Abstract
Hematopoietic stem cell transplantation (HSCT) for the treatment of severe autoimmune disorders continues to show great promise. The morbidity and mortality of the approach is relatively low and clinical benefit has been demonstrated in many, but not all patients. Furthermore, relapse following HSCT is not uncommon. Most centers now prefer onmyeloablative conditioning regimens using high dose cyclophosphamide prior to SCT; however, emerging data show that high dose cylophosphamide can be adminstered safely without the need for HSCT. Eliminating the use of HSCT after high dose cyclophosphamide shortens the duration of the procedure by several weeks, markedly reduces the cost of the procedure and eliminates the potential of reinfusing autoreactive lymphoctes with the autograft.
Keywords: High-dose cyclophosphamide, aldehyde dehydrogenase, autoimmunity
Introduction
Hematopoietic stem cell transplantation (HSCT) has shown great promise for treating severe autoimmune diseases [1,2]. The goal of this therapy is to eliminate the autoimmune response, and to provide long-term benefit or cure. The source of hematopoietic stem cells for HSCT can be allogeneic or autologous; however, most transplants performed for autoimmune diseases use autologous donors due to the relatively high risk of morbidity and mortality from allogeneic HSCT. There are three major components to autologous HSCT: (1) stem cell mobilization/collection; (2) conditioning with high doses of chemotherapy; and (3) hematopoietic stem cell infusion. The therapeutic potency of autologous HSCT is almost entirely derived from the immunosuppressive properties of the conditioning regimen, with the stem cells primarily a rescue procedure for hastening hemotopoietic recovery. A concern with autologous HSCT for autoimmunity is that the mobilized product contains numerous effector cells (auto-reactive lymphocytes) that may contribute to relapse. To reduce the risk of re-infusing auto-reactive effector cells, several groups have attempted to purge the autograft of lymphocytes.
High-dose cyclophosphamide (CY; 200 mg/kg divided over 4 consecutive days) forms the foundation of most conditioning regimens used for autoimmunity, because of its potent immunosuppressive activity. However, while high-dose CY produces near lymphoablation, it spares hematopoietic stem cells as full hematopoietic reconstitution occurs within 2 weeks after treatment. Differentiation of hematopoietic stem cells into lymphocytes in the presence of the autoantigen can lead to immunological tolerance, as it does during ontogeny in effect “rebooting” the immune system [3]. This novel approach to the treatment of severe autoimmune disease is relatively safe and effective; and long-term disease control for some, but not all, autoimmune diseases has been demonstrated. This review will discuss the rationale and the potential advantages and disadvantages of high-dose CY for the treatment of severe autoimmune disease.
Rationale for high-dose CY without BMT [4]
Pharmacology of high-dose CY
CY is a prodrug, and is converted to 4-hydroxycyclophosphamide and its tautomer aldophosphamide by the hepatic cytochrome P-450 system. These compounds diffuse into cells and are converted into the active alkylating compound, phosphoramide mustard, through simple intracellular decomposition. The major mechanism of CY detoxification appears to be inactivation of aldophosphamide by cellular aldehyde dehydrogenase (ALDH) to form the inert compound, carboxyphosphamide. ALDH1A1 (also known as ALDH1 or retinaldehyde dehydrogenase 1) appears to be the ALDH isoenzyme most responsible for CY detoxification [5]. ALDH1A1 also plays an important role in ethanol metabolism, but its major function appears to be the biosynthesis of retinoic acid from vitamin A (retinol) [4]. After alcohol dehydrogenase oxidizes retinol to retinaldehyde, ALDH1A1 oxidizes retinaldehyde to retinoic acid. Retinoic acid is essential for cellular growth and differentiation. Cells with high proliferative potential and thus a requirement retinoic acid, such as hematopoietic stem cells, are relatively resistant to CY because they express high levels of ALDH1A1 [6–8]. Lymphocytes have low levels of ALDH1A1 and are rapidly killed by high-dose CY. High-dose CY is therefore highly immunosuppressive, but not myeloablative, allowing endogenous hematopoietic stem cells to rapidly reconstitute hematopoiesis. Although high-dose CY as a single agent is non-myeloablative, it is also a component of most commonly employed myeloablative regimens, such as busulfan (Bu)/CY and CY/TBI.
