potential studies. Prog. Brain Res. 156, 31–51 10.1016/S0079-6123(06)56002-9 [DOI] [PubMed] [Google Scholar]
- Schupp H. T., Junghöfer M., Weike A. I., Hamm A. O. (2003). Emotional facilitation of sensory processing in the visual cortex. Psychol. Sci. 14, 7–13 [DOI] [PubMed] [Google Scholar]
- Schupp H. T., öhman A., Junghöfer M., Weike A. I., Stockburger J., Hamm A. O. (2004). The facilitated processing of threatening faces: an ERP analysis. Emotion 4, 189–200 10.1037/1528-3542.4.2.189 [DOI] [PubMed] [Google Scholar]
- Slovic P., Peters E. (2006). Risk perception and affect. Curr. Dir. Psychol. Sci. 15, 322–325 [Google Scholar]
- Sergent C., Baillet S., Dehaene S. (2005). Timing of the brain events underlying access to consciousness during the attentional blink. Nat. Neurosci. 8, 1391–1400 [DOI] [PubMed] [Google Scholar]
- Shuper P. A., Fisher W. A. (2008). The role of sexual arousal and sexual partner characteristics in HIV+ MSM's intentions to engage in unprotected sexual intercourse. Health Psychol. 27, 445–454 10.1037/0278-6133.27.4.445 [DOI] [PubMed] [Google Scholar]
- Stacy A. W., Ames S. L., Ullman J. B., Zogg J. B., Leigh B. C. (2006). Spontaneous cognition and HIV risk behavior. Psychol. Addict. Behav. 20, 196–206 [DOI] [PubMed] [Google Scholar]
- Strack F., Deutsch R. (2004). Reflective and impulsive determinants of social behavior. Pers. Soc. Psychol. Rev. 8, 220–247 [DOI] [PubMed] [Google Scholar]
- Swann W. B., Silvera D. H., Proske C. U. (1995). On “knowing your partner”: dangerous illusions in the age of AIDS? Pers. Relatsh. 2, 173–186 10.1111/j.1475-6811.1995.tb00084.x [DOI] [Google Scholar]
- Thompson S. C., Anderson K., Freedman D., Swan J. (1996). Illusions of safety in a risky world: a study of college students’ condom use. J. Appl. Soc. Psychol. 26, 189–210 [Google Scholar]
- Thompson S. C., Kent D. R., Thomas C., Vrungos S. (1999). Real and illusory control over exposure to HIV in college students and gay men. J. Appl. Soc. Psychol. 29, 1128–1150 [Google Scholar]
- Thompson S. C., Kyle D., Swan J., Thomas C., Vrungos S. (2002). Increasing condom use by undermining perceived invulnerability to HIV. AIDS Educ. Prev. 14, 505–514 [DOI] [PubMed] [Google Scholar]
- Thorpe S., Fize D., Marlot C. (1996). Speed of processing in the human visual system. Nature 381, 520–522 10.1038/381520a0 [DOI] [PubMed] [Google Scholar]
- Todorov A., Engell A. (2008). The role of the amygdala in implicit evaluation of emotionally neutral faces. Soc. Cogn. Affect. Neurosci. 3, 303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A., Mandisodza A. N., Goren A., Hall C. C. (2005). Inferences of competence from faces predict election outcomes. Science 308, 1623–1626 10.1126/science.1110589 [DOI] [PubMed] [Google Scholar]
- Todorov A., Said C. P., Engell A. D., Oosterhof N. N. (2008). Understanding evaluation of faces on social dimensions. Trends Cogn. Sci. (Regul. Ed.) 12, 455–460 [DOI] [PubMed] [Google Scholar]
- Van Berkum J. J., Holleman B., Nieuwland M., Otten M., Murre J. (2009). Right or wrong? The brain's fast response to morally objectionable statements. Psychol. Sci. 20, 1092–1099 [DOI] [PubMed] [Google Scholar]
- Wagner A. D., Schacter D. L., Rotte M., Koutstaal W., Maril A., Dale A. M., Rosen B. R., Buckner R. L. (1998). Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 281, 1188–1191 10.1126/science.281.5380.1188 [DOI] [PubMed] [Google Scholar]
- Weinstein N. D. (2000). Perceived probability, perceived severity, and health-protective behavior. Health Psychol. 19, 65–74 10.1037/0278-6133.19.1.65 [DOI] [PubMed] [Google Scholar]
- Wessel J. R., Danielmeier C., Ullsperger M. (2011). Error awareness revisited: accumulation of multimodal evidence from central and autonomic nervous systems. J. Cogn. Neurosci. [Epub ahead of print]. 10.1162/jocn.2011.21635 [DOI] [PubMed] [Google Scholar]
- Williams S. S., Kimble D. L., Covell N. H., Weiss L. H., Newton K. J., Fisher J. (1992). College students use implicit personality theory instead of safer sex. J. Appl. Soc. Psychol. 22, 921–933 [Google Scholar]
- Willis J., Todorov A. (2006). First impressions: making up your mind after a 100-ms exposure to a face. Psychol. Sci. 17, 592–598 [DOI] [PubMed] [Google Scholar]
- Yeung N., Botvinick M. M., Cohen J. D. (2004). The neural basis of error-detection: conflict monitoring and the error-related negativity. Psychol. Rev. 111, 931–959 [DOI] [PubMed] [Google Scholar]
- Yeung N., Sanfey A. G. (2004). Independent coding of reward magnitude and valence in the human brain. J. Neurosci. 24, 6258–6264 10.1523/JNEUROSCI.4537-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowitz L. A., McDonald S. (1991). The impact of litigants’ babyfacedness and attractiveness on adjudications in small claims courts. Law Hum. Behav. 15, 603–623 [Google Scholar]