Myeloablative versus non-myeloablative conditioning for HSCT in autoimmune diseases
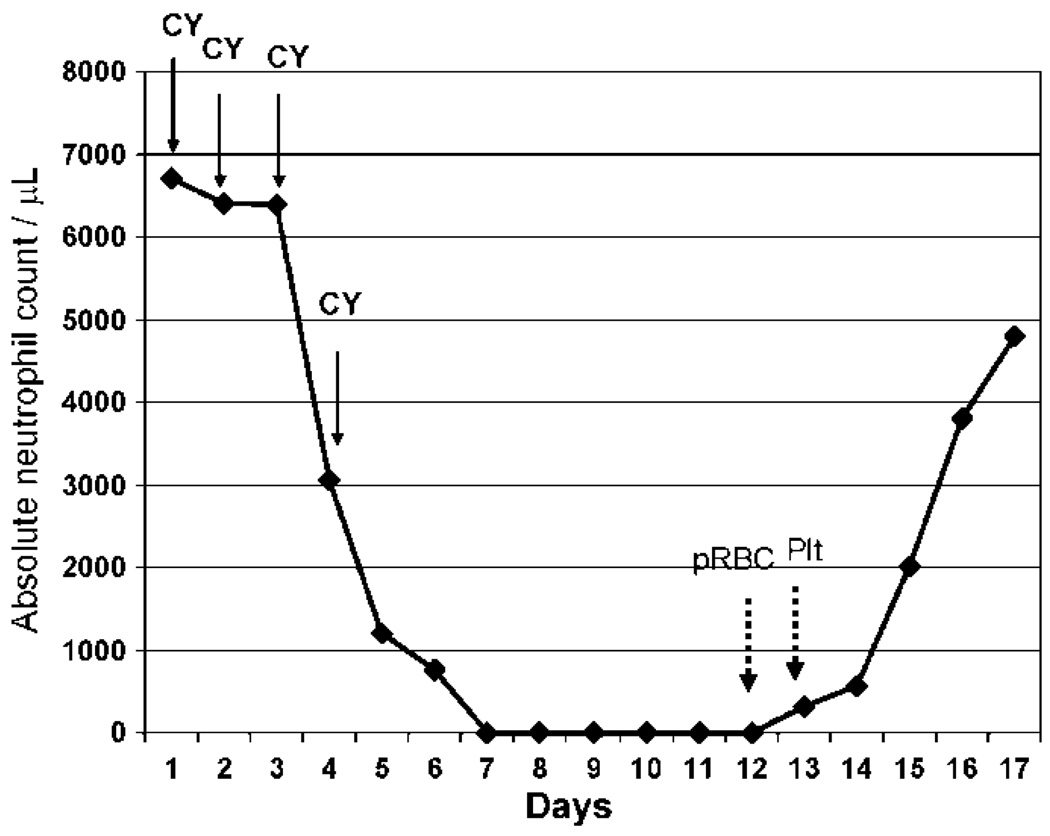
The European Group for Blood and Marrow Transplantation (EBMT) and European League Against Rheumatism (EULAR) has established a registry to compile the results of numerous phase I/II studies of HSCT for the treatment of autoimmune disease. In the United States, the results of HSCT for the treatment of autoimmune disease are captured by the International Bone Marrow Transplantation Registry (IBMTR). Together, these groups have compiled data on more than 700 patients. Although the diseases, conditioning regimens, source of stem cells, and selection of patients have been heterogenous, a tremendous amount of information has been collected. A 2005 EBMT/EULAR report on 473 patients found an overall mortality rate of 11% and a treatment related mortality of 7% [9]. The most common autoimmune diseases transplanted, accounting for roughly 50% of the cases, were multiple sclerosis and systemic sclerosis. Interestingly, a high-dose CY based, non-myeloablative, conditioning regimen has been used in over 50% of the HSCT performed by the EMBT/EULAR and the IBMTR allowing for subset analysis between myeloablative and non-myeloablative conditioning regimens. So far, there has been an increase in treatment-related mortality with myeloablative regimens and no clear advantage to the use of myeloablative conditioning regimens in terms of remission induction and relapse rate leading many investigators to favor an evolution from myeloablative to lymphoablative (non-myeloablative) regimens [1,2]. Thus, high-dose CY (200 mg/kg divided over 4 consecutive days), often with other non-myeloablative agents such as anti-thymocyte globulin (ATG), has become the preferred conditioning for HSCT in autoimmune diseases. However, it is now clear that prompt hematopoietic reconstitution occurs after high-dose CY with or without the HSCT (Figure 1).
Figure 1.
Kinetics of recovery after high-dose cyclophosphamide (CY) without HSCT. Neutrophil recovery in a 29 year-old lupus patient after high-dose CY. CY (50mg/kg/day) was administered intravenously on days 1 through 4. The patient received 2 units of packed red blood cells pRBC on day 12 and 1 platelet transfusion (Plt) on day 13. No other blood products were required.
Role of autologous HSCT after non-myeloablative conditioning for autoimmune diseases
The clinical activity of autologous HSCT is derived entirely from the conditioning regimen, and thus the primary reason to give autologous HSCT after non-myeloablative conditioning is to reduce morbidity and mortality by shortening the duration of hematologic cytopenias. Conversely, autologous HSCT carries the potential risk of reinfusing autoreactive effector cells with the autograft. However, it is not clear from the published literature whether the duration of neutropenia and thrombocytopenia or the need blood transfusions after high-dose CY is improved by HSCT support. This is made more difficult by the different methods of counting days to hematologic recovery after high CY depending on whether HSCT is used. Specifically, day 1 after HSCT is 3 days after completion of the last dose of CY: day-1 is often a “day of rest” and day 0 is the transplant. In fact, by including the 2–4 days of neutropenia that typically occur with mobilizing doses of CY, HSCT may actually be associated with more days of hematologic cytopenias than high-dose CY without HSCT.
Accordingly, the treatment-related mortality was 4%, with patients requiring a mean of 5.4 units of packed red cells, in 50 patients with SLE treated with high-dose CY followed by HSCT [10]. In contrast, in over 40 patients with SLE treated with high-dose CY without HSCT by the John Hopkins group, there has been no treatment related mortality and patients required a median of only 2 units of packed red cells (unpublished data) [11]. Moreover, the median number of hospitalized days was more than 7 days less in the Hopkins patients. Thus, it does not appear that HSCT improves on the rapid hematopoietic recovery and low morbidity seen after high-dose CY therapy; in fact, it appears that when stem cell mobilization is taken into account, there may actually be an increase in duration of cytopenias and length of hospitalization associated with HSCT after high-dose CY. Elimination of the mobilization procedure and infusion of stem cells not only avoids any risk of infusing autoreactive effector cells with the autograft, but also significantly reduces the cost of the procedure. It has been suggested that the mobilization dose of CY adds to the therapeutic efficacy of HSCT [2]. Currently, there is no data to support this claim, but even if true, a single dose of CY without collecting stem cells or transplanting them could easily be administered 1 month before high-dose CY.
Results of high-dose CY in autoimmune disease
Acquired severe aplastic anemia
Acquired severe aplastic anemia (SAA) manifests with severe pancytopenia and a hypocellular bone marrow [12]. In the majority of cases, bone marrow failure is the consequence of autoimmunity; cytotoxic T cells serve as the principal effector cells and hemopoietic stem cells are the target cells. Without treatment, over 50% of SAA patients will die within 2 years of diagnosis usually from infectious complications. In 1972, Thomas and colleagues [13] reported the first successful allogeneic HSCT in a human being. The patient was a 16-year-old boy with SAA and the conditioning regimen was high-dose CY (200 mg/kg divided over 4 consecutive days). Several years later, autologous hematopoietic recovery after allogeneic HSCT for SAA was reported by several groups [14–17]. These reports suggested that the immunosuppressive properties of high-dose CY alone could restore hematopoiesis in patients with SAA. Indeed, in 1976, Baran reported a case of SAA treated with high-dose CY without HSCT [18]. Since then, numerous reports have confirmed that high-dose CY therapy can produce durable remissions, without need for other drug therapy, in most patients with SAA [19–25]. With a median follow-up of 41 months, investigators from Johns Hopkins reported an 86% probability of survival and a 74% chance of achieving remission in 38 SAA patients after treatment with high-dose CY [26]. While it remains controversial whether high-dose CY is more effective than traditional immunosuppressive therapy for SAA patients, the potential for high-dose CY therapy to produce durable complete remissions in SAA is irrefutable.
Other severe autoimmune diseases
The success of high-dose CY therapy in SAA stimulated interest in studying this treatment in other severe refractory autoimmune diseases. The first report was from investigators at Johns Hopkins and Drexel who treated 8 patients with a variety of severe refractory autoimmune disorders (2 systemic lupus erythematosus, 2 Felty’s syndrome, 1 immune thrombocytopenia, 2 autoimmune hemolytic anemia and 1 chronic inflammatory demyelinating polyneuropathy) with high-dose CY [3]. Seven patients showed marked clinical improvement: 5 achieved complete remission and 2 achieved partial remission. Hematopoietic reconstitution was rapid with median times to a neutrophil count of 500/µl and platelet independence of 17 and 16 days respectively. Although there are fewer reports of high-dose CY for treatment of autoimmune disease compared to HSCT, durable remissions have been reported in patients with SLE [11,27], autoimmune hemolytic anemia [28], pemphigus vulgaris [29,30], myasthenia gravis [31,32], chronic inflammatory polyneuropathy [33], multiple sclerosis [34], immune thrombocytopenic purpura [3], autoimmune neutropenia [3], severe autoimmune enteritis [35] and autoimmune hepatitis [24]. Petri et al. [10] use high-dose CY to treat 14 patients with severe refractory SLE. There was no mortality with 5 patients achieving a complete remission and 6 patients achieving a partial remission. Moyo et al. [27] treated 9 patients with refractory, transfusion dependent autoimmune hemolytic anemia. All 9 patients became transfusion independent with 6 patients achieving complete remission (normal hemoglobin without need for prednisone or other immunosuppressive therapy). There were no reported relapses with a median follow-up of 15 months and 7 of the 9 patients were able to discontinue steroid therapy. High-dose CY treatment in 12 patients with refractory multiple sclerosis demonstrated stabilization or improvement in the expanded disability severity scale scores (EDSS) in 11 of 12 patients [34]. Neurologic improvement involved changes in gait, bladder control and visual function. There was no mortality and patients received a median of 1 unit of red cells and 1 platelet transfusion; absolute neutropenia lasted a median of 9 (range, 6–12) days.
High-dose CY also has the potential to eradicate alloimmunization [36]. Five patients with SAA who were refractory to platelet transfusions due to HLA-specific antibodies were studied before and after treatment with high-dose CY. Complete remission of SAA was achieved in 4 of the 5 patients. All four responders demonstrated a marked reduction in anti-HLA antibody; in three of the patients the antibody was complete eradicated.
Concluding remarks
High-dose CY is emerging as the conditioning regimen of choice for HSCT in autoimmune disease; however, compelling data now demonstrates that HSCT is not necessary after high-dose CY and may increase the morbidity and mortality of the procedure. Thus, randomized trials comparing high-dose CY with and without HSCT are clearly warranted. The burden of proof is for HSCT to demonstrate a significant advantage in terms of response, risk of relapse, or morbidity/mortality since high-dose CY alone would shorten the duration of the procedure by several weeks, reduce the cost of the procedure by up to 50%, and eliminate the potential reinfusing autoagressive lymphocytes with the autograft.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Tyndall A, Saccardi R. Haematopoietic stem cell transplantation in the treatment of severe autoimmune disease: Results from phase I/II studies, prospective randomized trials and future directions. Clin Exp Immunol. 2005;141(1):1–9. doi: 10.1111/j.1365-2249.2005.02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burt RK, Marmont A, Oyama Y, et al. Randomized controlled trials of autologous hematopoietic stem cell transplantation for autoimmune diseases: The evolution from myeloablative to lymphoablative transplant regimens. Arthritis Rheum. 2006;54(12):3750–3760. doi: 10.1002/art.22256. [DOI] [PubMed] [Google Scholar]
- 3.Brodsky RA, Petri M, Smith BD, et al. Immunoablative high-dose cyclophosphamide without stem-cell rescue for refractory, severe autoimmune disease. Ann Intern Med. 1998;129(12):1031–1035. doi: 10.7326/0003-4819-129-12-199812150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Abbott JD, Huang Y, Liu D, et al. Stromal cell-derived factor-1 alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 5.Duester G. Genetic dissection of retinoid dehydrogenases. Chem Biol Interact. 2001;130–132(1–3):469–480. doi: 10.1016/s0009-2797(00)00292-1. [DOI] [PubMed] [Google Scholar]
- 6.Hilton J. Role of aldehyde dehydrogenase in cyclophosphamide-resistant L1210 leukemia. Cancer Res. 1984;44(11):5156–5160. [PubMed] [Google Scholar]
- 7.Kastan MB, Schlaffer E, Russo JE, et al. Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood. 1990;75(10):1947–1950. [PubMed] [Google Scholar]
- 8.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85(10):2742–2746. [PubMed] [Google Scholar]
- 9.Gratwohl A, Passweg J, Bocelli-Tyndall C, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transplant. 2005;35(9):869–879. doi: 10.1038/sj.bmt.1704892. [DOI] [PubMed] [Google Scholar]
- 10.Burt RK, Traynor A, Statkute L, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295(5):527–535. doi: 10.1001/jama.295.5.527. [DOI] [PubMed] [Google Scholar]
- 11.Petri M, Jones RJ, Brodsky RA. High-dose cyclophosphamide without stem cell transplantation in systemic lupus erythematosus. Arthritis Rheum. 2003;48(1):166–173. doi: 10.1002/art.10752. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky RA, Jones RJ. Aplastic anaemia. Lancet. 2005;365(9471):1647–1656. doi: 10.1016/S0140-6736(05)66515-4. [DOI] [PubMed] [Google Scholar]
- 13.Thomas ED, Storb R, Fefer A, et al. Aplastic anaemia treated by marrow transplantation. Lancet. 1972;1(7745):284–289. doi: 10.1016/s0140-6736(72)90292-9. [DOI] [PubMed] [Google Scholar]
- 14.Thomas ED, Storb R, Giblett ER, et al. Recovery from aplastic anemia following attempted marrow transplantation. Exp Hematol. 1976;4(2):97–102. [PubMed] [Google Scholar]
- 15.Sensenbrenner LL, Steele AA, Santos GW. Recovery of hematologic competence without engraftment following attempted bone marrow transplantation for aplastic anemia: Report of a case with diffusion chamber studies. Exp Hematol. 1977;5(1):51–58. [PubMed] [Google Scholar]
- 16.Gmur J, Vonfelten A, Rhyner K, et al. Autologous hematologic recovery from aplastic anemia following high dose cyclophosphamide and HLA-matched allogeneic bone marrow transplantation. Acta Haematol. 1979;62:20–24. [Google Scholar]
- 17.Speck B, Cornu P, Jeannet M, et al. Autologous marrow recovery following allogeneic marrow transplantation in a patient with severe aplastic anemia. Exp Hematol. 1976;4(3):131–137. [PubMed] [Google Scholar]
- 18.Baran DT, Griner PF, Klemperer MR. Recovery from aplastic anemia after treatment with cyclophosphamide. N Engl J Med. 1976;295(27):1522–1523. doi: 10.1056/NEJM197612302952708. [DOI] [PubMed] [Google Scholar]
- 19.Brodsky RA, Sensenbrenner LL, Jones RJ. Complete remission in severe aplastic anemia after high-dose cyclophosphamide without bone marrow transplantation. Blood. 1996;87(2):491–494. [PubMed] [Google Scholar]
- 20.Brodsky RA, Sensenbrenner LL, Smith BD, et al. Durable treatment-free remission after high-dose cyclophosphamide therapy for previously untreated severe aplastic anemia. Ann Intern Med. 2001;135(7):477–483. doi: 10.7326/0003-4819-135-7-200110020-00006. [DOI] [PubMed] [Google Scholar]
- 21.Jaime-Perez JC, Gonzalez-Llano O, Gomez-Almaguer D. High-dose cyclophosphamide in the treatment of severe aplastic anemia in children. Am J Hematol. 2001;66(1):71. doi: 10.1002/1096-8652(200101)66:1<71::AID-AJH1019>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Yin S, Xie S, et al. Treatment of severe aplastic anemia using high-dose cyclophosphamide alone in China. Haematologica. 2000;85:E06. [PubMed] [Google Scholar]
- 23.Tisdale JF, Dunn DE, Geller N, et al. High-dose cyclophosphamide in severe aplastic anaemia: A randomised trial. Lancet. 2000;356(9241):1554–1559. doi: 10.1016/S0140-6736(00)03126-3. [DOI] [PubMed] [Google Scholar]
- 24.Brodsky RA, Chen AR, Brodsky I, Jones RJ. High-dose cyclophosphamide as salvage therapy for severe aplastic anemia. Exp Hematol. 2004;32(5):435–440. doi: 10.1016/j.exphem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Savage WJ, DeRusso PA, Resar LM, et al. Treatment of hepatitis-associated aplastic anemia with high-dose cyclophosphamide. Pediatr Blood Cancer. 2007;49(7):947–951. doi: 10.1002/pbc.21143. [DOI] [PubMed] [Google Scholar]
- 26.Brodsky R, Chen A, Dorr D. High dose cyclophosphamide for severe aplastic anemia: Safety and long term follow-up [abstract] Blood. 2005;106:41a–42a. doi: 10.1182/blood-2009-06-225375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gladstone DE, Prestrud AA, Pradhan A, et al. High-dose cyclophosphamide for severe systemic lupus erythematosus. Lupus. 2002;11(7):405–410. doi: 10.1191/0961203302lu229oa. [DOI] [PubMed] [Google Scholar]
- 28.Moyo VM, Smith D, Brodsky I, et al. High-dose cyclophosphamide for refractory autoimmune hemolytic anemia. Blood. 2002;100(2):704–706. doi: 10.1182/blood-2002-01-0087. [DOI] [PubMed] [Google Scholar]
- 29.Nousari HC, Brodsky RA, Jones RJ, et al. Immunoablative high-dose cyclophosphamide without stem cell rescue in paraneoplastic pemphigus: Report of a case and review of this new therapy for severe autoimmune disease. J Am Acad Dermatol. 1999;40(5 Pt 1):750–754. doi: 10.1016/s0190-9622(99)70157-x. [DOI] [PubMed] [Google Scholar]
- 30.Hayag MV, Cohen JA, Kerdel FA. Immunoablative high-dose cyclophosphamide without stem cell rescue in a patient with pemphigus vulgaris. J Am Acad Dermatol. 2000;43(6):1065–1069. doi: 10.1067/mjd.2000.110397. [DOI] [PubMed] [Google Scholar]
- 31.Drachman DB, Jones RJ, Brodsky RA. Treatment of refractory myasthenia: “Rebooting” with high-dose cyclophosphamide. Ann Neurol. 2003;53(1):29–34. doi: 10.1002/ana.10400. [DOI] [PubMed] [Google Scholar]
- 32.Gladstone DE, Brannagan TH, 3rd, Schwartzman RJ, et al. High dose cyclophosphamide for severe refractory myasthenia gravis. J Neurol Neurosurg Psychiatry. 2004;75(5):789–791. doi: 10.1136/jnnp.2003.019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brannagan TH, 3rd, Pradhan A, Heiman-Patterson T, et al. High-dose cyclophosphamide without stem-cell rescue for refractory CIDP. Neurology. 2002;58(12):1856–1858. doi: 10.1212/wnl.58.12.1856. [DOI] [PubMed] [Google Scholar]
- 34.Gladstone DE, Zamkoff KW, Krupp L, et al. High-dose cyclophosphamide for moderate to severe refractory multiple sclerosis. Arch Neurol. 2006;63(10):1388–1393. doi: 10.1001/archneur.63.10.noc60076. [DOI] [PubMed] [Google Scholar]
- 35.Oliva-Hemker MM, Loeb DM, Abraham SC, Lederman HM. Remission of severe autoimmune enteropathy after treatment with high-dose cyclophosphamide. J Pediatr Gastroenterol Nutr. 2003;36(5):639–643. doi: 10.1097/00005176-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Brodsky RA, Fuller AK, Ratner LE, et al. Elimination of alloantibodies by immunoablative high-dose cyclophosphamide. Transplantation. 2001;71(3):482–484. doi: 10.1097/00007890-200102150-00025. [DOI] [PubMed] [Google Scholar]